Highlights
-
•
Nuclear FOXO3a significantly correlates with glutamine synthetase expression.
-
•
FOXO3a nuclear localisation correlates with a DNA damage response.
-
•
Glutamine synthetase expression correlates with increasing Alzheimer pathology.
Keywords: FOXO3a, Glutamine synthetase, DNA damage response, Astrocyte, neurone, Alzheimer’s, Pathology, Ageing brain
Abstract
The accumulation of reactive oxygen species leading to oxidative damage and cell death plays an important role in a number of neurodegenerative disorders. FOXO3a, the main isoform of FOXO transcription factors, mediates the cellular response to oxidative stress by regulating the expression of genes involved in DNA repair and glutamine metabolism, including glutamine synthetase (GS). Immunohistochemical investigation of the population-based neuropathology cohort of the Medical Research Council’s Cognitive Function and Ageing Study (MRC CFAS) demonstrates that nuclear retention of FOXO3a significantly correlates with a DNA damage response and with GS expression by astrocytes. Furthermore, we show that GS expression correlates with increasing Alzheimer-type pathology in this ageing cohort. Our findings suggest that in response to oxidative stress, the nuclear retention of FOXO3a in astrocytes upregulates expression of GS as a neuroprotective mechanism. However, the activity of GS may be compromised by increasing levels of oxidative stress in the ageing brain resulting in dysfunctional enzyme activity, neuronal excitotoxic damage and cognitive impairment.
1. Introduction
Alzheimer’s disease (AD) is the most common form of dementia and is pathologically characterised by the extracellular deposition of β-amyloid (Aβ) protein, intracellular neurofibrillary tangles (NFT) of hyperphosphorylated tau, neuronal loss and extensive synaptic changes in the cerebral cortex. Given the currently limited success of Aβ-based therapies [25], it is likely that the successful treatment of AD will also include modulation of mechanisms which protect against other processes causing neuronal dysfunction and neurodegeneration, including excitotoxicity and oxidative stress. Characterisation of these mechanisms is essential to develop novel neuroprotective targets aimed at preventing neuronal dysfunction and cognitive impairment in the ageing population.
Glial pathology occurs in the ageing brain and is a major contributor to age-related neurodegeneration [26], [38]. Astrocytes play a key role maintaining homeostasis in the CNS, including the uptake and recycling of neurotransmitters such as glutamate [20]. Extracellular glutamate levels are mainly regulated through re-uptake from the synaptic cleft via glial excitatory amino acid transporters (EAATs) [43]. A functional glutamate-glutamine metabolic cycle between astrocytes and neurones is vital for preventing excessive extracellular accumulation of the neurotransmitter leading to neuronal excitotoxicity [8], [11]. Loss of astrocyte-associated EAAT2 and glutamate excitotoxicity are features of brain ageing and neurodegenerative diseases, including AD [18], [21], [36].
Forkhead box class O (FOXO) proteins form a family of transcription factors which are phosphorylated and regulated by Akt, resulting in their nuclear exclusion and the termination of their activity [42]. Activation of FOXO, depending on cell type, regulates a wide range of biological processes including stress resistance, cell cycle regulation, development and ageing [19]. Glutamine synthetase (GS), which catalyses the conversion of glutamate to glutamine, is highly expressed in astrocytes and is transcriptionally regulated by the phosphoinositide-3-kinase (PI3K)-Akt-FOXO pathway [41]. In contrast to Akt signalling, oxidative stress induces the nuclear retention of FOXO which, depending on the severity of the stimulus, results in either apoptosis or a protective response, including the transcription of anti-oxidant genes and activation of a DNA damage response [3], [15], [17], [39]. One member of the forkhead transcription factors, FOXO3a, has been implicated in a number of neurodegenerative disorders, including AD [3], [29], [33], [45], motor neuron disease [24], Parkinson’s disease [12] and stroke [13], and is expressed throughout the cortex and hippocampus [14].
The Medical Research Council’s Cognitive Function and Ageing Study (CFAS) is a well characterised prospective, longitudinal, population-based neuropathological study of the aging population (over 65 yrs) [44]. Studies performed on this population-representative cohort we have previously demonstrated a reduction in EAAT2 expression [36] and down-regulation of the PI3K-Akt pathway by astrocytes associated with increasing levels of Alzheimer-type pathology [37], and quantitated oxidative stress and the associated DNA damage response in this ageing cohort [35]. Given the proposed role of excitotoxicity in age-related neurodegeneration, we have now investigated the subcellular localisation of FOXO3a and its correlation with GS expression, astrogliosis and the DNA damage response in the ageing brain.
2. Materials and methods
2.1. Human CNS cases
Human autopsy brain tissue was obtained from one centre of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) [1], [44], following multi-centre research ethics committee (REC) approval (REC Reference number 11/H0308/2). Neuropathological lesions were assessed as part of the core CFAS neuropathology study using a modified protocol from the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD) [23] (wwws.cfas.ac.uk) and Braak neurofibrillary tangle staging [7]. The cases were categorised into groups representing entorhinal stages (Braak stages 0-II; 30 cases), limbic stages (Braak stages III-IV; 50 cases) and isocortical stages of tangle pathology (Braak stages V-VI; 17 cases). The mean age of death was 85.6 (SEM 7.4) years. Dementia status at death had been previously determined, based on all information available for each participant, including algorithmic (AGECAT) assessment in life, information from death certification and a Retrospective Informant Interview (RINI) developed by CFAS [34]. 59 participants had clinical dementia, 37 did not and in 2 cases clinical dementia status was undetermined. The median post-mortem delay was 17 h (IQR 10–32 h) and brain pH 6.49 (IQR 6.25–6.75). Formalin-fixed and frozen lateral temporal cortex samples (superior/middle temporal gyrus, Brodmann areas 22/21) were available for all cases and were used in the immunohistochemistry and western blotting experiments, respectively. The neuronal and astrocyte DNA damage response (DDR) (γH2AX and DNA-PKcs nuclear immunoreactivity), astrogliosis (GFAP immunoreactivity), and local measures of AD-type pathology (Aβ and AT8) were previously assessed in these cases [35], [36]. A total of 98 participants were included in these analyses, where 61 are females.
2.2. Immunohistochemistry
Immunohistochemistry was performed using a standard avidin-biotin complex (ABC) method. Sections were deparaffinised, rehydrated to water and endogenous peroxidase activity quenched by placing the sections in 0.3% H2O2/methanol for 20 min at room temperature (RT). Sections were subjected to antigen retrieval (0.01 M tri-sodium citrate pH 6.5, pressure cooker). Following incubation with 1.5% normal serum for 30 min at RT, the sections were incubated overnight at 4 °C with the well characterised, commercially available antibodies against FOXO3a (1:100; AbCam, UK), or glutamine synthetase (1:500; Millipore, UK). As phosphorylation of FOXO3a leads to the nuclear exclusion of the transcription factor and the termination of its activity, we elected to use an antibody which was raised against the N-terminus of the protein, as opposed to an antibody to specifically detect the phosphorylated form. To visualise antibody binding, the horse-radish peroxidase avidin biotin complex was used (Vectastain Elite kit, Vector Laboratories, UK) with 3,3′-diaminobenzidine (DAB) as the chromagen (Vector Laboratories, UK; brown).
To investigate astrocyte association with FOXO3a, dual labelling with the astrocyte marker GFAP was performed. Following incubation with the avidin-biotin blocking kit (Vector Laboratories, UK), FOXO3A immunostained sections were incubated overnight at 4 °C with anti-GFAP (1:500; DakoCytomation, UK), followed by the alkaline-phosphatase-conjugated avidin-biotin complex (Vectastain Elite kit, Vector Laboratories, UK), developed with alkaline phosphatase substrate 1 (Vector Laboratories, UK; red) and lightly counterstained with Mayer’s haematoxylin. Negative controls, either omission of the primary antibody or isotype controls, were included in every run.
2.3. Quantitative analysis of FOXO3a and GS
Assessment of FOXO3a and GS-specific immunoreactivity was performed by capturing bright-field microscopic images in 3 adjacent 350 μm-wide cortical ribbons, consisting of contiguous fields to cover the total cortical thickness through the apex of the gyrus, using a x20 objective (Nikon Eclipse Ni-U microscope, Nikon, UK) and analysed using the Analysis ^D software (Olympus Biosystems, Watford, UK). For GS, the image was thresholded and the immunoreactive area of the field determined per total area examined. The number of FOXO3a positive pyramidal neuronal nuclei was determined using a size exclusion of >450 pixels, and the number of positive glial nuclei determined by subtracting the number of pyramidal neuronal nuclei from the total number of positive nuclei.
2.4. Statistical Analysis
As our data was skewed, median and inter-quartile range (IQR) was used for descriptive analyses. To test if dementia, sex, age or post-mortem delay (PMD) were risk factors for GS and/or FOXO3a nuclear retention, linear regressions were used. Spearman's correlation coefficient (r) was calculated to verify the strength of correlations between continuous variables. All tests were 2-tailed. 95% confidence intervals (CI) were calculated for the linear regression coefficients (β). Statistical analyses were performed using statistical package STATA, version 12.
3. Results
3.1. Expression of FOXO3a and GS in the ageing temporal cortex
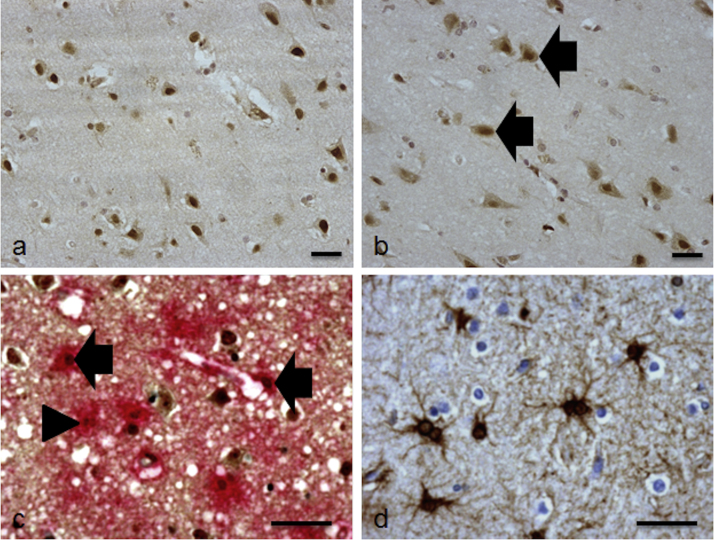
Specific nuclear immunostaining of FOXO3a was evenly distributed throughout all layers of the temporal cortex associated with cells morphologically resembling neurones and glia, and was seldom observed in the white matter (Fig. 1a). In a subgroup of 14 cases, faint FOXO3a immunoreactivity was also detected in the cytoplasmic compartment of cells (Fig. 1b). Dual staining with GFAP demonstrated association of FOXO3a with astrocytes (Fig. 1c), but a proportion of FOXO3a+ glia were not GFAP+. GS was exclusively associated with astrocytes throughout the cohort, predominantly staining the astrocyte cell body and their primary radiating processes within the cortex (Fig. 1d).
Fig. 1.
Expression of FOXO3a and GS in the lateral temporal cortex. (a) Specific nuclear immunostaining of FOXO3a was associated with cells morphologically resembling neurones and glia. (b) In a subgroup of cases increased levels of FOXO3a were detected in the cytoplasmic compartment, examples are indicated by the arrow. (c) FOXO3a co-localised with some (arrow), but not all (arrow head) GFAP+ astrocytes. (d) GS was exclusively associated with cells with an astrocytic morphology. Scale bar represents 100 μm in (a) and (b), and 50 μm in (c) and (d).
The median number of FOXO3A+ neurones within the temporal cortex was 28.9 (IQR = 11.4–51.8), FOXO3A+ glia was 86.6 (IQR = 62.6–118.6) and area GS immunoreactivity was 0.5 (IQR = 0.23–0.97). Neither GS nor FOXO3a (neuronal or glia immunoreactivity) significantly related to age, sex or PMD, but FOXO3a neuronal immunoreactivity did relate to tissue pH (Table 1).
Table 1.
Linear regression analyses investigating the relationship between GS and FOXO3a (glial or neuronal immunoreactivity) with demographics and brain pH.
| β | 95%CI(β) | p | ||
|---|---|---|---|---|
| GS | Age | 0.01 | (−0.01; 0.03) | 0.440 |
| pH | −0.12 | (−0.46; 0.22) | 0.474 | |
| PMD | 0.00 | (−0.01; 0.01) | 0.448 | |
| Sex | −0.23 | (−0.59; 0.12) | 0.191 | |
| FOXO3a glial | Age | −0.25 | (−1.72; 1.23) | 0.741 |
| pH | 20.15 | (−8.00; 48.29) | 0.158 | |
| PMD | 0.09 | (−0.49; 0.68) | 0.749 | |
| Sex | 7.22 | (−15.11; 29.56) | 0.522 | |
| FOXO3a neuronal | Age | −0.44 | (−1.32; 0.44) | 0.321 |
| pH | 25.16 | (8.10; 42.22) | 0.004 | |
| PMD | -0.16 | (−0.52; 0.19) | 0.357 | |
| Sex | 5.30 | (−8.07; 18.68) | 0.433 |
3.2. GS but not FOXO3a significantly correlates with Alzheimer-type pathology
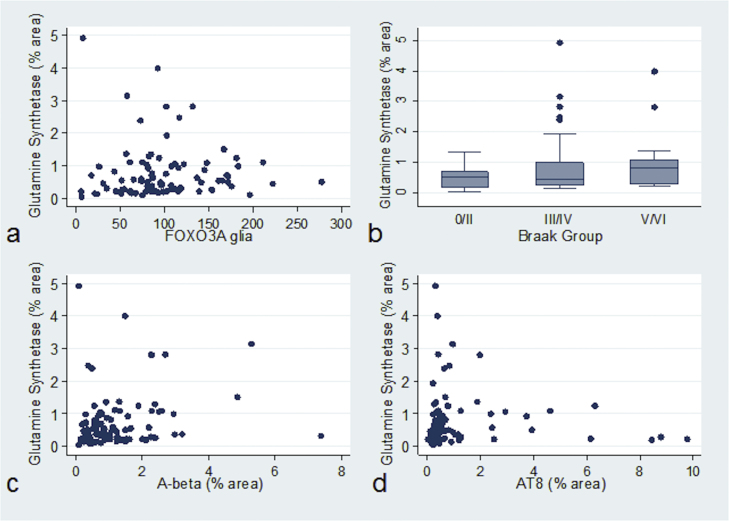
Nuclear localisation of FOXO3a in glia significantly correlated with increased expression of GS (n = 85, r = 0.23, p = 0.035, Fig. 2a). Increasing levels of GS+ astrocytes did not significantly correlate with astrogliosis (n = 92, r = 0.15, p = 0.163) but did with global measures of brain Alzheimer-type pathology (Braak neurofibrillary tangle stage) (n = 93, r = 0.23, p = 0.029, Fig. 2b), and with local measures of AD pathology in the temporal cortex, namely Aβ (n = 90, r = 0.24, p = 0.020, Figure 2c) and tau area immunoreactivity (n = 89, r = 0.24, p = 0.022, Fig. 2d). In contrast, neither FOXO3a+ glia nor FOXO3a+ neurones significantly correlated with Braak stage (n = 89, r = 0.06, p = 0.571; n = 89, r = -0.08, p = 0.437, respectively), Aβ (n = 86, r = 0.09, p = 0.405; n = 86, r = 0.16, p = 0.142, respectively), or tau pathology (n = 84, r = 0.14, p = 0.194; n = 84, r = 0.08, p = 0.482). The number of FOXO3a+ neurones, FOXO3a+ glia, and GS immunoreactive area (%) within each Braak stage is shown in Table 2.
Fig. 2.
GS expression associates with Alzheimer-type pathology. Increasing GS+ astrocytes associated with (a) FOXO3a+ glia, (b) Braak stage, (c) β-amyloid plaques and (d) levels of tau (AT8 immunoreactivity).
Table 2.
Number of FOXO3A+ neurones, FOXO3A+ glia, and GS immunoreactive area (%) within each Braak group.
| Braak group | Entorhinal | Limbic | Isocortical | |
|---|---|---|---|---|
| FOXO3A+ neurones | Median (IQR) | 31.0 (14.5–58.7) | 28.9 (11.9–45.5) | 20.2 (9.7–52.3) |
| FOXO3A+ glia | Median (IQR) | 86.8 (51.9–149.7) | 85.9 (61.5–118.0) | 98.4 (68.2–115.3) |
| GS | Median (IQR) | 0.5 (0.1–0.6) | 0.4 (0.2–1.0) | 0.8 (0.3–1.2) |
IQR: inter-quartile range.
3.3. FOXO3a nuclear expression significantly correlates with a DNA damage response
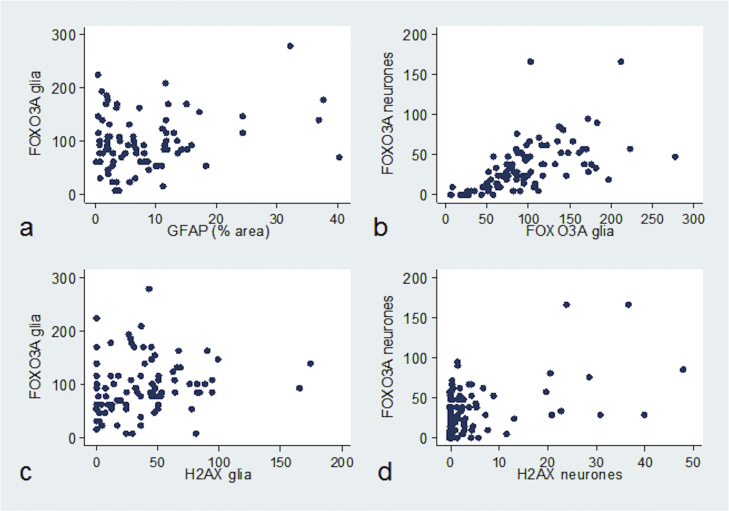
We investigated the relationships between FOXO3a neuronal and glial expression with astrogliosis and levels of a DNA damage response. FOXO3a+ glia did not correlate with astrogliosis (n = 88, r = 0.09, p = 0.377), but significantly correlated with FOXO3a+ neurones (n = 89, r = 0.68, p < 0.001, Fig. 3b). FOXO3a+ glia correlated with γH2AX+ glia (n = 87, r = 0.23, p = 0.030, Fig. 3c), and FOXO3a+ neurones significantly correlated with γH2AX+ neurones (n = 87, r = 0.27, p = 0.010, Fig.3d).
Fig. 3.
FOXO3a expression correlates with a DNA damage response. (a) FOXO3a+ glia showed no association with astrogliosis, (b) but did correlate with FOXO3a+ neurones. (c) FOXO3a+ glia significantly correlated with γH2AX+ glia, while (d) FOXO3a+ neurones significantly correlated with γH2AX+ neurones.
3.4. Relationship to dementia status
Neither GS (OR = 2.37, 95%CI (OR) = 0.96; 5.86, p = 0.061), FOXO3a neuronal (OR = 0.99, 95%CI (OR) = 0.98;1.01, p = 0.591) nor FOXO3a glial expression (OR = 1.00, 95%CI(OR) = 0.99; 1.01, p = 0.279) were significant predictors of dementia when the analyses were controlled for age and sex.
4. Discussion
FOXO transcription factors control various biological functions, including apoptosis, and the expression of genes involved in the regulation of glutamine metabolism, DNA repair and resistance to oxidative stress [4], [19], [41]. In the present study we demonstrate that nuclear retention of FOXO3a significantly correlates with a DNA damage response and with GS expression by astrocytes, but not with Alzheimer-type pathology in the ageing brain. Furthermore, we show that GS expression correlates with local burdens of Aβ plaques and tau pathology in this ageing cohort.
The generation of reactive oxygen species (ROS) leading to oxidative damage and neuronal cell death plays an important role in the pathogenesis of neurodegenerative disorders, suggesting that anti-oxidant defence mechanisms are unable to cope with increasing ROS levels in these diseases [22]. Glutamate and Aβ are two oxidative stressors: at high concentrations glutamate elevates intracellular calcium levels and increases the formation of ROS [30]; while Aβ induces mitochondrial dysfunction resulting in the generation of ROS [2]. FOXO3a, the main isoform of FOXO transcription factors, mediates cellular responses to oxidative stress and modulates adaptive responses. Post-translational phosphorylation of FOXO3a regulates the translocation of FOXO3a from the nucleus to the cytosol, resulting in the repression of the transcription of genes associated with protection against oxidative stress, DNA repair and anti-apoptosis [42]. The redox potential of neurones and glia is essential to protect against neurotoxic ROS levels which result in neuronal dysfunction and are associated with cognitive impairment. In the present study we demonstrate increased nuclear retention of FOXO3a by neurones and glia significantly correlates with a DNA damage response suggesting that, in response to oxidative DNA damage, FOXO3a may play a key role regulating the expression of neuroprotective anti-oxidant genes.
Cognitive decline is associated with synaptic loss and impaired synaptic connectivity, which may occur as a result of impaired neurotransmitter recycling and associated neuronal excitotoxicity [27]. We previously showed a reduction in astrocyte expression of the glutamate transporter EAAT2 associated with increasing levels of Alzheimer pathology, which likely results in the accumulation of excitotoxic levels of glutamate [36]. Levels of glutamate, the major excitatory neurotransmitter in the CNS, are primarily regulated by GS which is expressed by astrocytes in all cortical layers [32]. An increase in GS expression has been reported in the prefrontal cortex in AD and in the CSF of vascular dementia, motor neuron disease and AD patients [5], [9], [40]. In support of these findings, we demonstrate a significant increase in GS expression associated with increasing levels of Alzheimer-type pathology in the ageing brain. In contrast, other studies have reported a reduction in the astrocytic expression of this enzyme in AD which shows no association with Aβ pathology [16], [32]. Complicating the interpretation of these conflicting reports GS is significantly oxidised in both AD and mild cognitive impairment cases [10], with oxidation significantly reducing its activity [31]. Furthermore, while Aβ has been shown to induce astrocyte expression of GS in vitro [28], proteomic analysis has identified GS as being susceptible to oxidation after exposure to Aβ1 – 42 [6]. Our findings suggest that in response to oxidative stress, the nuclear retention of FOXO3a increases the expression of GS as a neuroprotective mechanism by astrocytes to maintain homeostasis of the synaptic environment and protect against accumulating levels of glutamate and neuronal excitotoxicity in the ageing brain. However while levels of GS may rise, its activity may be compromised by increasing levels of oxidative stressors, including glutamate and local Aβ pathology, resulting in dysfunctional enzyme activity, neuronal excitotoxic damage and cognitive impairment.
Astrocytes play a key role in maintaining homeostasis within the synaptic and neuronal environments. Understanding which factors control and modulate FOXO3a-mediated neuroprotection is crucial to identify potential therapeutic targets in the treatment of AD.
Acknowledgements
This study was supported by the Medical Research Council (MRJ004308/1). CG is supported by ARUK (ART:PG2010-5). CFAS study is supported by the Department of Health and the Medical Research Council (grants MRC/G9901400 and MRC U.1052.00.0013); the UKNIHR Biomedical Research Centre for Ageing and Age-related Disease Award to the Newcastle upon Tyne Hospitals Foundation Trust; the Cambridge Brain Bank is supported by the NIHR Cambridge Biomedical Research Centre; The Cambridgeshire and Peterborough NIHR CLAHRC; Nottingham University Hospitals NHS Trust; University of Sheffield and the Sheffield Teaching Hospitals NHS Foundation Trust; The Thomas Willis Oxford Brain Collection, supported by the Oxford Biomedical Research Centre; The Walton Centre NHS Foundation Trust, Liverpool. We would like to acknowledge the essential contribution of the liaison officers, the general practitioners, their staff, and nursing and residential home staff. We are grateful to our respondents and their families for their generous gift to medical research, which has made this study possible.
References
- 1.Cognitive function and dementia in six areas of England and Wales: the distribution of MMSE and prevalence of GMS organicity level in the MRC CFA Study. The Medical Research Council Cognitive Function and Ageing Study (MRC CFAS), Psychol. Med. 28 (1998) 319–335. [DOI] [PubMed]
- 2.Abramov A.Y., Canevari L., Duchen M.R. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J. Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akhter R., Sanphui P., Biswas S.C. The essential role of p53-up-regulated modulator of apoptosis (Puma) and its regulation by FoxO3a transcription factor in beta-amyloid-induced neuron death. J. Biol. Chem. 2014;289:10812–10822. doi: 10.1074/jbc.M113.519355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkenkamp K.U., Coffer P.J. Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem. Soc. Trans. 2003;31:292–297. doi: 10.1042/bst0310292. [DOI] [PubMed] [Google Scholar]
- 5.Bos I.W., Hoogland G., Meine Jansen C.F., Willigen G., Spierenburg H.A., van den Berg L.H., de Graan P.N. Increased glutamine synthetase but normal EAAT2 expression in platelets of ALS patients. Neurochem. Int. 2006;48:306–311. doi: 10.1016/j.neuint.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Boyd-Kimball D., Castegna A., Sultana R., Poon H.F., Petroze R., Lynn B.C., Klein J.B., Butterfield D.A. Proteomic identification of proteins oxidized by Abeta(1-42) in synaptosomes: implications for Alzheimer’s disease. Brain Res. 2005;1044:206–215. doi: 10.1016/j.brainres.2005.02.086. [DOI] [PubMed] [Google Scholar]
- 7.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 8.Broer S., Brookes N. Transfer of glutamine between astrocytes and neurons. J. Neurochem. 2001;77:705–719. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- 9.Burbaeva G., Boksha I.S., Tereshkina E.B., Savushkina O.K., Starodubtseva L.I., Turishcheva M.S. Glutamate metabolizing enzymes in prefrontal cortex of Alzheimer’s disease patients. Neurochem. Res. 2005;30:1443–1451. doi: 10.1007/s11064-005-8654-x. [DOI] [PubMed] [Google Scholar]
- 10.Butterfield D.A., Poon H.F., St Clair D., Keller J.N., Pierce W.M., Klein J.B., Markesbery W.R. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol. Dis. 2006;22:223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Choi D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 12.Desplats P., Patel P., Kosberg K., Mante M., Patrick C., Rockenstein E., Fujita M., Hashimoto M., Masliah E. Combined exposure to Maneb and Paraquat alters transcriptional regulation of neurogenesis-related genes in mice models of Parkinson’s disease. Mol. Neurodegener. 2012;7:49. doi: 10.1186/1750-1326-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y., Zhang X., Ji H., Liu H., Li S., Li L. Probucol and atorvastatin in combination protect rat brains in MCAO model: upregulating Peroxiredoxin2, Foxo3a and Nrf2 expression. Neurosci. Lett. 2012;509:110–115. doi: 10.1016/j.neulet.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 14.Hoekman M.F., Jacobs F.M., Smidt M.P., Burbach J.P. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr. Patterns: GEP. 2006;6:134–140. doi: 10.1016/j.modgep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Kops G.J., Dansen T.B., Polderman P.E., Saarloos I., Wirtz K.W., Coffer P.J., Huang T.T., Bos J.L., Medema R.H., Burgering B.M. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 16.Kulijewicz-Nawrot M., Sykova E., Chvatal A., Verkhratsky A., Rodriguez J.J. Astrocytes and glutamate homoeostasis in Alzheimer's disease: a decrease in glutamine synthetase, but not in glutamate transporter-1, in the prefrontal cortex. ASN Neuro. 2013;5:273–282. doi: 10.1042/AN20130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehtinen M.K., Yuan Z., Boag P.R., Yang Y., Villen J., Becker E.B., DiBacco S., Gygde la Iglesia N., Gygi T.K., Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 18.Li S., Mallory M., Alford M., Tanaka S., Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J. Neuropathol. Exp. Neurol. 1997;56:901–911. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Maiese K., Chong Z.Z., Shang Y.C. Sly as a FOXO: new paths with Forkhead signaling in the brain. Curr. Neurovasc. Res. 2007;4:295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maragakis N.J., Dykes-Hoberg M., Rothstein J.D. Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann. Neurol. 2004;55:469–477. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- 21.Masliah E., Alford M., DeTeresa R., Mallory M., Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann. Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 22.Melo A., Monteiro L., Lima R.M., Oliveira D.M., Cerqueira M.D., El-Bacha R.S. Oxidative stress in neurodegenerative diseases: mechanisms and therapeutic perspectives. Oxid. Med. Cell. Longevity. 2011;2011:467180. doi: 10.1155/2011/467180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirra S.S. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol. Aging. 1997;18:S91–94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 24.Mojsilovic-Petrovic J., Nedelsky N., Boccitto M., Mano I., Georgiades S.N., Zhou W., Liu Y., Neve R.L., Taylor J.P., Driscoll M., Clardy J., Merry D., Kalb R.G. FOXO3a is broadly neuroprotective in vitro and in vivo against insults implicated in motor neuron diseases. J. Neurosci. 2009;29:8236–8247. doi: 10.1523/JNEUROSCI.1805-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panza F., Solfrizzi V., Imbimbo B.P., Tortelli R., Santamato A., Logroscino G. Amyloid-based immunotherapy for Alzheimer’s disease in the time of prevention trials: the way forward. Expert Rev. Clin. Immunol. 2014;10:405–419. doi: 10.1586/1744666X.2014.883921. [DOI] [PubMed] [Google Scholar]
- 26.Parpura V., Heneka M.T., Montana V., Oliet S.H., Schousboe A., Haydon P.G., Stout R.F., Jr., Spray D.C., Reichenbach A., Pannicke T., Pekny M., Pekna M., Zorec R., Verkhratsky A. Glial cells in (patho) physiology. J. Neurochem. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paula-Lima A.C., Brito-Moreira J., Ferreira S.T. Deregulation of excitatory neurotransmission underlying synapse failure in Alzheimer's disease. J. Neurochem. 2013;126:191–202. doi: 10.1111/jnc.12304. [DOI] [PubMed] [Google Scholar]
- 28.Pike C.J., Ramezan-Arab N., Miller S., Cotman C.W. Beta-Amyloid increases enzyme activity and protein levels of glutamine synthetase in cultured astrocytes. Exp. Neurol. 1996;139:167–171. doi: 10.1006/exnr.1996.0091. [DOI] [PubMed] [Google Scholar]
- 29.W. Qin W., Zhao L., Ho J., Wang K., Walsh S., Gandy G.M. Pasinetti. Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer’s disease-type amyloid neuropathology and spatial memory deterioration. Ann. N. Y. Acad. Sci. 2008;1147:335–347. doi: 10.1196/annals.1427.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds I.J., Hastings T.G. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivett A.J. Preferential degradation of the oxidatively modified form of glutamine synthetase by intracellular mammalian proteases. J. Biol. Chem. 1985;260:300–305. [PubMed] [Google Scholar]
- 32.Robinson S.R. Changes in the cellular distribution of glutamine synthetase in Alzheimer’s disease. J. Neurosci. Res. 2001;66:972–980. doi: 10.1002/jnr.10057. [DOI] [PubMed] [Google Scholar]
- 33.Sahin P., McCaig C., Jeevahan J., Murray J.T., Hainsworth A.H. The cell survival kinase SGK1 and its targets FOXO3a and NDRG1 in aged human brain. Neuropathol. Appl. Neurobiol. 2013;39:623–633. doi: 10.1111/nan.12023. [DOI] [PubMed] [Google Scholar]
- 34.Savva G.M., Wharton S.B., Ince P.G., Forster G., Matthews F.E., Brayne C., Medical Research Council Cognitive F., Ageing S. Age, neuropathology, and dementia. N. Engl. J. Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 35.Simpson J.E., Ince P.G., Haynes L.J., Theaker R., Gelsthorpe C., Baxter L., Forster G., Lace G.L., Shaw P.J., Matthews F.E., Savva G.M., Brayne C., Wharton S.B., MRC Cognitive Function and Ageing Neuropathology Study Group Population variation in oxidative stress and astrocyte DNA damage in relation to Alzheimer-type pathology in the ageing brain. Neuropathol. Appl. Neurobiol. 2010;36:25–40. doi: 10.1111/j.1365-2990.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 36.Simpson J.E., Ince P.G., Lace G., Forster G., Shaw P.J., Matthews F., Savva G., Brayne C., Wharton S.B., MRC Cognitive Function and Ageing Neuropathology Study Group Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol. Aging. 2010;31:578–590. doi: 10.1016/j.neurobiolaging.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Simpson J.E., Ince P.G., Shaw P.J., Heath P.R., Raman R., Garwood C.J., Gelsthorpe C., Baxter L., Forster G., Matthews F.E., Brayne C., Wharton S.B., MRC Cognitive Function and Ageing Neuropathology Study Group Microarray analysis of the astrocyte transcriptome in the aging brain: relationship to Alzheimer's pathology and APOE genotype. Neurobiol. Aging. 2011;32:1795–1807. doi: 10.1016/j.neurobiolaging.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran H., Brunet A., Grenier J.M., Datta S.R., Fornace A.J., Jr, DiStefano P.S., Chiang L.W., Greenberg M.E. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 40.Tumani H., Shen G., Peter J.B., Bruck W. Glutamine synthetase in cerebrospinal fluid, serum, and brain: a diagnostic marker for Alzheimer disease? Arch. Neurol. 1999;56:1241–1246. doi: 10.1001/archneur.56.10.1241. [DOI] [PubMed] [Google Scholar]
- 41.van der Vos K.E., Eliasson P., Proikas-Cezanne T., Vervoort S.J., van Boxtel R., Putker M., van Zutphen I.J., Mauthe M., Zellmer S., Pals C., Verhagen L.P., Groot Koerkamp M.J., Braat A.K., Dansen T.B., Holstege F.C., Gebhardt R., Burgering B.M., Coffer P.J. Modulation of glutamine metabolism by the PI(3)K-PKB-FOXO network regulates autophagy. Nat. Cell Biol. 2012;14:829–837. doi: 10.1038/ncb2536. [DOI] [PubMed] [Google Scholar]
- 42.Vogt P.K., Jiang H., Aoki M. Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle. 2005;4:908–913. doi: 10.4161/cc.4.7.1796. [DOI] [PubMed] [Google Scholar]
- 43.Wadiche J.I., Arriza J.L., Amara S.G., Kavanaugh M.P. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 44.Wharton S.B., Brayne C., Savva G.M., Matthews F.E., Forster G., Simpson J., Lace G., Ince PG; Medical Research Council Cognitive Function and Aging Study Epidemiological neuropathology: the MRC Cognitive Function and Aging Study experience. J. Alzheimer’s Dis.: JAD. 2011;25:359–372. doi: 10.3233/JAD-2011-091402. [DOI] [PubMed] [Google Scholar]
- 45.Wong H.K., Veremeyko T., Patel N., Lemere C.A., Walsh D.M., Esau C., Vanderburg C., Krichevsky A.M. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer's disease. Hum. Mol. Genet. 2013;22:3077–3092. doi: 10.1093/hmg/ddt164. [DOI] [PubMed] [Google Scholar]