Abstract
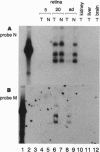
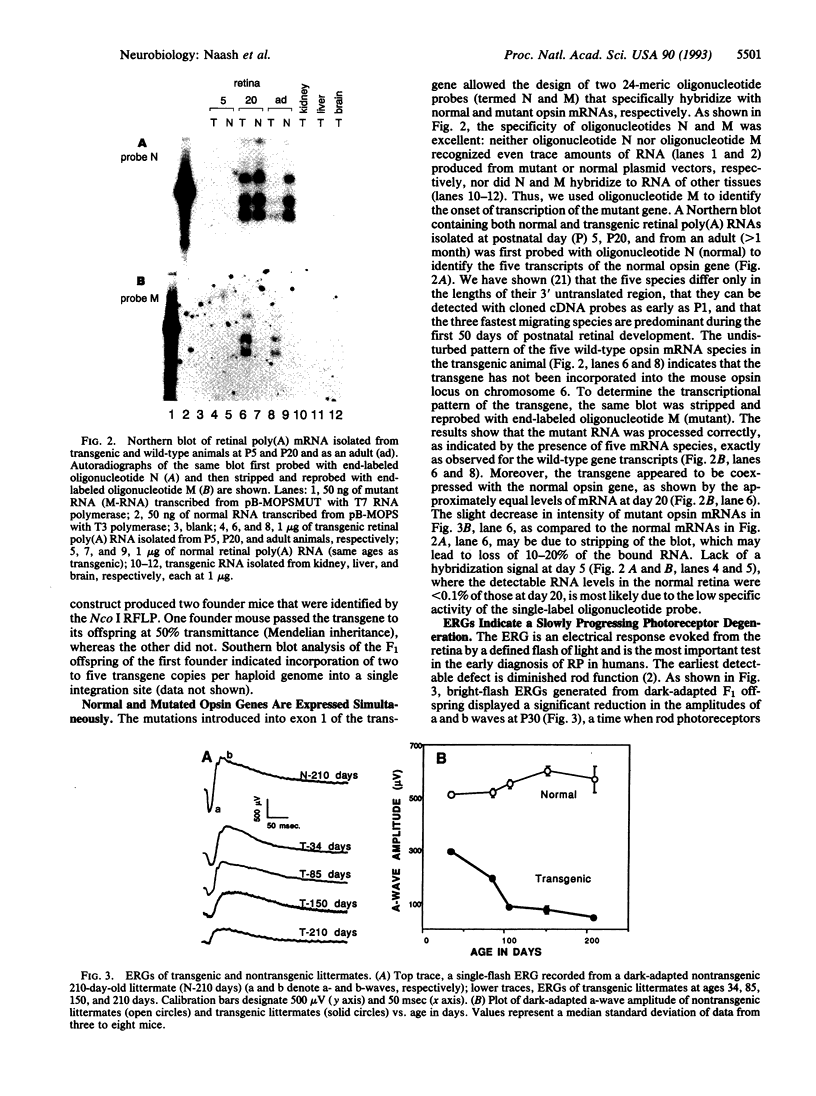
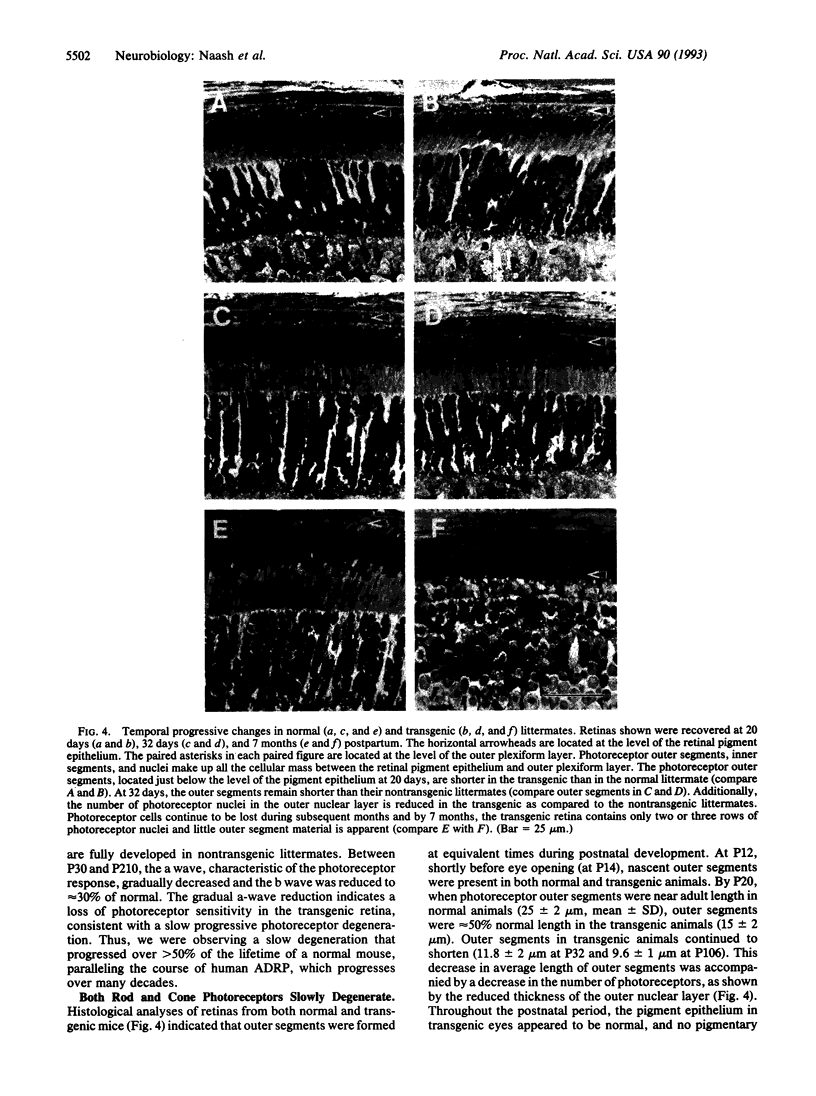
Autosomal dominant retinitis pigmentosa (ADRP), slowly progressing over decades, leads to severe visual impairment and in some cases to complete blindness. More than 40 mutations in the human opsin gene have been linked to some forms of this genetically heterogeneous disease. In photoreceptor cells of ADRP patients with mutations in the opsin gene, normal rhodopsin is thought to be synthesized concomitantly with mutated rhodopsin, which, by an unknown mechanism, causes the slow degeneration of the photoreceptor cells. To establish a transgenic mouse line that carries a mutated mouse opsin gene in addition to the endogenous opsin gene, we introduced a mouse opsin gene containing mutations in exon 1 into the germ line of a normal mouse. The alterations consisted of three amino acid substitutions near the N terminus of rhodopsin, Val-20-->Gly (V20G), Pro-23-->His (P23H), and Pro-27-->Leu (P27L). The P23H mutation is the most prevalent mutation in human ADRP patients. During early postnatal development, mice heterozygous for the mutated opsin gene appear to develop normal photoreceptors, but their light-sensitive outer segments never reach normal length. With advancing age, both rod and cone photoreceptors are reduced progressively in number. The slow degeneration of the transgenic retina is associated with a gradual decrease of light-evoked electroretinogram responses. Our results show that simultaneous expression of mutated and normal opsin genes induces a slow degeneration of both rod and cone photoreceptors and that the course of the retinal degeneration of the mutant mouse retina mimics the course of human ADRP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Falk J. D., Bugra K., Triantafyllos J. T., McGinnis J. F. Isolation and analysis of the mouse opsin gene. FEBS Lett. 1988 Oct 10;238(2):253–256. doi: 10.1016/0014-5793(88)80490-3. [DOI] [PubMed] [Google Scholar]
- Berson E. L., Rosner B., Sandberg M. A., Dryja T. P. Ocular findings in patients with autosomal dominant retinitis pigmentosa and a rhodopsin gene defect (Pro-23-His). Arch Ophthalmol. 1991 Jan;109(1):92–101. doi: 10.1001/archopht.1991.01080010094039. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Lester D., Keen J., Bashir R., Lauffart B., Inglehearn C. F., Jay M., Bird A. C. Retinitis pigmentosa and mutations in rhodopsin. Lancet. 1991 Jan 19;337(8734):185–185. doi: 10.1016/0140-6736(91)90858-m. [DOI] [PubMed] [Google Scholar]
- Bowes C., Li T., Danciger M., Baxter L. C., Applebury M. L., Farber D. B. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990 Oct 18;347(6294):677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- Connell G., Bascom R., Molday L., Reid D., McInnes R. R., Molday R. S. Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):723–726. doi: 10.1073/pnas.88.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Hahn L. B., Cowley G. S., McGee T. L., Berson E. L. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9370–9374. doi: 10.1073/pnas.88.20.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Reichel E., Hahn L. B., Cowley G. S., Yandell D. W., Sandberg M. A., Berson E. L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990 Jan 25;343(6256):364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Farrar G. J., Kenna P., Jordan S. A., Kumar-Singh R., Humphries M. M., Sharp E. M., Sheils D. M., Humphries P. A three-base-pair deletion in the peripherin-RDS gene in one form of retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):478–480. doi: 10.1038/354478a0. [DOI] [PubMed] [Google Scholar]
- Gal A., Artlich A., Ludwig M., Niemeyer G., Olek K., Schwinger E., Schinzel A. Pro-347-Arg mutation of the rhodopsin gene in autosomal dominant retinitis pigmentosa. Genomics. 1991 Oct;11(2):468–470. doi: 10.1016/0888-7543(91)90159-c. [DOI] [PubMed] [Google Scholar]
- Humphries P., Kenna P., Farrar G. J. On the molecular genetics of retinitis pigmentosa. Science. 1992 May 8;256(5058):804–808. doi: 10.1126/science.1589761. [DOI] [PubMed] [Google Scholar]
- Inglehearn C. F., Bashir R., Lester D. H., Jay M., Bird A. C., Bhattacharya S. S. A 3-bp deletion in the rhodopsin gene in a family with autosomal dominant retinitis pigmentosa. Am J Hum Genet. 1991 Jan;48(1):26–30. [PMC free article] [PubMed] [Google Scholar]
- Kajiwara K., Hahn L. B., Mukai S., Travis G. H., Berson E. L., Dryja T. P. Mutations in the human retinal degeneration slow gene in autosomal dominant retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):480–483. doi: 10.1038/354480a0. [DOI] [PubMed] [Google Scholar]
- Keen T. J., Inglehearn C. F., Lester D. H., Bashir R., Jay M., Bird A. C., Jay B., Bhattacharya S. S. Autosomal dominant retinitis pigmentosa: four new mutations in rhodopsin, one of them in the retinal attachment site. Genomics. 1991 Sep;11(1):199–205. doi: 10.1016/0888-7543(91)90119-y. [DOI] [PubMed] [Google Scholar]
- Olsson J. E., Gordon J. W., Pawlyk B. S., Roof D., Hayes A., Molday R. S., Mukai S., Cowley G. S., Berson E. L., Dryja T. P. Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa. Neuron. 1992 Nov;9(5):815–830. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- Penn J. S., Naash M. I., Anderson R. E. Effect of light history on retinal antioxidants and light damage susceptibility in the rat. Exp Eye Res. 1987 Jun;44(6):779–788. doi: 10.1016/s0014-4835(87)80041-6. [DOI] [PubMed] [Google Scholar]
- Pittler S. J., Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittler S. J., Baehr W., Wasmuth J. J., McConnell D. G., Champagne M. S., vanTuinen P., Ledbetter D., Davis R. L. Molecular characterization of human and bovine rod photoreceptor cGMP phosphodiesterase alpha-subunit and chromosomal localization of the human gene. Genomics. 1990 Feb;6(2):272–283. doi: 10.1016/0888-7543(90)90567-e. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Fishman G. A., Beck J. S., Kimura A. E., Stone E. M. Identification of novel rhodopsin mutations associated with retinitis pigmentosa by GC-clamped denaturing gradient gel electrophoresis. Am J Hum Genet. 1991 Oct;49(4):699–706. [PMC free article] [PubMed] [Google Scholar]
- Sung C. H., Davenport C. M., Hennessey J. C., Maumenee I. H., Jacobson S. G., Heckenlively J. R., Nowakowski R., Fishman G., Gouras P., Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C. H., Schneider B. G., Agarwal N., Papermaster D. S., Nathans J. Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8840–8844. doi: 10.1073/pnas.88.19.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis G. H., Sutcliffe J. G., Bok D. The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron. 1991 Jan;6(1):61–70. doi: 10.1016/0896-6273(91)90122-g. [DOI] [PubMed] [Google Scholar]
- al-Ubaidi M. R., Hollyfield J. G., Overbeek P. A., Baehr W. Photoreceptor degeneration induced by the expression of simian virus 40 large tumor antigen in the retina of transgenic mice. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1194–1198. doi: 10.1073/pnas.89.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Ubaidi M. R., Pittler S. J., Champagne M. S., Triantafyllos J. T., McGinnis J. F., Baehr W. Mouse opsin. Gene structure and molecular basis of multiple transcripts. J Biol Chem. 1990 Nov 25;265(33):20563–20569. [PubMed] [Google Scholar]