Abstract
AIM: To assess the diagnostic concordance between endoscopic and histological atrophy in the United Kingdom and Japan.
METHODS: Using published data, a total of 252 patients, 126 in the United Kingdom and 126 in Japan, aged 20 to 80 years, were evaluated. The extent of endoscopic atrophy was classified into five subgroups according to a modified Kimura-Takemoto classification system and was compared with histological findings of atrophy at five biopsy sites according to the updated Sydney system.
RESULTS: The strength of agreement of the extent of atrophy between histology and visual endoscopic inspection showed good reproducibility, with a weighted kappa value of 0.76 (P < 0.001). Multivariate analysis showed that three factors were associated with decreased concordance: Japanese ethnicity [odds ratio (OR) 0.22, 95% confidence interval (CI) 0.11-0.43], older age (OR = 0.32, 95%CI: 0.16-0.66) and endoscopic atrophy (OR = 0.10, 95%CI: 0.03-0.36). The strength of agreement between endoscopic and histological atrophy, assessed by cancer risk-oriented grading, was reproducible, with a kappa value of 0.81 (95%CI: 0.75-0.87). Only nine patients (3.6%) were endoscopically underdiagnosed with antral predominant rather than extensive atrophy and were considered false negatives.
CONCLUSION: Endoscopic grading can predict histological atrophy with few false negatives, indicating that precancerous conditions can be identified during screening endoscopy, particularly in patients in western countries.
Keywords: Gastritis, Atrophy, Histology, Endoscopy, Diagnosis
Core tip: Gastric atrophy is generally regarded as a precancerous condition. Thus, improvements in methods used to diagnose atrophy may identify patients at risk for gastric cancer. Our data on endoscopic assessment could be compared with diagnosis by an expert histopathologist with a weighted kappa value. Our data of this agreement is better than the inter-observer agreement between two histopathologists reported before. Thus, our results suggest that endoscopic atrophy grading can predict extensive histological atrophy and may serve as a practical assessment of precancerous conditions during endoscopy in routine clinical practice, especially for patients in western countries.
INTRODUCTION
Gastric adenocarcinoma continues to be a leading cause of cancer-related deaths in many parts of the world. Patients at increased risk of gastric adenocarcinoma may be identified by endoscopic screening for precancerous conditions. According to the Correa hypothesis, gastric carcinogenesis is a progressive process, from chronic gastritis to gastric atrophy and then to dysplasia or cancer. Thus, gastric atrophy is generally regarded as a precancerous condition[1,2].
Following the identification of Helicobacter pylori (H. pylori) in 1983[3], it became apparent that longstanding infection often leads to atrophic gastritis and that infection and the development of gastric cancer are strongly associated. These observations were confirmed by a long-term prospective trial, showing not only that the histological severity of gastritis, atrophy and intestinal metaplasia was predictive of cancer but that the anatomical distribution of the gastritis was even more important[4]. Patients with corpus predominant or pangastritis were at much higher risk of cancer than patients with antral predominant gastritis[4,5]. Thus, endoscopic evaluation should assess both the presence and anatomic location of atrophic gastritis in determining the potential risk of future cancer.
In Japan, where a large number of patients have atrophic gastritis caused by H. pylori infection, endoscopy has long been used to assess the macroscopic changes associated with gastritis. An endoscopic scoring system was developed to determine the extent of atrophy by identifying the atrophic border[6,7]. defined as the transition zone between non-atrophic and atrophic gastritis, in the stomach. Endoscopic atrophy was found to closely correlate with gastric cancer[4,8]. This approach, however, is not widely used in western countries, with few endoscopists taking multiple biopsies during routine upper digestive endoscopy. Moreover, it is unclear whether histological atrophy correlates with endoscopic atrophy in western patients.
Improvements in methods used to diagnose atrophic gastritis may identify patients at risk for gastric cancer. This study, using previously published data[5], compared the endoscopic and histological diagnosis of gastric atrophy in patients in the United Kingdom and Japan. Endoscopic atrophy, determined using the Kimura-Takemoto classification system, was compared with histological atrophy, determined using the updated Sydney classification system.
MATERIALS AND METHODS
Study population
The study was performed at Leeds General Infirmary in the United Kingdom and the National Cancer Centre Hospital in Tokyo, Japan. The data were from a cross sectional, cluster sampling study of patients[5], and were used as a historical study. Twenty-one patients in each 10-year age group from 20 to 80 years were recruited in both Leeds and Tokyo between May 2000 and April 2002. Other inclusion criteria were epigastric pain as the predominant symptom and no endoscopic evidence of reflux esophagitis, peptic ulcer disease, or malignancy. Patients were excluded if they had received H. pylori eradication therapy in the past; had been treated with any non-steroidal anti-inflammatory drug, proton pump inhibitor, antibiotic, or bismuth-containing compound during the previous 2 wk; or had received H2 receptor antagonists in the previous 2 d.
Three endoscopists (T Gotoda in Tokyo and L Gatta and G Naylor in Leeds) trained together for 2 mo to ensure standardization of patient selection, recruitment, biopsy sites, processing of histological and microbiological specimens, blood sampling, and storage. The study protocol was approved by the local ethics committees, and all patients provided written informed consent.
Histology
Biopsy samples were obtained using standard endoscopy biopsy forceps from the five sites specified in the updated Sydney system (Figure 1). Each tissue sample was placed in a separate bottle of 10% formalin and embedded in paraffin for sectioning. Sections were stained with hematoxylin and eosin (HE) and evaluated. In this study, results diagnosed by one super-expert histopathologist in Leeds were considered histological atrophy. The pathologist was blinded to the age and sex of the subjects. The graded features were scored according to the updated Sydney system for atrophy. Patients were considered positive for histological atrophy if the score was mild, moderate or marked in each location.
Figure 1.
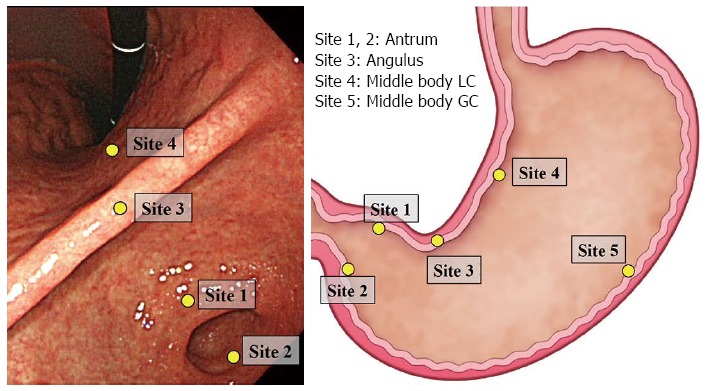
Location of biopsy sites according to the updated Sydney system. GC: Greater curvature; LC: Lesser curvature.
Endoscopy examination
Endoscopies were performed by the three previously described experienced endoscopists using GIF-Q240 conventional gastroscopes (Olympus Co., Tokyo, Japan). Atrophic features included discoloration of the mucosa, greater visibility of a submucosal vascular pattern due to thinning of the mucosa, uneven surfaces and fold disappearance. The border between atrophic and non-atrophic mucosa was defined endoscopically by differences in the color and height of the gastric mucosa. This atrophic border has been confirmed by assessments of sequential biopsy specimens[6].
The atrophic border crosses the angulus on the lesser curvature in the C-1 pattern, the lower and middle parts of the corpus in the C-2 pattern, and the upper part of the corpus in the C-3 pattern (Figure 2). The atrophic border, which is parallel to the vertical axis of the stomach, is on the lesser curvature in the O-1 pattern, on the anterior and posterior walls in the O-2 pattern, and on the greater curvature in the O-3 pattern. The C-1 pattern represents highly localized antral atrophy, with subsequent lines representing increasing extension through the lesser and greater curvatures. The O-3 pattern represents extensive atrophy, affecting almost the entire stomach.
Figure 2.
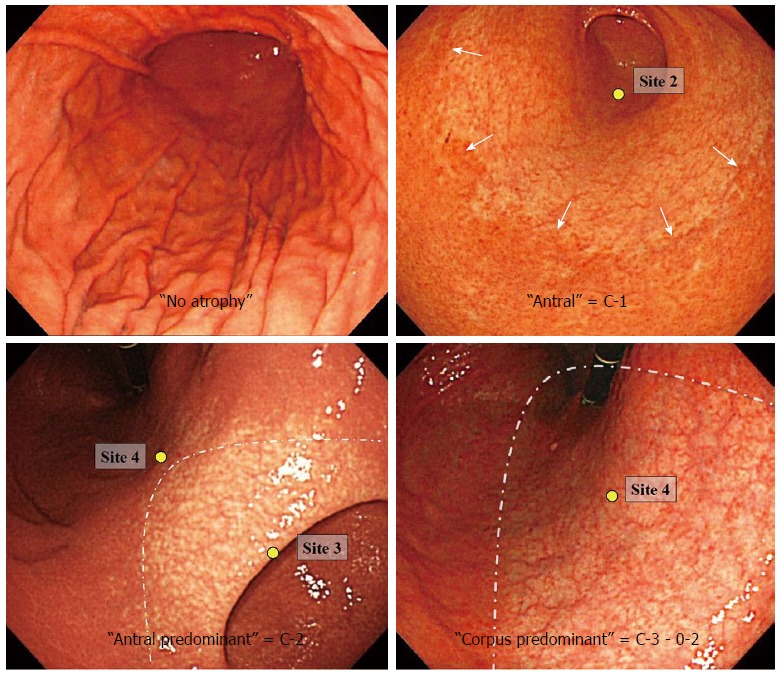
Endoscopic atrophy grades.
Definition of concordance
The endoscopic findings of the extent of atrophy were compared with the histological findings of glandular atrophy at five biopsy sites (Figure 1). To compare the extent of atrophy strictly, both classifications were modified to five grades according to definitions of those anatomical locations. Histological grading was scored as 1, none; 2, antrum (site 1 and/or 2); 3, angulus (up to site 3); 4, the middle body of the lesser curvature (up to site 4) and 5, the middle body of the greater curvature (up to site 5). Endoscopic atrophic grading according to the modified Kimura-Takemoto classification was scored as 1, none; 2; antral (C-1); 3, antral predominant (C-2); 4, corpus predominant (C-3, O-1, O-2) and 5, pan-atrophy (O-3) (Figure 3).
Figure 3.
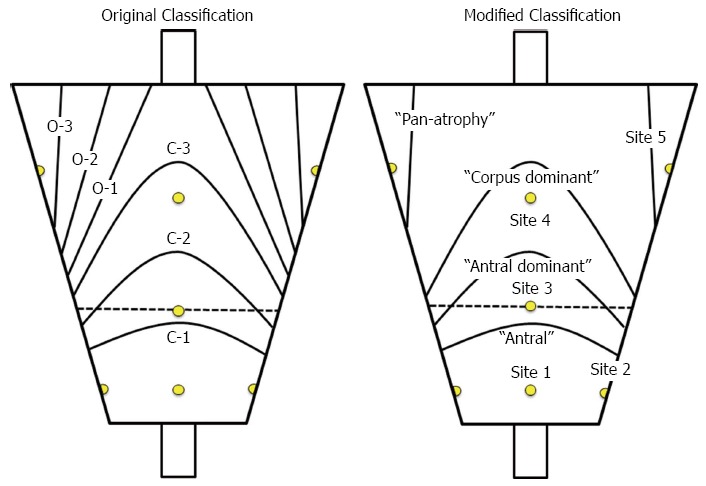
Original and modified Kimura - Takemoto classifications.
Inasmuch as extensive atrophy is associated with a much higher cancer risk than limited atrophy[4], the Kimura-Takemoto classification was simplified to three grades of cancer risk oriented atrophy as: normal (no atrophy), limited atrophy (antral and antral predominant atrophy; C-1, C-2) and extended atrophy (corpus predominant and pan-atrophy; C-3, O-1, O-2, O-3).
Concordance was defined as matching of endoscopic and histologic grades, with all other findings defined as discordance.
Serum pepsinogen levels
Fasting serum was collected from all subjects. The samples were centrifuged immediately at 4 °C and serum stored at -70 °C until used. Serum concentrations of pepsinogen (PG) I and II were measured using a latex-enhanced turbidimetric immunoassay, and the PG I to PG II ratios (PG I/II) were calculated.
H. pylori
Serum samples from all patients were tested by enzyme linked immunosorbent assay for the presence and concentration of IgG antibodies to H. pylori (HM-CAP; Enteric Products Inc., Westbury, NY). A concentration ≥ 1.8 was defined as positive (sensitivity 98.7%, specificity 100%).
Statistical analysis
Analyses were performed with Microsoft Office Exel spreadsheet and SPSS software, version 22 (SPSS Inc, Chicago, IL). χ2 tests and t tests were used to evaluate endoscopic, histological, and serological parameters in patients with gastric atrophy. Agreement between endoscopic and histologic evaluations of the grade of gastric atrophy was assessed by determining the weighted kappa value. Factors associated with extensive atrophy were estimated by univariate logistic regression analysis. Covariates showing a significant association with extensive atrophy by the χ2 or t test were included in multiple logistic regression analyses. A P value < 0.05 was considered statistically significant.
RESULTS
Demographic data
A total of 252 patients aged 20 to 80 years were included in this study, including 126 patients from the United Kingdom and 126 from Japan. Mean (± SD) patient age was 49.4 (± 16.9) years. Of these patients, 107 were male and 145 female; and 103 (40.9%) were serologically H. pylori-positive. The detailed characteristics of the two subgroups are shown in Table 1.
Table 1.
Patient demographic and clinical characteristics n (%)
| Clinical parameters | United Kingdom (n = 126) | Japan (n = 126) | Total (n = 252) | P value (United Kingdom vs Japan) |
| Sex | ||||
| Male | 63 (50.0) | 44 (35.0) | 107 (42.5) | 0.019 |
| Female | 63 (50.0) | 82 (65.0) | 145 (57.5) | |
| Age (yr) | ||||
| ≥ 50 | 63 (50.0) | 65 (51.6) | 128 (50.8) | 0.801 |
| < 50 | 63 (50.0) | 61 (48.4) | 124 (49.2) | |
| Helicobacter pylori IgG | ||||
| Positive | 47 (37.3) | 56 (44.4) | 103 (40.9) | 0.733 |
| Negative | 79 (62.7) | 70 (55.6) | 149 (59.1) | |
| Serologic features (ng/mL), mean ± SD | ||||
| Pepsinogen I | 51.9 ± 40.0 | 50.7 ± 26.8 | 51.3 ± 33.8 | 0.780 |
| Pepsinogen II | 13.0 ± 8.9 | 13.7 ± 9.7 | 13.4 ± 9.3 | 0.552 |
| Pepsinogen I/II ratio | 4.4 ± 1.8 | 4.6 ± 2.2 | 4.5 ± 2.0 | 0.364 |
| Histological atrophy | ||||
| None | 56 (44.4) | 38 (30.2) | 94 (37.3) | < 0.001 |
| Antrum | 49 (38.8) | 19 (15.0) | 68 (27.0) | |
| Angulus | 19 (15.0) | 20 (15.8) | 39 (15.5) | |
| Lesser curvature of middle body | 2 (1.6) | 25 (19.8) | 27 (10.7) | |
| Greater curvature of middle body | 0 (0) | 24 (19.0) | 24 (9.5) | |
| Endoscopic atrophy | ||||
| No atrophy | 56 (44.4) | 27 (21.4) | 83 (32.9) | < 0.001 |
| Antral (C-1) | 40 (31.8) | 33 (26.2) | 73 (29.0) | |
| Antral predominant (C-2) | 24 (19.0) | 20 (15.8) | 44 (17.5) | |
| Corpus predominant (C-3-O-2) | 6 (4.8) | 43 (34.2) | 49 (19.4) | |
| Pan-atrophy (O-3) | 0 (0) | 3 (2.4) | 3 (1.2) |
There were significant differences between the groups from the United Kingdom and Japan, especially in the extent of atrophy. In the United Kingdom, only two patients (1.6%) were diagnosed histologically as having corpus atrophy, whereas, in Japan, 49 of 126 (38.9%) patients had corpus atrophy. Fifty-six (44.4%) patients in the United Kingdom and 38 (30.2%) in Japan showed no evidence of histological atrophy.
Concordance between endoscopic and histological atrophy
Table 2 shows comparisons between endoscopic and histological atrophy scores. Of the 252 patients, 176 (69.8%) showed complete concordance. The strength of agreement between the modified Kimura-Takemoto classification and histological atrophy, as defined by the updated Sydney system, showed good reproducibility, with a weighted kappa value of 0.76 (95%CI: 0.71-0.80).
Table 2.
Cross-tabulation of endoscopic and histological atrophy
| Endoscopic atrophy |
Histological atrophy |
Total | Weighted κ value | ||||
| None | Antrum | Angulus | Middle body LC | Middle body GC | |||
| No atrophy | 79 | 4 | 83 | 0.76 | |||
| Antrum (C-1) | 13 | 51 | 9 | 73 | |||
| Antrum predominant (C-2) | 2 | 12 | 21 | 5 | 4 | 44 | |
| Corpus predominant (C-3-O-2) | 1 | 9 | 22 | 17 | 49 | ||
| Pan-atrophy(O-3) | 3 | 3 | |||||
| Total | 94 | 68 | 39 | 27 | 24 | 252 | |
| Misdiagnosis | 15 | 17 | 18 | 5 | 21 | 76 (30.2%) | |
GC: Greater curvature; LC: Lesser curvature.
A total of 76 patients were endoscopically misdiagnosed, including 37 (14.7%) who were over-diagnosed and 39 (15.5%) who were under-diagnosed. Of the 24 patients histologically diagnosed with atrophy in the middle of the body of the greater curvature, 21 (87.5%) were under-diagnosed endoscopically. Moreover, 15 of 94 (16.0%) patients without histological atrophy were endoscopically over-diagnosed with antral or antral predominat atrophy (Table 2).
Factors affecting concordance
To identify factors affecting the concordance between endoscopic and histological atrophy, univariate analyses were performed; factors analyzed included the patient populations (United Kingdom vs Japan), age, sex, H. pylori infection, endoscopic atrophy (no atrophy vs others) and PG I/II ratios. Factors significantly associated with reduced concordance included Japanese ethnicity (P < 0.001), older age (P < 0.001), low pepsinoge I/II ratio (P = 0.015), endoscopic atrophy (P < 0.001) and H. pylori infection (P = 0.001) (Table 3). In contrast, sex was not significantly associated with reduced concordance (P = 0.143). Multivariate analysis showed that only three factors were independently associated with reduced concordance: Japanese ethnicity, older age and endoscopic atrophy (Table 4).
Table 3.
Univariate analysis of factors significantly associated with reduced concordance between endoscopic and histological atrophy n (%)
| Concordant group (n = 180) | Discordant group (n = 72) | OR | 95%CI | P values | |
| Populations | |||||
| United Kingdom | 107 (84.9) | 19 (15.1) | 1 | Referent | < 0.001 |
| Japan | 69 (54.8) | 57 (45.2) | 0.22 | 0.12-0.39 | |
| Helicobacter pylori IgG | |||||
| Negative | 113 (78.5) | 31 (21.5) | 1 | Referent | 0.001 |
| Positive | 60 (58.3) | 43 (41.7) | 0.38 | 0.22-0.67 | |
| Age (yr) | |||||
| < 50 | 102 (82.3) | 22 (17.7) | 1 | Referent | < 0.001 |
| ≥ 50 | 74 (57.8) | 54 (42.2) | 0.30 | 0.17-0.53 | |
| Sex | |||||
| Male | 80 (74.8) | 27 (25.2) | 1 | Referent | 0.143 |
| Female | 96 (66.2) | 49 (33.8) | 0.66 | 0.38-1.15 | |
| Pepsinogen I/II ratio | |||||
| > 3.0 | 141 (73.8) | 50 (26.2) | 1 | Referent | 0.015 |
| ≤ 3.0 | 35 (57.4) | 26 (42.6) | 0.48 | 0.26-0.87 | |
| Endoscopic atrophy | |||||
| No atrophy | 79 (95.2) | 4 (4.8) | 1 | Referent | < 0.001 |
| Others | 97 (57.4) | 72 (42.6) | 0.07 | 0.02-0.20 |
Table 4.
Multivariate analysis of factors significantly associated with reduced concordance between endoscopic and histological atrophy
| Adjusted OR | 95%CI | P value | |
| Populations | |||
| United Kingdom | 1 | Referent | < 0.001 |
| Japan | 0.22 | 0.11-0.43 | |
| Helicobacter pylori IgG | |||
| Negative | 1 | Referent | 0.268 |
| Positive | 0.6 | 0.31-1.38 | |
| Age (yr) | |||
| < 50 | 1 | Referent | 0.008 |
| ≥ 50 | 0.32 | 0.16-0.66 | |
| Pepsinogen I/II ratio | |||
| > 3.0 | 1 | Referent | 0.328 |
| ≤ 3.0 | 1.50 | 0.67-3.35 | |
| Endoscopic atrophy | |||
| No atrophy | 1 | Referent | < 0.001 |
| Others | 0.10 | 0.03-0.36 |
Greater atrophy: Antral predominant, corpus predominant and pan-atrophy.
Further assessments according to factors significant on multivariate analysis
To further assess the disagreement between histological and endoscopic atrophy, patients were cross-tabulated by each factor found to be significant (Tables 5 and 6). Although lower concordance rates for extensive atrophy were observed in both populations, 19 (15.1%) of the 126 British patients were misdiagnosed, including 15 who were over-diagnosed with histological atrophy of the antrum or angulus. In contrast, 57 (45.2%) of the 126 Japanese patients, of all histological grades, were misdiagnosed, including 22 who were over-diagnosed and 35 who were under-diagnosed. Of the 24 patients with histological atrophy at the greater curvature in the middle body, 21 were under-diagnosed (Table 5). Age also was significantly associated with concordance. A significantly higher percentage of older than of younger patients with histological atrophy of the antrum or angulus or without atrophy were over-diagnosed [27/91 (29.7%) vs 10/110 (9.1%), P < 0.001] (Table 6).
Table 5.
Cross-tabulation of endoscopic and histological atrophy according to populations
| Endoscopic atrophy |
Histological atrophy |
Total | Weighted κ value | ||||
| None | Antrum | Angulus | Middle body LC | Middle body GC | |||
| United Kingdom | |||||||
| No atrophy | 54 | 2 | 56 | 0.81 | |||
| Antrum | 1 | 37 | 2 | 40 | |||
| Antrum predominant | 1 | 9 | 14 | 24 | |||
| Corpus predominant | 1 | 3 | 2 | 6 | |||
| Pan-atrophy | 0 | ||||||
| Total | 56 | 49 | 19 | 2 | 0 | 126 | |
| Japan | |||||||
| No atrophy | 25 | 2 | 27 | 0.68 | |||
| Antrum | 12 | 14 | 7 | 33 | |||
| Antrum predominant | 1 | 3 | 7 | 5 | 4 | 20 | |
| Corpus predominant | 6 | 20 | 17 | 43 | |||
| Pan-atrophy | 3 | 3 | |||||
| Total | 38 | 19 | 20 | 25 | 24 | 126 | |
GC: Greater curvature; LC: Lesser curvature.
Table 6.
Cross-tabulation of endoscopic and histological atrophy according to age
| Endoscopic atrophy |
Histological atrophy |
Total | Weighted κ value | ||||
| None | Antrum | Angulus | Middle body LC | Middle body GC | |||
| Age < 50 yr | |||||||
| No atrophy | 61 | 2 | 63 | 0.81 | |||
| Antrum | 5 | 28 | 3 | 36 | |||
| Antrum predominant | 1 | 3 | 6 | 3 | 1 | 14 | |
| Corpus predominant | 1 | 7 | 3 | 11 | |||
| Pan-atrophy | 0 | ||||||
| Total | 67 | 33 | 10 | 10 | 4 | 124 | |
| Age ≥ 50 yr | |||||||
| No atrophy | 18 | 2 | 20 | 0.67 | |||
| Antrum | 8 | 23 | 6 | 37 | |||
| Antrum predominant | 1 | 9 | 15 | 2 | 3 | 30 | |
| Corpus predominant | 1 | 8 | 15 | 14 | 38 | ||
| Pan-atrophy | 3 | 3 | |||||
| Total | 27 | 35 | 29 | 17 | 20 | 128 | |
GC: Greater curvature; LC: Lesser curvature.
Cancer risk-oriented atrophy grading
When classified according to cancer risk-oriented atrophy grading defined above, 222 (88.1%) of the 252 patients were concordant (Table 7). The strength of agreement between endoscopic and histological assessment by cancer risk-oriented grading showed good reproducibility, with a weighted kappa value of 0.81 (95%CI: 0.75-0.87). However, nine patients (3.6%), all Japanese, were not diagnosed with extended atrophy but with limited atrophy (Table 8). Of these nine patients, seven were women and eight were negative for H. pylori infection. Furthermore, six of the nine patients had PG I levels < 70 ng/mL and eight of nine had PG I/II ratios ≤ 3.
Table 7.
Cancer risk oriented atrophy grade
| Endoscopic atrophy |
Histological atrophy |
Total | Weighted κ value | ||
| Normal | Limited | Extended | |||
| Normal | 79 | 4 | 83 | 0.81 | |
| Limited (C-1-2) | 15 | 93 | 9 | 117 | |
| Extended (C-3-O-3) | 10 | 42 | 52 | ||
| Total | 94 | 107 | 51 | 252 | |
Limited: Atrophy limited antrum and angulus; Extended: Atrophy with corps-predominant or pan-atrophy.
Table 8.
Characteristics of underdiagnosed patients at high-risk of gastric cancer
| No. | United Kingdom/Japan | Sex | Age | Helicobacter pylori antibody | Pepsinogen I (ng/mL) | Pepsinogen II (ng/mL) | Pepsinogen I/II ratio | Endoscopic atrophy |
| 1 | Japan | F | 24 | Negative | 45.1 | 21.3 | 2.1 | Antral predominant |
| 2 | Japan | F | 42 | Negative | 126.0 | 41.4 | 3.0 | Antral predominant |
| 3 | Japan | F | 49 | Negative | 44.8 | 40.9 | 1.1 | Antral predominant |
| 4 | Japan | F | 55 | Negative | 65.4 | 34.3 | 1.9 | Antral predominant |
| 5 | Japan | F | 58 | Negative | 71.4 | 34.3 | 2.1 | Antral predominant |
| 6 | Japan | F | 66 | Negative | 46.9 | 17.4 | 2.7 | Antral predominant |
| 7 | Japan | F | 67 | Negative | 26.7 | 12.2 | 2.2 | Antral predominant |
| 8 | Japan | M | 35 | Positive | 56.0 | 23.8 | 2.4 | Antral predominant |
| 9 | Japan | M | 65 | Negative | 82.4 | 19.6 | 4.2 | Antral predominant |
M: Male; F: Female.
DISCUSSION
Gastric atrophy has been found to progress, accompanied by repeated loss and regeneration of gastric mucosa due to chronic inflammation induced by persistent H. pylori infection[9]. The updated Sydney system was designed to assess histological gastritis and atrophy more objectively and has become the international standard[10]. Although this classification includes assessment of five biopsy sites, this extensive approach is not common in daily practice, because of patient discomfort, cost and time restrictions. Conversely, endoscopic classifications of atrophy are not common, except in Japan.
Several previous studies have assessed the relationship between endoscopic and histological diagnoses of gastritis and atrophy using a variety of evaluation methods. Negative results were reported in a prospective study, which found that endoscopy alone could not reliably diagnose H. pylori gastritis[11]. The absence of rugae and the presence of visible vessels in the gastric mucosa predict severe atrophy but with relatively low sensitivity[12]. Based on these results, the recent guidelines of the European Society of Gastrointestinal Endoscopy, the European Helicobacter Study Group, the European Society of Pathology, and the Sociedade Portuguesa de Endoscopia Digestiva stated that conventional white light endoscopy cannot accurately differentiate among and diagnose preneoplastic gastric conditions[13]. However, these guidelines were based in part on the results of a very old study from the 1950s[14].
Recent studies from Asian countries, however, have yielded different findings. For example, the sensitivity and specificity of endoscopy for the histological diagnosis of atrophy were reported to be 61.5% and 57.7%, respectively, in the antrum, and 46.8% and 76.4%, respectively, in the body of the stomach[15]. That study also reported that mucosal inflammation reduced the sensitivity of endoscopy at both sites, especially in individuals below 50 years of age. Significant correlations between endoscopic atrophy scores based on the Kimura-Takemoto classification and histological atrophy scores based on the Sydney classification were reported in the gastric corpus (r = 0.390) but correlations in the antrum were much weaker[16]. Furthermore, the severity of endoscopic gastric atrophy was found to be significantly associated with the severity of histological gastric atrophy, as assessed by OLGA gastritis stage[17]. The strength of agreement between endoscopic and histological atrophy scores was found to be good, with a weighted kappa value of 0.51[18].
As these methods differed, they are difficult to compare with each other and their results were inconsistent. Furthermore, although sensitivity, specificity or positive predictive value may have been high, there is no obvious standard to decide whether a classification is useful or not. Moreover, despite the updated Sydney system being the international standard for histological assessment, inter-observer agreements between pathologists have been reported to be relatively low[5,19,20]. Our data on endoscopic assessment could be compared with diagnosis by an expert histopathologist. We observed good agreement between endoscopic atrophy grades and histological expert assessment[21], with a weighted kappa value of 0.76. If agreement between a general and an expert histopathologist is not high, our results suggest that endoscopic atrophy grading may be comparable to histological assessment.
This study also assessed the factors affecting concordance. Extended endoscopic atrophy was associated with decreased concordance. Biopsies sample only a tiny area of the entire gastric mucosa and may therefore not be representative of the entire mucosa. It was not surprising, however, that concordance was better for distinguishing between normal and atrophic mucosa than among grades of abnormality.
Endoscopic features associated with atrophy included discoloration of the mucosa, visibility of a submucosal vascular pattern due to thinning of the mucosa, unevenness of the surface mucosa and fold disappearance. However, their presence may result in under-diagnosis, especially if they occur in the middle of the body at the greater curvature. The mucosa in this area is much thicker than on the lesser curvature, as well as being the most difficult area of the stomach to distend by air insufflation. Thus, discoloration of the mucosa and fold disappearance in this area may be more difficult to detect, with changes associated with atrophy perhaps being overlooked and underestimated.
The concordance rate for atrophy was significantly higher for patients in the United Kingdom than for those in Japan. However, many more patients in Japan had extended atrophy, which is more easily misdiagnosed. The two populations also had different concordances on multivariate analysis, suggesting that this difference may be associated with differences in the background of the two populations. Indeed, the two populations differed in host genetic factors, diet, and bacterial virulence. For example, there are ethnic differences in the protein encoded by the H. pylori cytotoxin associated gene A (CagA), one of the most important virulence factors for gastric mucosal injury and atrophy. The CagA gene is polymorphic and is primarily classified into East Asian and Western types based on sequences in its 3′ coding region[22]. Previous studies have clarified the differences in gastritis and atrophy among patients infected with East Asian CagA-positive, Western CagA-positive, and CagA-negative H. pylori[23], with differences in virulence perhaps causing differences in concordance rates.
The histological structures of the normal antral and corpus mucosa differ, with the border between these two areas located at the angulus[24,25]. This anatomically defined border is difficult to detect clearly using conventional endoscopy[25], although it can be better detected using high definition equipment. However, a slight difference in color between the antrum and the corpus may occur in the absence of histological atrophy. Mistaking this anatomical border for the atrophic border may result in over-diagnosis. We found that older patients, particularly older Japanese, tended to be over-diagnosed when their histological atrophy was not too extensive. Two possibilities may explain this finding. The first is that our study was open-labelled; thus there may have been observer bias because elderly Japanese are more likely to be infected with H. pylori. The second reason is that gastric mucosal blood flow decreases with age[26,27], which may affect mucosal appearance. Because of the slight differences in mucosal color, endoscopists may mistake normal for atrophic antral mucosa in older patients.
Advanced endoscopic techniques, such as chromoendoscopy, image-enhanced endoscopy, high definition and magnification, are likely to improve the accuracy of endoscopic diagnosis of atrophy. The endoscopic Congo red test was developed in the 1970s to diagnose the extent of atrophy[28]. However, this test requires gastrin injection to stimulate acid secretion and to observe changes in color. Autofluorescence imaging (AFI) is useful in the diagnosis of mucosal atrophy. Loss of fundic glands through atrophy increases the intensity of AFI, with the mucosa appearing green and the atrophic border more reproducibly identified[29]. The combination of AFI and narrow band imaging (NBI) also improves the detection of pre-cancerous conditions[30]. However, some of these techniques extend the time of the endoscopic procedure, generate additional workload and may reduce patient tolerance for the procedure. A greater number of attributes must be considered, with the increase in modalities employed for diagnosis making it more difficult for endoscopists to agree on a standard workable protocol. Thus their routine use cannot always be recommended. In contrast, conventional endoscopy diagnoses atrophy only by the Kimura-Takemoto classification. Thus, the newest endoscopes are not needed.
A previous study reported that extensive atrophic gastritis indicates a high risk for gastric cancer and that the main purpose of assessing the extent of atrophy is to identify patients at greatest risk for potential future endoscopic surveillance[4]. The results presented here indicate that cancer risk oriented atrophy grading into three groups is much more predictable and its use is acceptable in daily practice. However, it is potentially a serious oversight to assign a patient to an incorrect group. Patients with histological atrophy in the middle of the corpus should be diagnosed endoscopically as having extensive atrophy, indicating that they are at high risk for gastric cancer. Our results showed that nine patients (3.6%) were diagnosed with limited rather than extensive atrophy and were therefore false negatives. Inclusion of patients with antral predominant atrophy in those with extensive atrophy could include all patients with histological atrophy in the middle of the corpus. However, the number of false positive patients would dramatically increase, from 10 to 45; thus, specificity would decrease considerably and the workload increase.
These results suggest a benefit of including additional assessments, such as biopsy, for patients diagnosed with antral predominant atrophy, particularly Japanese women infected with H. pylori. Identification of patients with low pepsinogen I levels and I/II ratios may also improve outcomes.
This study had several limitations. First, it was based on historical study data. Therefore, there was no intent to recruit patients for this study, and these data were relatively old. In addition, the endoscopes used were not the newest type. However, the characteristics of these patients and devices may be similar to those of more recent studies. To support these conclusions, a prospective multicenter trial will be warranted. Second, gastric atrophy was assessed in all patients by three expert gastrointestinal endoscopists. Thus, inter-observer agreement may have been higher than in routine clinical practice. Third, gastritis in most patients was caused by H. pylori infection. The effects of other etiologies are unclear because patients taking non-steroidal anti-inflammatory drugs were excluded. No patient had a characteristic histological appearance of autoimmune gastritis but parietal cell antibodies were not measured.
In conclusion, endoscopic evaluation by the Kimura-Takemoto classification showed good histological evaluation of the risk of stomach cancer. Simplifying this classification to three groups was useful, enabling most patients at high risk to be identified, with relatively few false negatives and an acceptable number of false positives. Indeed histological assessment is fundamental, but endoscopic atrophy grading can predict extensive histological atrophy and may serve as a practical assessment of precancerous conditions during endoscopy in routine clinical practice, especially for patients in western countries.
COMMENTS
Background
Gastric atrophy is generally regarded as a precancerous condition. Thus, improvements in methods used to diagnose atrophy may identify patients at risk for gastric cancer. The updated Sydney system was designed to assess histological gastritis and atrophy more objectively and has become the international standard. Although this classification includes assessment of five biopsy sites, this extensive approach is not common in daily practice. Conversely, endoscopic classifications of atrophy are not common, except in Japan.
Research frontiers
Although routine endoscopy can assess precancerous conditions by evaluating the extent of gastric atrophy, concordance between endoscopic and histological atrophy is unclear. This study therefore assessed the diagnostic concordance between endoscopic and histological atrophy in the United Kingdom and Japan.
Innovations and breakthroughs
Several previous studies have assessed the relationship between endoscopic and histological diagnoses of atrophy using a variety of evaluation methods. As these methods differed, they are difficult to compare with each other and there is no obvious standard to decide whether a classification is useful or not. However, present data on endoscopic assessment could be compared with diagnosis by an expert histopathologist with a weighted kappa value. This study’s data of the agreement is better than the inter-observer agreement between two histopathologists reported before. Thus, present results suggest that endoscopic atrophy grading may be comparable to histological assessment.
Applications
Endoscopic atrophy grading can predict extensive histological atrophy and may serve as a practical assessment of precancerous conditions during endoscopy in routine clinical practice, especially for patients in western countries.
Terminology
Kimura-Takemoto classification: atrophic patterns classified into 7 types according to the location of the atrophic border.
Peer-review
This is a good descriptive study in which authors assess the agreements between endoscopic and histological atrophy by using weighted kappa value. The results are interesting and suggest that endoscopic atrophy grading can predict extensive histological atrophy and may serve as a practical assessment of precancerous conditions during endoscopy in routine clinical practice, especially for patients in western countries.
Footnotes
Institutional review board statement: The study protocol was approved by the local Ethics Committees, and all patients provided written informed consent.
Informed consent statement: All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement: The authors have none to declare.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 11, 2015
First decision: July 10, 2015
Article in press: October 13, 2015
P- Reviewer: Ding SG, Makhoul E S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 2.Correa P. Is gastric cancer preventable? Gut. 2004;53:1217–1219. doi: 10.1136/gut.2004.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. [PubMed] [Google Scholar]
- 4.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 5.Naylor GM, Gotoda T, Dixon M, Shimoda T, Gatta L, Owen R, Tompkins D, Axon A. Why does Japan have a high incidence of gastric cancer? Comparison of gastritis between UK and Japanese patients. Gut. 2006;55:1545–1552. doi: 10.1136/gut.2005.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87–97. [Google Scholar]
- 7.Takemoto T. Endoscopic diagnosis of chronic gastritis. Diagnosis and Treatment. 1966;54:1274–1285. [Google Scholar]
- 8.Yoshimura T, Shimoyama T, Fukuda S, Tanaka M, Axon AT, Munakata A. Most gastric cancer occurs on the distal side of the endoscopic atrophic border. Scand J Gastroenterol. 1999;34:1077–1081. doi: 10.1080/003655299750024850. [DOI] [PubMed] [Google Scholar]
- 9.Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525–1528. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 10.Price AB. The Sydney System: histological division. J Gastroenterol Hepatol. 1991;6:209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 11.Bah A, Saraga E, Armstrong D, Vouillamoz D, Dorta G, Duroux P, Weber B, Froehlich F, Blum AL, Schnegg JF. Endoscopic features of Helicobacter pylori-related gastritis. Endoscopy. 1995;27:593–596. doi: 10.1055/s-2007-1005764. [DOI] [PubMed] [Google Scholar]
- 12.Redéen S, Petersson F, Jönsson KA, Borch K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946–950. doi: 10.1055/s-2003-43479. [DOI] [PubMed] [Google Scholar]
- 13.Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O’Connor A, Pereira C, Pimentel-Nunes P, Correia R, Ensari A, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED) Virchows Arch. 2012;460:19–46. doi: 10.1007/s00428-011-1177-8. [DOI] [PubMed] [Google Scholar]
- 14.Atkins L, Benedict EB. Correlation of gross gastroscopic findings with gastroscopic biopsy in gastritis. N Engl J Med. 1956;254:641–644. doi: 10.1056/NEJM195604052541403. [DOI] [PubMed] [Google Scholar]
- 15.Eshmuratov A, Nah JC, Kim N, Lee HS, Lee HE, Lee BH, Uhm MS, Park YS, Lee DH, Jung HC, et al. The correlation of endoscopic and histological diagnosis of gastric atrophy. Dig Dis Sci. 2010;55:1364–1375. doi: 10.1007/s10620-009-0891-4. [DOI] [PubMed] [Google Scholar]
- 16.Takao T, Ishikawa T, Ando T, Takao M, Matsumoto T, Isozaki Y, Okita M, Nagao Y, Oyamada H, Yokoyama K, et al. Multifaceted Assessment of Chronic Gastritis: A Study of Correlations between Serological, Endoscopic, and Histological Diagnostics. Gastroenterol Res Pract. 2011;2011:631461. doi: 10.1155/2011/631461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quach DT, Le HM, Hiyama T, Nguyen OT, Nguyen TS, Uemura N. Relationship between endoscopic and histologic gastric atrophy and intestinal metaplasia. Helicobacter. 2013;18:151–157. doi: 10.1111/hel.12027. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Uemura N, Xiao SD, Tytgat GN, Kate FJ. Agreement between endoscopic and histological gastric atrophy scores. J Gastroenterol. 2005;40:123–127. doi: 10.1007/s00535-004-1511-x. [DOI] [PubMed] [Google Scholar]
- 19.el-Zimaity HM, Graham DY, al-Assi MT, Malaty H, Karttunen TJ, Graham DP, Huberman RM, Genta RM. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum Pathol. 1996;27:35–41. doi: 10.1016/s0046-8177(96)90135-5. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz B, Garay J, Johnson W, Li D, Rugge M, Dixon MF, Fiocca R, Genta RM, Hattori T, Lechago J, et al. Morphometric assessment of gastric antral atrophy: comparison with visual evaluation. Histopathology. 2001;39:235–242. doi: 10.1046/j.1365-2559.2001.01221.x. [DOI] [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 22.Yamaoka Y. Pathogenesis of Helicobacter pylori-Related Gastroduodenal Diseases from Molecular Epidemiological Studies. Gastroenterol Res Pract. 2012;2012:371503. doi: 10.1155/2012/371503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azuma T. Helicobacter pylori CagA protein variation associated with gastric cancer in Asia. J Gastroenterol. 2004;39:97–103. doi: 10.1007/s00535-003-1279-4. [DOI] [PubMed] [Google Scholar]
- 24.Kimura K. Chronological transition of the fundic-pyloric border determined by stepwise biopsy of the lesser and greater curvatures of the stomach. Gastroenterology. 1972;63:584–592. [PubMed] [Google Scholar]
- 25.Van Zanten SJ, Dixon MF, Lee A. The gastric transitional zones: neglected links between gastroduodenal pathology and helicobacter ecology. Gastroenterology. 1999;116:1217–1229. doi: 10.1016/s0016-5085(99)70025-9. [DOI] [PubMed] [Google Scholar]
- 26.Lee M. Age-related changes in gastric blood flow in rats. Gerontology. 1996;42:289–293. [PubMed] [Google Scholar]
- 27.Tarnawski AS, Ahluwalia A, Jones MK. Increased susceptibility of aging gastric mucosa to injury: the mechanisms and clinical implications. World J Gastroenterol. 2014;20:4467–4482. doi: 10.3748/wjg.v20.i16.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tatsuta M, Saegusa T, Okuda S. Extension of fundal gastritis studied by endoscopic Congo red test. Endoscopy. 1974;6:20–26. [Google Scholar]
- 29.Inoue T, Uedo N, Ishihara R, Kawaguchi T, Kawada N, Chatani R, Kizu T, Tamai C, Takeuchi Y, Higashino K, et al. Autofluorescence imaging videoendoscopy in the diagnosis of chronic atrophic fundal gastritis. J Gastroenterol. 2010;45:45–51. doi: 10.1007/s00535-009-0150-7. [DOI] [PubMed] [Google Scholar]
- 30.So J, Rajnakova A, Chan YH, Tay A, Shah N, Salto-Tellez M, Teh M, Uedo N. Endoscopic tri-modal imaging improves detection of gastric intestinal metaplasia among a high-risk patient population in Singapore. Dig Dis Sci. 2013;58:3566–3575. doi: 10.1007/s10620-013-2843-2. [DOI] [PubMed] [Google Scholar]


