Abstract
AIM: To compare robotic and three-dimensional (3D) laparoscopic colectomy based on the literature and our preliminary experience.
METHODS: This retrospective observational study compared operative measures and postoperative outcomes between laparoscopic 3D and robotic colectomy for cancer. From September 2013 to September 2014, 24 robotic colectomies and 23 3D laparoscopic colectomy were performed at our Department. Data were analyzed and reported both by approach and by colectomy side. Robotic left colectomy (RL) vs laparoscopic 3D left colectomy (LL 3D) and Robotic right colectomy (RR) vs laparoscopic 3D (LR 3D). Rectal cancer procedures were not included.
RESULTS: There were 18 RR and 11 LR 3D, 6 RL and 12 LL 3D. As regards LR 3D, extracorporeal anastomosis (EA) was performed in 7 patients and intracorporeal anastomosis (IA) in 4; the RR group included 14 IA and 4 EA. There was no mortality. Median operative time was higher for the robotic group while conversion rate (12.5% vs 13%) and lymph nodes removed (14 vs 13) were similar for both. First flatus time was 1 d for RR and 2 d the other patient groups. Oral intake was resumed in 1 d by LR and in 2 d by the other patients (P = 0.012). Overall cost was €4950 and €1950 for RL and LL 3D, and €4450 and €1450 for RR and LR 3D, respectively.
CONCLUSION: There were no differences between RR and LR 3D, except that IA was easier with RR, and probably contributed with the learning curve to the longer operative time recorded. Both techniques offer similar advantages for the patient with significantly different costs. In left colectomies robotic colectomy provided better outcomes, especially in resections approaching the rectum.
Keywords: Three-dimensional vision systems, Robotic surgery, Laparoscopic surgery, Colectomy, Costs, Short-term outcomes
Core tip: Three-dimensional (3D) vision systems (Robotic and 3D laparoscopy) have been applied recently in the field of general colorectal surgery. They have brought a lot of benefits not only for the surgeon but also for the patient. However the robotic technique is very expensive, and no study has been published comparing these two techniques. Here, we compared robotic colorectal surgery to 3D laparoscopic with respect to short term outcomes and costs.
INTRODUCTION
Laparoscopy has been a reliable approach for colon cancer surgery for a number of years[1,2]. Technological advances have introduced significant improvements. Currently available technologies include robotically assisted surgery, three-dimensional (3D) vision, computer graphics, 4k laparoscopic systems, and other methods[3]. 3D surgical imaging systems provide stereoscopic depth information that conventional 2D display systems cannot supply. Stereoscopic projection technology improves the performance, proficiency, as well as teaching of minimally invasive surgery[4,5]. Robotic systems derive from military medical research directed at improving procedure feasibility, safety and efficacy[6,7]. Since their introduction they have been applied to several surgical specialties, to overcome the inherent limitations of laparoscopy[6]. The main advantages of robotic systems include high-definition 3D vision, magnification up to 10 ×, and an endo-wrist with 360° range of movement. However, reports of robotic colectomy (RC) involve small series[7-10], and there are no studies comparing laparoscopic colectomy (3DLC) to RC. According to the literature, either approach enhances surgeon performance; however, it is still unclear whether they also involve advantages for the patient. The aim of this study was to compare the benefits, short-term outcomes, and cost of 3DLC and RC.
MATERIALS AND METHODS
Study population
From June 2013 to September 2014, 47 of the colon cancer procedures performed at our Department used a minimally invasive 3D vision technique. For all patients, demographics, pathological examination, operative details and postoperative outcomes were retrospectively collected and analyzed.
Surgical procedure
Patient assignation to robotic colectomy or 3D laparoscopic colectomy depended exclusively on equipment availability. All procedures were performed by two skilled laparoscopic surgeons, that performs almost 200 laparoscopic operations per year, who had performed at least 100 3D laparoscopic procedures and who had recently begun using the robotic equipment. Right colectomy was defined as the procedure that involves removing the cecum, the ascending colon, the hepatic flexure, the first one third of the transverse colon and part of the terminal ileum along with fat and lymph nodes. Left colectomy was defined as the procedure that involves removing of the distal transverse colon, descending colon and sigmoid colon with colorectal anastomosis. Rectal cancer procedures were excluded because of their complexity while surgeons are in their learning curve.
Perioperative management
Post-operative management of patients included a specific fast track protocol. An early mobilization and an early re alimentation was guaranteed as soon as possible according to patients condition and co-operation. No patient was discharged earlier than 5 d as the local health care system does not provide home assistance.
Statistical analysis
The benefits, short-term outcomes, and cost of 3D laparoscopic colectomy and robotic colectomy were examined and compared. A retrospective analysis of all data was performed. Since right and left colectomy are technically different procedures, data were analyzed both in terms of approach used and of colectomy side (Figure 1). For the quantitative values the median was calculated as a measure of centrality and the 1st and 3rd quartiles as a measure of variability. The qualitative values were expressed as actual values and percentage. Data were divided into right and left colectomies. The Wilcoxon test for independent samples was applied to the quantitative values and the χ2 test and Fisher’s test to the qualitative values. Statistical significance was set at α < 0.05. The IBM SPSS Statistics program was used for all analyses.
Figure 1.
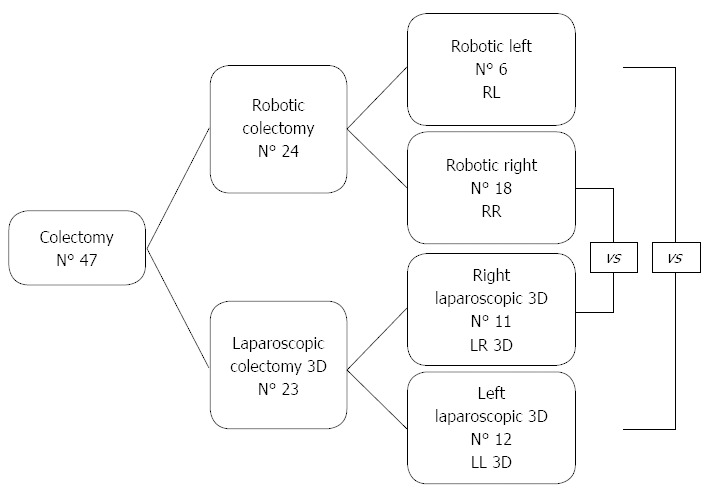
Study design. RL: Robotic left colectomy; LL: Laparoscopic left colectomy.
RESULTS
Patients were 24 men and 23 women with a mean age of 68 years. There were 24 robotic colectomy and 23 3D laparoscopic colectomy. The robotic colectomy and laparoscopic 3D groups were of comparable age and body mass index and were not significantly different in terms of disease stage, ASA class, or previous surgery. Patients underwent 18 right robotic colectomy (RR), 11 right 3D laparoscopic colectomy (LR 3D), 6 left robotic colectomy (RL), and 12 left laparoscopic colectomy (LL 3D) (Figure 1). As regards LR 3D, extracorporeal anastomosis (EA) was performed in 7 patients and intracorporeal anastomosis (IA) in 4; the RR group included 14 IA and 4 EA. The benefits, short-term outcomes, and cost of laparoscopic 3D colectomy and robotic colectomy are analyzed and reported by side and technique.
Left colectomy
Operative results: As regards the 18 left colectomies - 6 RL and 12 LL 3D - there were no significant differences between patients in terms of operative results such as operative time, intraoperative blood loss, complications and number of lymph nodes removed. These data are reported in Table 1 and Figure 2. The conversion rate was higher in RC, albeit without significant differences: 2 RC were converted to 2D laparoscopy and one 3DLC to open surgery. EA was performed in 4 LL and 1 RL patient; in the other cases the Knight Griffen method was adopted. Median operative time was 100 min for RL and 167 min for LL.
Table 1.
Qualitative and quantitative characteristics of left colectomies n (%)
| RL (n = 6) | LL 3D (n = 12) | P value | |
| Gender | |||
| Male | 1 (16.7) | 5 (41.7) | 0.600 |
| Female | 5 (83.3) | 7 (58.3) | |
| Anastomosis | |||
| Extracorporeal | 1 (16.7) | 4 (33.3) | 0.615 |
| Intracorporeal | 5 (83.3) | 8 (66.7) | |
| Conversions | 2 (33.3) | 1 (8.3) | 0.245 |
| Complications | 1 (16.7) | 1 (8.0) | 1.000 |
| Pain medications | 5 (83.3) | 9 (75.0) | 1.000 |
| Age1 | 65 (63-72) | 66 (57-74) | 0.708 |
| BMI1 | 26 (23-28) | 27 (26-29) | 0.353 |
| Overall time1 (min) | 175 (146-196) | 167 (144-186) | 0.672 |
| Surgical time1 (min) | 114 (99-149) | 113 (94-130) | 0.707 |
| Anesthesia time1 (min) | 159 (133-176) | 153 (118-166) | 0.605 |
| Hospital stay1 (d) | 6 (6-9) | 6 (6-9) | 0.526 |
| Solid food1 (d) | 1 (1-2) | 2 (2-2) | 0.012 |
| First flatus1 (d) | 2 (1-5) | 2 (1-4) | 0.567 |
| Mobilization1 (d) | 2 (1-2) | 1 (1-2) | 0.610 |
| Lymph nodes removed1 | 13 (9-15) | 13 (9-16) | 0.189 |
| Duration of pain medications1 (d) | 3 (2-4) | 2 (1-2) | 0.316 |
| DRG1 | 7326 (7326-10570) | 7326 (7326-11660) | 0.911 |
Median (1st-3rd quartile). RL: Robotic left colectomy; LL: Laparoscopic left colectomy; BMI: Body mass index; DRG: Disease related group.
Figure 2.
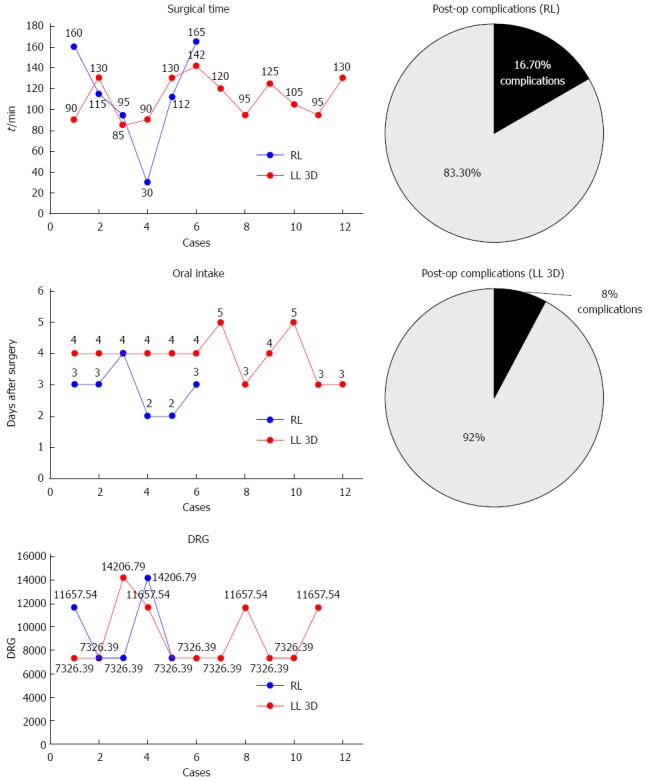
Left colectomy: Surgical time, oral intake, complications, and disease related group.
Postoperative results and complications: Postoperative results were comparable in terms of amount of pain medications, first passage of flatus, mobilization, hospital stay or disease-related group (DRG). The 6 RL patients began to eat solid food significantly earlier (P = 0.012) than the 12 LL patients (median 1 d vs 2 d). The only complication in RL was an anastomotic leakage that was treated with re-intervention; the sole complication in the LL group was an abdominal abscess that was treated with percutaneous drainage.
Costs: The two left colectomies fall under the same DRG despite the fact that their cost at discharge may vary due to possible complications and hospital stay. The cost of RC was higher. In fact, even excluding equipment purchase and maintenance costs, RL cost €4950 and LL cost €1950.
Right colectomy
Operative results: As regards the right colectomies, there were 18 RR and 11 LR. There were no significant differences between these patients in terms of operative results such as intraoperative blood loss and number of lymph nodes removed. These data are reported in Table 2 and Figure 3. Median operative time was 173 min for RR and 145 min for LR; the difference was significant (P = 0.006). One RR and 2 LR required conversion to open procedures, and the difference was not significant. All conversions involved patients with previous abdominal surgery. IA were 14 in RR and 4 in LR; the remaining 11 patients had EA performed with a mechanical suture technique.
Table 2.
Qualitative and quantitative characteristics of right colectomies n (%)
| RR (n = 18) | LR 3D (n = 11) | P value | |
| Sex | |||
| Male | 9 (50) | 9 (81.8) | 0.187 |
| Female | 9 (50) | 2 (18.2) | |
| Anastomosis | |||
| Extracorporeal | 4 (22.2) | 7 (63.6) | 0.070 |
| Intracorporeal | 14 (77.8) | 4 (36.4) | |
| Conversions | 1 (5.6) | 2 (18.2) | 0.649 |
| Complications | 3 (16.7) | 4 (36.4) | 0.449 |
| Pain medication | 11 (61.1) | 6 (54.5) | 0.449 |
| Age1 | 74 (57-80) | 65 (59-75) | 0.559 |
| BMI1 | 26 (24-28) | 26 (23-28) | 0.654 |
| Overall time1 (min) | 173 (156-189) | 145 (130-155) | 0.006 |
| Surgical time1 (min) | 130 (106-139) | 100 (95-120) | 0.033 |
| Anesthesia time1 (min) | 155 (134-164) | 125 (118-140) | 0.008 |
| Hospital stay1 (d) | 5 (5-7) | 5 (5-10) | 0.723 |
| Solid food1 (d) | 2 (2-3) | 2 (2-2) | 0.137 |
| First flatus1 (d) | 1 (1-3) | 2 (2-4) | 0.927 |
| Mobilization1 (d) | 2 (1-2) | 1 (1-2) | 0.345 |
| Lymph nodes removed1 | 14 (8-20) | 14 (9-20) | 0.829 |
| Duration of pain medication1 (d) | 1 (0-2) | 1 (0-2) | 0.832 |
| DRG1 | 7326 (7326-7326) | 7326 (7326-9492) | 0.588 |
Median (1st-3rd quartile). RL: Robotic left colectomy; LL: Laparoscopic left colectomy; BMI: Body mass index; DRG: Disease related group.
Figure 3.
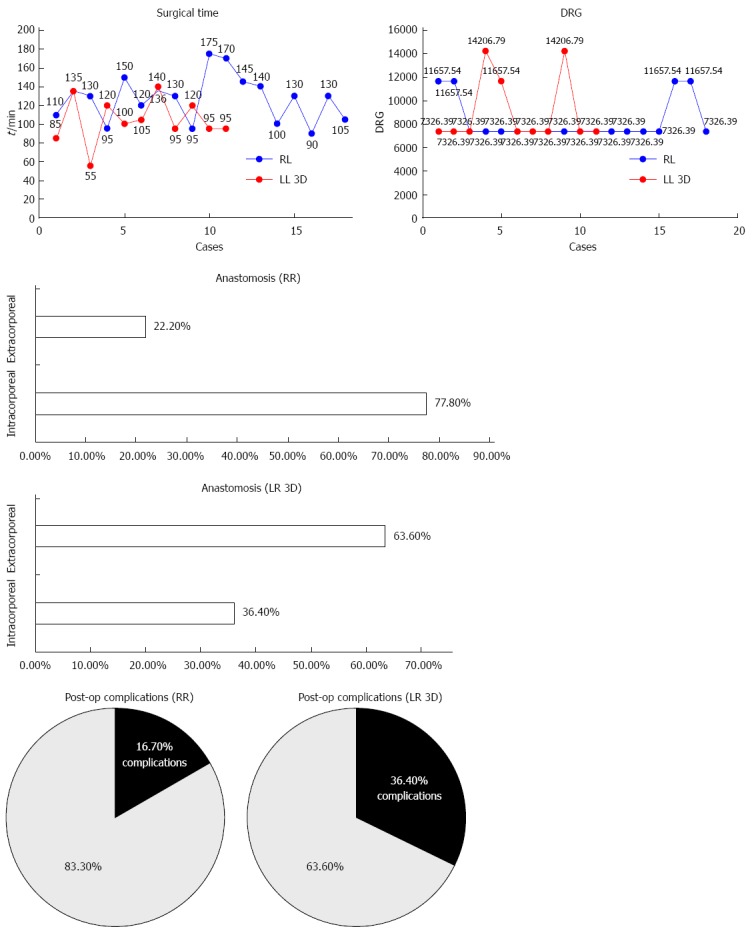
Right colectomy: Surgical time, oral intake, complications, and disease related group, and type of anastomosis.
Postoperative results and complications: Postoperative results were comparable in terms of hospital stay, complications, amount of pain medications, first flatus time, mobilization and time until tolerance of solid food. Three RR patients had anastomosis bleeding that was managed with transfusion, since all had roughly 10 g/dL hemoglobin before surgery. As regards LR, one patient had postoperative bleeding that required transfusion and another had wound dehiscence.
Costs: These two operations also fall under the same DRG, despite their different final cost at discharge. The cost of the procedure was €4450 for RR and €1450 for LR.
DISCUSSION
Since its introduction, robotics surgery has been proved to be a safe and effective technique for various procedures, including complex ones[8,10,11]. The first RC for colorectal cancer was performed almost 14 years ago[12]. Since then the technology has spread widely, and results have been published by several groups[10,13-15]. However, most of the studies assessing the benefits of robotic surgery have compared it to open surgery, not to laparoscopy[16-18]. The main advantages of robotic surgery have been documented in gastrectomy and colorectal resections[7]. The few studies comparing robotic and (2D) laparoscopy found similar benefits, but a higher cost and longer operative time for the former technique. Finally, there are no studies comparing robotic and 3D laparoscopy. Two ongoing randomized trials, the ACOSOG Z6051 and the ROLARR (Robotic vs Laparoscopic Resection for Rectal cancer) are evaluating RC and 3DLC. While awaiting their findings, laparoscopy remains the optimal approach in terms of patient outcomes and use of healthcare resources[19]. Where the oncological results are concerned, the robot likely performs superior total mesorectal excisions (TME) in a narrow and deep space[20]. With regard to surgeon benefits, a multicenter study by Pigazzi et al[21] found an interesting 97% survival rate 3 years after robotic TME. The literature suggests that there are no significant differences in the other oncological measures between the two techniques, thus confirming the safety of robotic surgery[22]. The majority of studies refer mainly to rectal cancer or colorectal cancer and there is poor evidence in the literature concerning colon cancer alone.
Despite the small sample, our data agree with the above reports; for instance the number of lymph nodes removed was similar for both approaches in all resections. None of the short-term outcomes evaluated in our study were significantly different, except the interval until solid oral intake, which was 1 d for RL and 2 d for all the other procedures (P = 0.012). Operating time was significantly longer between RR and LR 3D (P = 0.006) (median 173 min vs 145 min). This is easily explained by the higher rate of IA in the RR compared with the LR 3D group (14 vs 4).
Some studies have suggested that in RR patients IA may result in superior postoperative outcomes and lower extraction site morbidity, such as herniation[23,24]. Moreover, a hand-sewn laparoscopic IA is quite complex to perform: this part of the procedure is enhanced by the robot, which provides considerable benefits, especially in the case of obese patients with a short and heavy mesentery[24]. The shorter RC incision is also an advantage, especially to the extent that it reduces the risk of wound infection[25]. Nevertheless, the feasibility of robotic surgery needs further evaluation.
Despite these advantages, the price of robotic equipment is a problem for stressed western economies. Previous publications have described the poor cost-effectiveness of RC. Other studies have stressed that the steep purchase cost is accompanied by expensive dedicated disposable instruments and tools[19]. In our experience and according to the literature, RC costs almost three times as much as 3DLC procedure even without factoring in the purchase cost[26,27]. Our study provides no clear evidence of postoperative benefits that would help offset such greater cost[19]. 3D vision is one of the main advantages of robotics technology, but based on our data 3DLC provided similar short-term outcomes at a lower cost.
However, the present study refers to a preliminary experience and has several limitations. Most importantly, this was a retrospective study and with a small sample size. In addition, results can be influenced by surgeon being in their learning curve of robotic surgery. The lack of high quality data in literature in this field demonstrates the need for randomized clinical trials.
Why then should we invest in this new technology? For example, the United Kingdom has adopted an integrated national teaching program to extend laparoscopic surgery, including 3D laparoscopy, instead of RC technology, to achieve a more efficient and effective use of healthcare resources[28]. Yet robotic should be viewed as the natural evolution of laparoscopy, even though its indications are still to be defined. Robotics surgery overcomes the limitations of laparoscopy, also by reducing the conversion rate[29,30] and improving nerve function[19]. The current state of healthcare resources suggests that 3D laparoscopy should be extended to treat the majority of patients with colon cancer, but it should be stressed that RC provides an opportunity to develop the technology further with the same safety profile and efficiency of treatment. Finally, a Dutch study[31] suggests that evaluation of a new technology should take into account its affordance. The Affordances approach “can capture empirically the contextual dynamics of technology adoption in health care by exploring in depth actor’s interaction with the technology while considering the interpretative spaces created in situation of use. This is the best way to elicit real-life values of innovations, values as defined through the eyes of the (potential) users”[31]. According to this approach, robotics surgery is both rational and inevitable.
Further evaluation is required to establish the clinical benefits and real cost of RC before its introduction into routine practice. The two techniques fall under the same DRG despite the fact that RC is more expensive. This currently limits its use to cases where laparoscopy is least effective and RC provides real advantages to patients, but this requires further comparative studies. Robotics should be considered as the logical evolution of laparoscopy. The only way to develop it is to use it.
COMMENTS
Background
Colorectal cancer is one of the most common cancers worldwide. Surgery is the only potentially curative therapeutic option. Laparoscopy has been a reliable approach for colon cancer surgery for a number of years. Three-dimensional (3D) laparoscopy represents the latest evolution of this technique. Recently, robotics has been introduced in general surgery. Robotics surgery has been proved to be a safe and effective technique for various procedures, including complex ones. The first robotic colectomy for colorectal cancer was performed almost 14 years ago.
Research frontiers
Technological advances have introduced significant improvements in general surgery. 3D vision systems have brought many benefits to the surgeon. Robotic surgery remains the latest challenge for the surgeon. Actually the hot topic in literature is how much these new technologies can bring benefits not only for the surgeon, but also for the patient with acceptable costs.
Innovations and breakthroughs
Various studies in the literature have compared robotic surgery to open surgery, and only a few have compared it with other types of mini-invasive surgery. No study has compared 3D laparoscopic surgery with Robotics in the field of colorectal surgery. This study evaluate the short outcomes of Robotic vs laparoscopic 3D surgery in colorectal cancer, as well as its economic feasibility.
Applications
The data in this study suggested that there are no significant advantages for the patient performing robotic vs 3D laparoscopic resections for colon cancer. Furthermore, this study also provided readers with important information regarding the economic obstacles. This study did not evaluate rectal cancer, where significant advantages exist according to the literature and robotics should be adopted. The authors suggest that economic resources should be more properly invested to extend laparoscopic surgery, especially 3D rather than robotics, in the field of colon cancer. Nowadays robotic colectomy provides an opportunity to develop the technology further with the same safety profile and efficiency of treatment.
Terminology
Currently available technologies include robotically assisted surgery, 3D vision, computer graphics, 4k laparoscopic systems, and other methods. 3D surgical imaging systems provide stereoscopic depth information that conventional 2D display systems cannot supply. Stereoscopic projection technology improves the performance, proficiency, as well as teaching of minimally invasive surgery.
Peer-review
To date, no study has compared 3D laparoscopic surgery with Robotics in the field of colorectal surgery. This study evaluated the short outcomes of Robotic vs laparoscopic 3D surgery in colorectal cancer checking, as well as its economic feasibility. These findings could be applied in clinical practice right away. They are likely to be of great interest to the vision of surgeons, researchers, clinicians, and trainees.
Footnotes
Institutional review board statement: The internal ethic committee approved this research.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: Authors have no conflict of interest to declare.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 12, 2015
First decision: June 19, 2015
Article in press: September 15, 2015
P- Reviewer: Chow CFK S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma S
References
- 1.Kang CY, Halabi WJ, Luo R, Pigazzi A, Nguyen NT, Stamos MJ. Laparoscopic colorectal surgery: a better look into the latest trends. Arch Surg. 2012;147:724–731. doi: 10.1001/archsurg.2012.358. [DOI] [PubMed] [Google Scholar]
- 2.Delaney CP, Chang E, Senagore AJ, Broder M. Clinical outcomes and resource utilization associated with laparoscopic and open colectomy using a large national database. Ann Surg. 2008;247:819–824. doi: 10.1097/SLA.0b013e31816d950e. [DOI] [PubMed] [Google Scholar]
- 3.Hashizume M, Tsugawa K. Robotic surgery and cancer: the present state, problems and future vision. Jpn J Clin Oncol. 2004;34:227–237. doi: 10.1093/jjco/hyh053. [DOI] [PubMed] [Google Scholar]
- 4.Smith R, Day A, Rockall T, Ballard K, Bailey M, Jourdan I. Advanced stereoscopic projection technology significantly improves novice performance of minimally invasive surgical skills. Surg Endosc. 2012;26:1522–1527. doi: 10.1007/s00464-011-2080-8. [DOI] [PubMed] [Google Scholar]
- 5.Bhayani SB, Andriole GL. Three-Dimensional (3D) Vision: Does It Improve Laparoscopic Skills? An Assessment of a 3D Head-Mounted Visualization System. Rev Urol. 2005;7:211–214. [PMC free article] [PubMed] [Google Scholar]
- 6.Mirnezami AH, Mirnezami R, Venkatasubramaniam AK, Chandrakumaran K, Cecil TD, Moran BJ. Robotic colorectal surgery: hype or new hope? A systematic review of robotics in colorectal surgery. Colorectal Dis. 2010;12:1084–1093. doi: 10.1111/j.1463-1318.2009.01999.x. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi PP, Pigazzi A, Choi GS. Clinical Robotic Surgery Association Fifth Worldwide Congress, Washington DC, 3-5 October 2013: Robotic Colorectal Surgery. Ecancermedicalscience. 2014;8:385. doi: 10.3332/ecancer.2014.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pucci MJ, Beekley AC. Use of robotics in colon and rectal surgery. Clin Colon Rectal Surg. 2013;26:39–46. doi: 10.1055/s-0033-1333660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkiewicz W, Zawadzki M, Rząca M, Obuszko Z, Czarnecki R, Turek J, Marecik S. Robot-assisted right colectomy: surgical technique and review of the literature. Wideochir Inne Tech Maloinwazyjne. 2013;8:253–257. doi: 10.5114/wiitm.2011.33761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–784. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 11.Giulianotti PC, Buchs NC, Caravaglios G, Bianco FM. Robot-assisted lung resection: outcomes and technical details. Interact Cardiovasc Thorac Surg. 2010;11:388–392. doi: 10.1510/icvts.2010.239541. [DOI] [PubMed] [Google Scholar]
- 12.Ballantyne GH. The pitfalls of laparoscopic surgery: challenges for robotics and telerobotic surgery. Surg Laparosc Endosc Percutan Tech. 2002;12:1–5. doi: 10.1097/00129689-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 13.D’Annibale A, Pernazza G, Morpurgo E, Monsellato I, Pende V, Lucandri G, Termini B, Orsini C, Sovernigo G. Robotic right colon resection: evaluation of first 50 consecutive cases for malignant disease. Indian J Surg Oncol. 2012;3:279–285. doi: 10.1007/s13193-012-0193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JS, Choi GS, Lim KH, Jang YS, Jun SH. Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol. 2010;17:3195–3202. doi: 10.1245/s10434-010-1162-5. [DOI] [PubMed] [Google Scholar]
- 15.Sebajang H, Trudeau P, Dougall A, Hegge S, McKinley C, Anvari M. The role of telementoring and telerobotic assistance in the provision of laparoscopic colorectal surgery in rural areas. Surg Endosc. 2006;20:1389–1393. doi: 10.1007/s00464-005-0260-0. [DOI] [PubMed] [Google Scholar]
- 16.Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, Fowler WC. A case-control study of robot-assisted type III radical hysterectomy with pelvic lymph node dissection compared with open radical hysterectomy. Am J Obstet Gynecol. 2008;199:357.e1–357.e7. doi: 10.1016/j.ajog.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 17.Krambeck AE, DiMarco DS, Rangel LJ, Bergstralh EJ, Myers RP, Blute ML, Gettman MT. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009;103:448–453. doi: 10.1111/j.1464-410X.2008.08012.x. [DOI] [PubMed] [Google Scholar]
- 18.Park JS, Choi GS, Lim KH, Jang YS, Jun SH. S052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc. 2011;25:240–248. doi: 10.1007/s00464-010-1166-z. [DOI] [PubMed] [Google Scholar]
- 19.Keller DS, Senagore AJ, Lawrence JK, Champagne BJ, Delaney CP. Comparative effectiveness of laparoscopic versus robot-assisted colorectal resection. Surg Endosc. 2014;28:212–221. doi: 10.1007/s00464-013-3163-5. [DOI] [PubMed] [Google Scholar]
- 20.Buchs NC, Pugin F, Bucher P, Morel P. Totally robotic right colectomy: a preliminary case series and an overview of the literature. Int J Med Robot. 2011;7:348–352. doi: 10.1002/rcs.404. [DOI] [PubMed] [Google Scholar]
- 21.Pigazzi A, Luca F, Patriti A, Valvo M, Ceccarelli G, Casciola L, Biffi R, Garcia-Aguilar J, Baek JH. Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol. 2010;17:1614–1620. doi: 10.1245/s10434-010-0909-3. [DOI] [PubMed] [Google Scholar]
- 22.Hellan M, Ouellette J, Lagares-Garcia JA, Rauh SM, Kennedy HL, Nicholson JD, Nesbitt D, Johnson CS, Pigazzi A. Robotic Rectal Cancer Resection: A Retrospective Multicenter Analysis. Ann Surg Oncol. 2015;22:2151–2158. doi: 10.1245/s10434-014-4278-1. [DOI] [PubMed] [Google Scholar]
- 23.Pigazzi A, Garcia-Aguilar J. Robotic colorectal surgery: for whom and for what? Dis Colon Rectum. 2010;53:969–970. doi: 10.1007/DCR.0b013e3181db8055. [DOI] [PubMed] [Google Scholar]
- 24.Hellan M, Anderson C, Pigazzi A. Extracorporeal versus intracorporeal anastomosis for laparoscopic right hemicolectomy. JSLS. 2009;13:312–317. [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura T, Mitomi H, Ihara A, Onozato W, Sato T, Ozawa H, Hatade K, Watanabe M. Risk factors for wound infection after surgery for colorectal cancer. World J Surg. 2008;32:1138–1141. doi: 10.1007/s00268-008-9528-6. [DOI] [PubMed] [Google Scholar]
- 26.deSouza AL, Prasad LM, Park JJ, Marecik SJ, Blumetti J, Abcarian H. Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum. 2010;53:1000–1006. doi: 10.1007/DCR.0b013e3181d32096. [DOI] [PubMed] [Google Scholar]
- 27.Choi DJ, Kim SH, Lee PJ, Kim J, Woo SU. Single-stage totally robotic dissection for rectal cancer surgery: technique and short-term outcome in 50 consecutive patients. Dis Colon Rectum. 2009;52:1824–1830. doi: 10.1007/DCR.0b013e3181b13536. [DOI] [PubMed] [Google Scholar]
- 28.Coleman MG, Hanna GB, Kennedy R. The National Training Programme for Laparoscopic Colorectal Surgery in England: a new training paradigm. Colorectal Dis. 2011;13:614–616. doi: 10.1111/j.1463-1318.2011.02643.x. [DOI] [PubMed] [Google Scholar]
- 29.Baek JH, Pastor C, Pigazzi A. Robotic and laparoscopic total mesorectal excision for rectal cancer: a case-matched study. Surg Endosc. 2011;25:521–525. doi: 10.1007/s00464-010-1204-x. [DOI] [PubMed] [Google Scholar]
- 30.Patriti A, Ceccarelli G, Bartoli A, Spaziani A, Biancafarina A, Casciola L. Short- and medium-term outcome of robot-assisted and traditional laparoscopic rectal resection. JSLS. 2009;13:176–183. [PMC free article] [PubMed] [Google Scholar]
- 31.Abrishami P, Boer A, Horstman K. Understanding the adoption dynamics of medical innovations: affordances of the da Vinci robot in the Netherlands. Soc Sci Med. 2014;117:125–133. doi: 10.1016/j.socscimed.2014.07.046. [DOI] [PubMed] [Google Scholar]


