Abstract
Peliosis hepatis (PH) is a disease characterized by multiple and small, blood-filled cysts within the parenchymatous organs. PH is a very rare disease, more common in adults, and when it affects the liver, it comes to the surgeon’s attention only in an extremely urgent situation after the lesion’s rupture with the resulting hemoperitoneum. This report describes the case of a 29-year-old woman affected by recurring abdominal pain. Computed tomography scans showed a hepatic lesion formed by multiple hypodense areas, which showed an early acquisition of the contrast during the arterial phase. Furthermore, it remained isodense with the remaining parenchyma during the late venous phase. We decided on performing a liver resection of segment VII while avoiding a biopsy for safety reasons. The histopathologic examination confirmed the diagnosis of focal PH. PH should always be considered in the differential diagnosis of hepatic lesions. Clinicians should discuss the possible causes and issues related to the differential diagnosis in addition to the appropriate therapeutic approach. The fortuitous finding of a lesion, potentially compatible with PH, requires elective surgery with diagnostic and therapeutic intents. The main aim is to prevent the risk of a sudden bleeding that, in absence of properly equipped structures, may have a fatal outcome.
Keywords: Hemoperitoneum, Hemorrhagic hepatic cysts, Liver mass, Peliosis hepatis, Surgical treatment
Core tip: This report describes the case of a 29-year-old woman affected by recurring abdominal pain. Computed tomography scans showed a hepatic lesion formed by multiple hypodense areas, which showed an early acquisition of the contrast during the arterial phase. We performed a liver resection of segment VII while avoiding biopsy for safety reasons. The histopathologic examination confirmed the diagnosis of focal hepatic peliosis. Surgery was successful and the patient had a good recovery.
INTRODUCTION
Peliosis hepatis (PH) is a disease characterized by multiple, small, blood-filled cysts within the parenchymatous organs. PH is a very rare disease that comes to the surgeon’s attention just after the rupture, even spontaneous, of the lesion, resulting in a massive hemoperitoneum. In this particular case, PH becomes a potentially fatal disease[1-5].
CASE REPORT
This case involves a 29-year-old woman who was hospitalized at the Hepato-Pancreato Biliary Surgery Unit at Rome “Regina Elena” Cancer Institute because of recurrent, though discontinuous over the years, abdominal pain. The pain initiated from the back and had become chronic since 2006, when she was subjected to a laparoscopic cholecystectomy for gallbladder stones and without any postoperative complications.
The patient came to our hospital for persistent symptoms after cholecystectomy, characterized by epigastric pain that spread to the shoulder blade area. Past medical history was negative for other kinds of abdominal diseases. The ultrasound did not show the presence of hepatic masses, except for the presence of biliary sand. The patient did not report chronic use of drugs, such as steroids or oral contraceptives, antibiotics, estrogens, or tamoxifen. The past medical history was negative for infection with Rickettsia, hepatitis, human immunodeficiency virus (HIV), tuberculosis, and other diseases of the hematopoietic system. Abdominal ultrasound showed multiple, low echogenicity liver cysts in the right lobe in addition to some other high echogenicity areas, mainly located in the seventh segment (total diameter = 4 cm). These features suggested the presence of multiple abscesses or a newly formed process of indeterminable nature. A contrast Computed tomography (CT) was also performed: the lesion appeared to be comprised of multiple low-attenuation areas, and an early acquisition of contrast (Figure 1) with centrifugal progression was noticed during the arterial phase. The entire parenchyma appeared as homogeneously isodense as large vessels during the late venous phase such that the lesion was indistinguishable.
Figure 1.
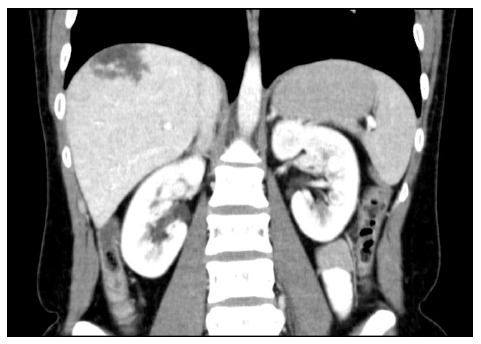
Computed tomography (arterial phase). The lesion is composed of greater areas of low attenuation and shows an early acquisition of the contrast.
The patient was also subjected to magnetic resonance imaging (MRI) examination both with and without contrast. In T2-weighted sequences, the lesions were as hyperintense as the remaining parenchyma with multiple high-signal spots (Figure 2A); in T1-weighted sequences the lesions were hypointense (Figure 2B). As in CT, an early enhancement was found. CT and MRI images did not guide the diagnosis for any of the hepatic lesions most commonly identified. The only plausible hypothesis seemed to be that of a hemangioma, even though the presence of necrotic areas of persistent attenuation requires a differential diagnosis with abscesses, hematomas, and liver tumors.
Figure 2.
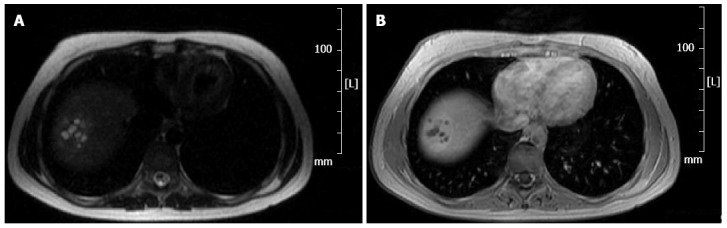
Magnetic resonance. A: T2-weighted sequence. The lesion is as hyperintense as the remaining parenchyma with multiple foci of high-signal intensity; B: T1-weighted sequence. The lesion appears hypointense.
Percutaneous biopsy was not performed because the mass was located in the liver and because of the remarkable vascularization of the lesions.
Due to the variety of pathologies, including cancer, that may determine a biliary duct dilatation with pain and/or segmental Caroli’s disease, and the impossibility to establish or exclude the presence of PH in the differential diagnosis, we decided to operate on the patient, fully removing the lesion via the resection of segment VII with diagnostic and therapeutic intent.
The histopathologic examination confirmed the presence of hemorrhagic cyst cavities in the liver parenchyma, sized from less than one to several millimeters in diameter, and excluded the presence of neoplastic cells. The histopathologic features were useful for the diagnosis of the focal PH (Figures 3 and 4).
Figure 3.
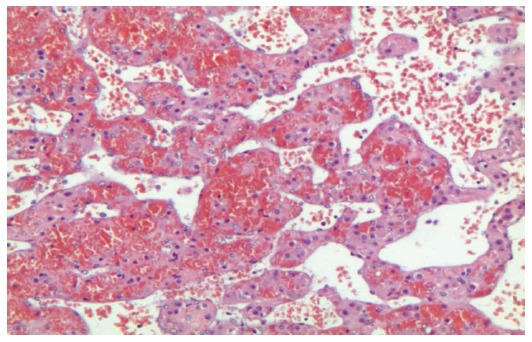
Hepatic tissue with pseudovascular pattern (without endothelium) filled with debris and erythrocytes. Note the presence of rich intratissutal capillary vessels (Hematoxylin and eosin; magnification × 200).
Figure 4.
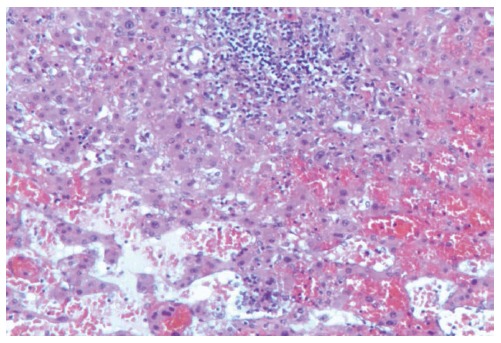
Pseudovascular spaces associated with reactive lymphoid aggregates with macrophage cells and tissutal hystiocytes (Hematoxylin and eosin, magnification × 200).
DISCUSSION
PH was first described in 1861 by E. Wagner. In 1916, W. Schoenlack denominated it peliosis from the Greek word “pelios” that means “reddish” or “bluish”[6-8]. Its pathogenesis remains uncertain, though the triggering event that produces the dilation of sinusoids might be due to an altered outflow with the consequent damage of the sinusoids’ walls and the dilatation of the central vein of the hepatic lobule[9,10]. A pathogenesis determined by the hepatocellular necrosis with the subsequent formation of blood-filled cavities is also supposed[10,11].
PH could have a focal development within liver parenchyma, even though more often it is widespread[6,12]. Even the possibility of hepatic rupture varies in percentage considering the different sites of the disease: on the right liver in 75% of the cases, on the left liver in 11% of the cases, and on both in 14% of the cases[1].
Although most commonly identified in the liver, the same process may occur in the spleen, bone marrow, lymph nodes, and more rarely in lungs, stomach, intestine, parathyroid, pancreas, pituitary gland, and kidneys[2,5-7,13]. Its incidence does not vary according to sex, and a greater frequency is observed among adults, even though it can also be found in pediatric patients[11,14,15]. PH pathogenesis remains uncertain, but Pan et al[14] divided the etiologic factors between: (1) factors related with drugs; (2) factors related with autoimmune mechanisms; and (3) factors related with infectious cases.
Over time, many drugs have been associated with PH onset, such as steroids, oral contraceptives, tamoxifen, methotrexate, thiopurine, azathioprine, and iron chelators, in addition to several toxins, such as arsenic or thorium. Even alcohol consumption facilitates the onset of PH, as it reduces the amount of glutathione that would have a protective and detoxifying effect for sinusoidal endothelial cells, especially during the metabolism of immunomodulatory drugs. As previously reported[7,14,16], patients who take these kinds of drugs are more likely to develop PH[7,8,17,18]. There is also severe risk in association with neoplastic diseases (such as hepatocellular carcinoma), in particular myeloproliferative disease, or in those diseases involving the lymphatic system[13,14,19-21], and Castleman’s disease[13,22]. Some authors have reported associations between arteritis, telangiectasia, and intestinal lymphangiectasia[10,23,24] and cases of portal circulation hypertension in un-transplanted patients[25] and cases of myopathy tubular[26,27], cystic fibrosis[2], and celiac disease[13]. Other authors have described associations between PH and leprosy, tuberculosis, or sifilidis, or more in general, infectious cases of HIV[2,8,11,13,28,29], such as the bacillus infection of Rickettsia family (Rochalimaea henselae and R. quintana)[2,6,13,30]. In 20%-50% of the cases it is not possible to identify any comorbidity.
Imaging studies (CT angiography, ETG and MRI angiography), though carried out using hepatospecific contrast in hepatic sites of the disease, did not allow a clear diagnosis of PH[11,30-37]. The differential diagnoses in hepatic localizations include adenomas, hemangioma, focal nodular hyperplasia, Caroli’s disease, multiple abscesses, and metastatic adenocarcinomas[3,11,20,32,38,39]. Lesions may show a variable diameter from a few millimeters up to more than 4 cm[5,38,40]. Ultrasound scans may show a pseudocystic lesion of the hepatic parenchyma, which may correspond to venous lakes that are commonly described upon histopathologic examination[5]. Even the condition of the hepatic parenchyma determines the sonographic features of PH, which appears hyperechoic in presence of steatosis, and hypoechoic in a normal parenchyma. Through Doppler examination, it is possible to show the intra- or perilesional vascularity[11,32,37]. Some authors have also reported the use of ultrasound with contrast, though an effective higher sensitivity was not emphasized[37,38]. Angiography examination shows multiple hypervascularized nodules during the late arterial phase, and the enhancement is more pronounced during the parenchymal phase and persists during the venous phase[1,32]. CT images may show small lesions ranging from a few millimeters to 1-4 cm in diameter[5]. These lesions appear as multiple areas with low signal. Their aspect depends on the degree of thrombosis or hemorrhage inside the cavity, as they can appear with variable density (though more often hypodense) and contain some calcifications[2,7,11,32]. In the early arterial phase, they may appear hyperdense and then become isodense with the remaining parenchyma[5,32]. The dimensions of the cavities can have a density comparable to that of vessel or they can even appear without enhancement when thrombosed[5,24,32].
CT scans cannot detect lesions smaller than 1 cm in diameter, and this method does not appear to be more sensitive compared with the ultrasound in PH diagnosis[6,24]. On the other hand, MRI examination represents the gold standard in radiologic diagnosis for this disease[32], especially if it is combined with the use of a hepatospecific-contrast medium[30]. The imaging varies considerably depending on the blood component of the lesion[1,3,5,8]. Usually, lesions are never exophytic[32]; in T2-weighted-sequences, they may appear as hyperintense lesions compared with the surrounding parenchyma with high-signal multiple spots that are attributable to hemorrhagic necrosis[5,8,24]. In T1-weighted-sequences, different types of lesions can be detected (hypo, iso, or hyperintense), which show enhancement at the time of contrast medium administration. This enhancement is generally centrifugal (as for the CT), but can also be centripetal[5].
In T1 acquisitions with fat suppression, during the late stage and after the use of gadobenate dimeglumine, a strong contrast with ramifications due to the vascular component is observed[24,32]. The acquisition of the contrast medium during the late phase can sometimes indicate a hemangioma as differential diagnosis. The uncertainty can only be resolved after the surgery[3,4]. For the differential diagnosis with proliferative lesions, the use of positron emission tomography allows one to exclude a high metabolic activity of the lesion[39].
Some authors claim that, due to the high risk of bleeding, an open biopsy is essential in order to realize the differential diagnosis under intraoperative ultrasound supervision[2,6,13,31,34,40]. Macroscopic examinations show that peliotic lesions in the cutting section present hemorrhagic cyst cavities of various sizes (from < 1 mm to several centimeters) with features of “Swiss cheese”[1,3]. From the histopathologic point of view, the differential diagnosis is used in order to diversify the sinusoids’ dilatation from Budd-Chiari syndrome, which determines a venous congestion of the liver due to a vascular occlusion[1,4,41].
Yanoff et al[42] described microscopically two different types of PH: “parenchymal peliosis,” which consists of irregular cavities that are surrounded neither by the sinusoidal cells nor by fibrous tissue, and “peliosis flebectasica,” which is characterized by spherical regular cavities coated by endothelium and/or fibrosis[3,5,32].
Even though the blood-filled cavities do not always have endothelial coverage, it has been observed that this is quickly reconstituted. The continuity and the new breaking of the sinusoids’ endothelium do not constitute a real classification parameter[5]; in this manner it is more adequate to describe such circumstances as temporally different aspects of the same pathology[33].
PH does not show a well-defined evolution. It can become worse asymptomatically and present as an accidental finding that occurs during investigations of other diseases or on the autopsy table[2,32]. Some authors claim that PH could be associated to liver failure with clinical features of hepatomegaly, portal hypertension, cholestasis, and, more rarely, in cirrhotic patients positive for hepatitis C virus[6,32,43] or in cases of rupture of the lesions with hemoperitoneum and hemodynamic decompensation with lethargy and abdominal pain[1,3,6,32]. In other cases, especially when PH occurs at very young age, the disease shows important effects of compression with stenosis of the vena cava[44]. In still other cases, after the interruption of the steroid therapy or the resolution of the subsequent infections, a total regression was noticed[5,6,32]. The regression can also occur without any connection with the past medical history, especially in those cases (20%-50%) that do not allow association of PH to any kind of etiology[30]. Even though some authors support possible transplantation in acute liver failure cases or to use hepatectomy for the diagnosis and treatment of PH, surgery is more and more often used in urgent cases as treatment for the bleeding that may occur. At the same time, for the same issue, some authors proposed embolization that may be performed by an interventional radiologist[45,46] or during the operation or the laparoscopic biopsy, as previously mentioned.
PH is a rare disease that often arises with atypical symptoms, but more often is asymptomatic and occurs in urgent situations after the spontaneous lesion’s rupture or related to minor trauma. The peculiarity of the present case is that it was not diagnosed in an urgent situation, but as a result of the preoperative investigations. We decided to operate by means of diagnostic and therapeutic means by which we could prevent future bleeding complications.
Patients are rarely treated with elective surgery because of the diagnostic difficulty and because none of the preoperative investigations are useful for making a certain diagnosis. As already described in the literature[11,32], the ultrasound (level I investigation) shows only the presence of a hypervascularized lesion, usually hypoechoic, without further features. Even CT showed only the presence of a hypervascular lesion in the early stage (Figure 1) with rapid washout of contrast, which was not useful for obtaining a differential diagnosis[5,32]. Even MRI with contrast material did not allow for diagnostic classification because in T2-weighted sequences, lesions were as hyperintense as the remaining parenchyma with multiple high-signal spots. On the contrary, in T1-weighted sequences, lesions were hypointense, and thus hardly distinguishable from that of the hemangiomatous lesions[4,32]. We decided not to perform a percutaneous biopsy because of the considerable vascularity of the lesion and the consequent high risk of bleeding[31,34,40].
We decide to perform an hepatic resection that, according to Samyn et al[6], permits better management of the prior hemostasis, also in concordance with Fowell et al’s[38] assertions and the patient’s good condition, such as her young age and the site of the lesion in site VII (that can be easily reached in surgery). The histopathologic examination of the entire mass confirmed the presence of hemorrhagic cyst cavities in the hepatic parenchyma, sizes from less than one to several millimeters in diameter[3,5,32]; it also confirmed the diagnosis of PH. In the case reported here, we could not identify any past medical history element because of the nature of the lesion. In 20%-50% of the cases, in fact, it is not possible to define the PH etiology[11,24,30,31]. In addition to permitting the diagnosis and the pathology therapy, the surgical intervention also allows for the prevention of possible hemorrhagic events that are very frequent in patients affected by PH.
In conclusion, PH is a rare and difficult to diagnose disease. From an iconographic point of view, it is almost impossible to carry out a differential diagnosis, especially during the preoperative phase, because of the presence of many other diseases with similar characteristics. The conspicuous vascularization, which is not only a PH feature, requires caution toward percutaneous biopsies. The PH is by definition an asymptomatic disease until a dramatic event, such as the hemorrhage, makes it obvious. We believe that the fortuitous finding of a lesion, potentially compatible with PH, requires elective surgery with diagnostic and therapeutic intents. The main aim is to prevent the risk of sudden bleeding that, in absence of properly equipped structures, may have a fatal outcome.
ACKNOWLEDGMENTS
We would like to thank the patients who participated in this study.
COMMENTS
Case characteristics
A 29-year old woman affected by recurring abdominal pain.
Laboratory diagnosis
Histopathological examination confirmed the presence of haemorrhagic cyst cavities in the liver parenchyma, with size from less than one to several millimetres in diameter, and excluded the presence of neoplastic cells.
Imaging diagnosis
Computed tomography scan showed a hepatic lesion formed by multiple hypodense areas, which showed an early acquisition of the contrast during the arterial phase.
Treatment
Liver resection of segment VII avoiding biopsy was performed for safety reasons.
Experiences and lessons
The fortuitous finding of a lesion, potentially compatible with peliosis hepatis, requires elective surgery with diagnostic and therapeutic intents. The main aim is to prevent the risk of a sudden bleeding that, in absence of properly equipped structures, may have a fatal outcome.
Peer-review
It is an interisting case mention about a 29 y/o woman with peliosis hepatis mimicking liver tumor, some revision needed.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing interests.
Informed consent statement: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images and the study was carried out according to the Helsinki Declaration. A copy of the written consent is available for review by the Editor-in-Chief of this journal. In our study, we treated the patient with standard, and not experimental, therapies, and therefore, the request for authorization to the Ethics Committee does not apply.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 20, 2015
First decision: May 18, 2015
Article in press: August 31, 2015
P- Reviewer: Chen LW S- Editor: Yu J L- Editor: Filopodia E- Editor: Ma S
References
- 1.Sommacale D, Palladino E, Tamby EL, Diebold MD, Kianmanesh AR. Spontaneous hepatic rupture in a patient with peliosis hepatis: A report of one case. Int J Surg Case Rep. 2013;4:508–510. doi: 10.1016/j.ijscr.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi SK, Jin JS, Cho SG, Choi SJ, Kim CS, Choe YM, Lee KY. Spontaneous liver rupture in a patient with peliosis hepatis: a case report. World J Gastroenterol. 2009;15:5493–5497. doi: 10.3748/wjg.15.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caremani M, Tacconi D, Lapini L. Acute nontraumatic liver lesions. J Ultrasound. 2013;16:179–186. doi: 10.1007/s40477-013-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsokos M, Erbersdobler A. Pathology of peliosis. Forensic Sci Int. 2005;149:25–33. doi: 10.1016/j.forsciint.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Wang SY, Ruggles S, Vade A, Newman BM, Borge MA. Hepatic rupture caused by peliosis hepatis. J Pediatr Surg. 2001;36:1456–1459. doi: 10.1053/jpsu.2001.26397. [DOI] [PubMed] [Google Scholar]
- 6.Samyn M, Hadzic N, Davenport M, Verma A, Karani J, Portmann B, Mieli-Vergani G. Peliosis hepatis in childhood: case report and review of the literature. J Pediatr Gastroenterol Nutr. 2004;39:431–434. doi: 10.1097/00005176-200410000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Yu CY, Chang LC, Chen LW, Lee TS, Chien RN, Hsieh MF, Chiang KC. Peliosis hepatis complicated by portal hypertension following renal transplantation. World J Gastroenterol. 2014;20:2420–2425. doi: 10.3748/wjg.v20.i9.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong WJ, Hu LJ, Jian YC, He Y, Zhou W, Guo XL, Zheng YX. Focal peliosis hepatis in a colon cancer patient resembling metastatic liver tumor. World J Gastroenterol. 2012;18:5999–6002. doi: 10.3748/wjg.v18.i41.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wanless IR, Huang WY. Vascular disorders. In: MacSween’s pathology of the liver., editor. 6th ed. New York: Elsevier; 2012. pp. 618–619. [Google Scholar]
- 10.Mungan Z, Pinarbasi B, Bakir B, Gulluoglu M, Baran B, Akyuz F, Demir K, Kaymakoglu S. Congenital portal vein aneurysm associated with peliosis hepatis and intestinal lymphangiectasia. Gastroenterol Res Pract. 2009;2009:479264. doi: 10.1155/2009/479264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brancatelli G, Vilgrain V, Federle MP, Hakime A, Lagalla R, Iannaccone R, Valla D. Budd-Chiari syndrome: spectrum of imaging findings. AJR Am J Roentgenol. 2007;188:W168–W176. doi: 10.2214/AJR.05.0168. [DOI] [PubMed] [Google Scholar]
- 12.Ben Hassen W, Wagner M, Lucidarme O. Unusual hepatic lesion in a patient with a lung tumor. Gastroenterology. 2014;147:e7–e8. doi: 10.1053/j.gastro.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 13.Kleger A, Bommer M, Kunze M, Klaus J, Leithaeuser F, Wegener M, Adler G, Dikopoulos N. First reported case of disease: peliosis hepatis as cardinal symptom of Hodgkin’s lymphoma. Oncologist. 2009;14:1088–1094. doi: 10.1634/theoncologist.2009-0215. [DOI] [PubMed] [Google Scholar]
- 14.Pan W, Hong HJ, Chen YL, Han SH, Zheng CY. Surgical treatment of a patient with peliosis hepatis: a case report. World J Gastroenterol. 2013;19:2578–2582. doi: 10.3748/wjg.v19.i16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sierra Solís A, Rincón Rodera P, Zamorano Pozo T, Castillo Cano-Cortés MT. [Solution to case 31. Peliosis hepatis] Radiologia. 2011;53:376–378. doi: 10.1016/j.rx.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Elsing C, Placke J, Herrmann T. Alcohol binging causes peliosis hepatis during azathioprine therapy in Crohn’s disease. World J Gastroenterol. 2007;13:4646–4648. doi: 10.3748/wjg.v13.i34.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tunç B, Tavil B, Karakurt N, Yarali N, Azik FM, Kara A, Culha V, Ozkasap S. Deferasirox therapy in children with Fanconi aplastic anemia. J Pediatr Hematol Oncol. 2012;34:247–251. doi: 10.1097/MPH.0b013e318249a4be. [DOI] [PubMed] [Google Scholar]
- 18.Lui WH, Chou TC, Chang SS, Hung CJ, Lin YJ, Lee PC. Peliosis hepatis in a kidney transplant recipient with manifestation as massive ascites and liver dysfunction: case report. Transplant Proc. 2014;46:630–633. doi: 10.1016/j.transproceed.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Corpa MV, Bacchi MM, Bacchi CE, Coelho KI. Peliosis hepatis associated with lymphoplasmacytic lymphoma: an autopsy case report. Arch Pathol Lab Med. 2004;128:1283–1285. doi: 10.5858/2004-128-1283-PHAWLL. [DOI] [PubMed] [Google Scholar]
- 20.Slim R, Hachem N, Smayra V, Yaghi C, Daniel F, Boujaoude J, Honein K, Sayegh R, Ghosn M. Education and Imaging. Hepatobiliary and pancreatic: peliosis hepatis associated with multiple myeloma. J Gastroenterol Hepatol. 2014;29:5. doi: 10.1111/jgh.12469. [DOI] [PubMed] [Google Scholar]
- 21.Tsirigotis P, Sella T, Shapira MY, Bitan M, Bloom A, Kiselgoff D, Levin M, Libster D, Abdul Hai A, Gesundheit B, et al. Peliosis hepatis following treatment with androgen-steroids in patients with bone marrow failure syndromes. Haematologica. 2007;92:e106–e110. doi: 10.3324/haematol.11343. [DOI] [PubMed] [Google Scholar]
- 22.Saritas U, Ustundag Y, Isitan G, Bastugrul S, Erekul S. Abdominal Castleman disease with mixed histopathology in a patient with iron deficiency anemia, growth retardation and peliosis hepatis. Am J Med Sci. 2006;331:51–54. doi: 10.1097/00000441-200601000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Durant C, Martin J, Hervier B, Gournay J, Hamidou M. Takayasu arteritis associated with hepatic sinusoidal dilatation. Ann Hepatol. 2011;10:559–561. [PubMed] [Google Scholar]
- 24.Alessandrino F, Felisaz PF, La Fianza A. Peliosis hepatis associated with hereditary haemorrhagic telangiectasia. Gastroenterol Rep (Oxf) 2013;1:203–206. doi: 10.1093/gastro/got021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berzigotti A, Magalotti D, Zappoli P, Rossi C, Callea F, Zoli M. Peliosis hepatis as an early histological finding in idiopathic portal hypertension: A case report. World J Gastroenterol. 2006;12:3612–3615. doi: 10.3748/wjg.v12.i22.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terlizzi JP, Azizi R, Chow MD, Underberg-Davis S, Nosher JL, Stafford PW, Pierre J. Peliosis hepatis in a child with myotubular myopathy: successful treatment using hepatic artery embolization. J Pediatr Surg. 2013;48:e9–e12. doi: 10.1016/j.jpedsurg.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Motoki T, Fukuda M, Nakano T, Matsukage S, Fukui A, Akiyoshi S, Hayashi YK, Ishii E, Nishino I. Fatal hepatic hemorrhage by peliosis hepatis in X-linked myotubular myopathy: a case report. Neuromuscul Disord. 2013;23:917–921. doi: 10.1016/j.nmd.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Sanz-Canalejas L, Gómez-Mampaso E, Cantón-Moreno R, Varona-Crespo C, Fortún J, Dronda F. Peliosis hepatis due to disseminated tuberculosis in a patient with AIDS. Infection. 2014;42:185–189. doi: 10.1007/s15010-013-0490-3. [DOI] [PubMed] [Google Scholar]
- 29.Chen JF, Chen WX, Zhang HY, Zhang WY. Peliosis and gummatous syphilis of the liver: a case report. World J Gastroenterol. 2008;14:1961–1963. doi: 10.3748/wjg.14.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savastano S, San Bortolo O, Velo E, Rettore C, Altavilla G. Pseudotumoral appearance of peliosis hepatis. AJR Am J Roentgenol. 2005;185:558–559. doi: 10.2214/ajr.185.2.01850558. [DOI] [PubMed] [Google Scholar]
- 31.Tallón García M, Cobelas Cobelas MC, Fernández Sanmartín M, Bao Corral A, Granja Martínez MC. [Peliosis hepatitis secondary to hormone treatment] An Pediatr (Barc) 2011;75:286–288. doi: 10.1016/j.anpedi.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Ferrozzi F, Tognini G, Zuccoli G, Cademartiri F, Pavone P. Peliosis hepatis with pseudotumoral and hemorrhagic evolution: CT and MR findings. Abdom Imaging. 2011;26:197–199. doi: 10.1007/s002610000131. [DOI] [PubMed] [Google Scholar]
- 33.Buelow B, Otjen J, Sabath AP, Harruff RC. Peliosis hepatis presenting as liver rupture in a vulnerable adult: a case report. Am J Forensic Med Pathol. 2012;33:307–310. doi: 10.1097/PAF.0b013e31823a8b38. [DOI] [PubMed] [Google Scholar]
- 34.Dai W, Zhong D. Peliosis hepatis mimicking cancer: A case report. Oncol Lett. 2013;6:960–962. doi: 10.3892/ol.2013.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battal B, Akgun V, Sari S. Peliosis hepatis: one pathology, a thousand faces, and a clinical and radiological diagnostic challenge. J Dig Dis. 2014;15:281–282. doi: 10.1111/1751-2980.12137. [DOI] [PubMed] [Google Scholar]
- 36.Kim EA, Yoon KH, Jeon SJ, Cai QY, Lee YW, Yoon SE, Yoon KJ, Juhng SK. Peliosis hepatis with hemorrhagic necrosis and rupture: a case report with emphasis on the multi-detector CT findings. Korean J Radiol. 2007;8:64–69. doi: 10.3348/kjr.2007.8.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu HX, Xie XY, Lu MD, Liu GJ, Xu ZF, Liang JY, Chen LD. Unusual benign focal liver lesions: findings on real-time contrast-enhanced sonography. J Ultrasound Med. 2008;27:243–254. doi: 10.7863/jum.2008.27.2.243. [DOI] [PubMed] [Google Scholar]
- 38.Fowell AJ, Mazhar D, Shaw AS, Griffiths WJ. Education and imaging. Hepatobiliary and pancreatic: peliosis hepatis. J Gastroenterol Hepatol. 2011;26:1082. doi: 10.1111/j.1440-1746.2011.06752.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, Pasupathy A, Weltman M. Multifocal peliosis hepatis simulating metastatic malignancy. Dig Liver Dis. 2014;46:862–863. doi: 10.1016/j.dld.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Battal B, Kocaoglu M, Atay AA, Bulakbasi N. Multifocal peliosis hepatis: MR and diffusion-weighted MR-imaging findings of an atypical case. Ups J Med Sci. 2010;115:153–156. doi: 10.3109/03009730903262118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picardi N, Pasta V, Monti M. [Possibility of hemodynamic compensation of the Retzius system and of the paravertebral veins in Budd-Chiari syndrome] Ann Ital Chir 1975- 1976;49:163–177. [PubMed] [Google Scholar]
- 42.Yanoff M, Rawson AJ. Peliosis hepatis. An anatomic study with demonstration of two varieties. Arch Pathol. 1964;77:159–165. [PubMed] [Google Scholar]
- 43.Hyodo M, Mogensen AM, Larsen PN, Wettergren A, Rasmussen A, Kirkegaard P, Yasuda Y, Nagai H. Idiopathic extensive peliosis hepatis treated with liver transplantation. J Hepatobiliary Pancreat Surg. 2004;11:371–374. doi: 10.1007/s00534-004-0908-5. [DOI] [PubMed] [Google Scholar]
- 44.Hiorns MP, Rossi UG, Roebuck DJ. Peliosis hepatis causing inferior vena cava compression in a 3-year-old child. Pediatr Radiol. 2005;35:209–211. doi: 10.1007/s00247-004-1311-8. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki S, Suzuki H, Mochida Y, Hirai H, Yoshida T, Ide M, Tani M, Shimura T, Morinaga N, Ishizaki M, et al. Liver hemorrhage due to idiopathic peliosis hepatis successfully treated with hepatic artery embolization. Int Surg. 2011;96:310–315. doi: 10.9738/cc43.1. [DOI] [PubMed] [Google Scholar]
- 46.Omori H, Asahi H, Irinoda T, Takahashi M, Kato K, Saito K. Peliosis hepatis during postpartum period: successful embolization of hepatic artery. J Gastroenterol. 2004;39:168–171. doi: 10.1007/s00535-003-1268-7. [DOI] [PubMed] [Google Scholar]


