Abstract
Maintaining blood glucose concentration within a relatively narrow range through periods of fasting or excess nutrient availability is essential to the survival of the organism. This is achieved through an intricate balance between glucose uptake and endogenous glucose production to maintain constant glucose concentrations. The liver plays a major role in maintaining normal whole body glucose levels by regulating the processes of de novo glucose production (gluconeogenesis) and glycogen breakdown (glycogenolysis), thus controlling the levels of hepatic glucose release. Aberrant regulation of hepatic glucose production (HGP) can result in deleterious clinical outcomes, and excessive HGP is a major contributor to the hyperglycemia observed in Type 2 diabetes mellitus (T2DM). Indeed, adjusting glycaemia as close as possible to a non-diabetic range is the foremost objective in the medical treatment of patients with T2DM and is currently achieved in the clinic primarily through suppression of HGP. Here, we review the molecular mechanisms controlling HGP in response to nutritional and hormonal signals and discuss how these signals are altered in T2DM.
2 Introduction
Abnormal concentrations of glucose in plasma result in deleterious effects at the whole organism level. Glucose is the main energy source for the brain and decreased plasma glucose levels (hypoglycemia) can lead to impaired brain function and death. Conversely, increased plasma glucose levels (hyperglycemia), a major clinical symptom of diabetes, dramatically increase the risk of various macrovascular and microvascular complications.
Glucose homeostasis is balanced by nutrient sensing and hormonal signaling intracellular mechanisms that control tissue-specific rates of glucose utilization and production. Among the tissues contributing to the maintenance of normal ranges of blood glucose levels are the liver, skeletal and cardiac muscle, fat and brain. After a carbohydrate meal, ~33% of the glucose is taken up by the liver, another ~33% is taken up by muscle and adipose tissues, and the remaining glucose is taken up by the brain, kidney and red blood cells (RBC) (Moore et al., 2012). Insulin and glucagon are two central glucose-dependent counterregulatory hormones that orchestrate the peripheral tissues’ responses to control rates of utilization and production of glucose to maintain glycaemia within narrow ranges. Indeed, the resistance of these tissues to insulin is the major contributor to impaired glucose homeostasis leading to hyperglycemia and to the development of type 2 diabetes mellitus (T2DM) (Samuel and Shulman, 2012).
The liver plays a major role in maintaining glucose homeostasis, as it is the main organ for glucose storage, in the form of glycogen, as well as endogenous glucose production. When nutrients are available, insulin is secreted from pancreatic β cells and promotes hepatic glycogen synthesis and lipogenesis. When nutrients become scarce, insulin levels are decreased and glucagon is secreted from pancreatic α cells to promote hepatic glucose production (HGP) to meet brain and RBC energetic demands. HGP is achieved by glycogen breakdown (glycogenolysis) as well as by de novo glucose synthesis from available precursors (gluconeogenesis). Increased rates of HGP, as observed in patients with T2DM, significantly impair glucose homeostasis and significantly contribute to hyperglycemia (Lin and Accili, 2011). Therefore, controlling the rates of HGP is one of the major targets for the treatment of T2DM patients. In this review, we will focus on the molecular mechanisms underlying the nutrient and hormonal regulatory control of HGP.
3 Whole body glucose homeostasis
Blood glucose concentrations in normal healthy individuals are normally maintained at ~90 mg/dl. This is a result of an intricate balance between endogenous glucose production and glucose removal from the blood stream, which are dynamically regulated by hormonal and nutritional signals. The primary tissue source for endogenous glucose production is the liver and under some conditions the kidney. The clearance of glucose from the blood stream is the net consumption primarily by the brain, muscle, adipose and liver tissues. In this section, we will briefly review the regulatory contribution of each tissue in maintaining the net blood glucose concentration.
Liver
The liver plays a major role in whole body glucose metabolism by maintaining a balance between glucose production and glucose storage in the form of glycogen. Approximately 80% of endogenous glucose production is accounted for by the liver and the remaining by the kidney (Gerich, 2010). In humans, the splanchnic bed (comprising the liver and gut) accounts for ~25% of glucose utilization under fasting conditions and for 35% after an oral glucose load (DeFronzo, 2004; Moore et al., 2012). When nutrients are available, as occurs after a meal, blood glucose concentrations rise. The effect of high glucose on the liver is dual. First, hyperglycemia per se promotes glucose absorption from the blood stream to be stored as glycogen. Second, it promotes insulin secretion from the pancreas that suppresses hepatic glucose production (HGP). Glucose entrance into hepatocytes is insulin-independent and is facilitated by the glucose transporter GLUT2. When nutrients become scarce, even after a few hours of fasting, the liver releases glucose to the blood by regulating the two primary glucose production metabolic pathways, glycogenolysis and gluconeogenesis. In order to achieve net glucose production or uptake, key enzymes in these pathways must be tightly regulated. These pathways are primarily regulated by insulin and glucagon secreted from the pancreas. Elevated insulin levels initiate the insulin signaling cascade in liver that suppresses glycogen breakdown and promotes glycogen storage and lipogenesis. Insulin's action in the liver is counter-regulated by glucagon, which promotes glycogen breakdown and gluconeogenesis when nutrients are not available. The production of glucose by the liver is also controlled by catecholamines secreted from the adrenal medulla (Rizza et al., 1980). In the liver, catecholamines increase glucose production by cyclic AMP (cAMP) activation of glycogen phosphorylase and gluconeogenesis.
Brain
A primary purpose of maintaining constant plasma glucose levels is to keep a steady energy supply to the brain, which depends on glucose as its sole energy source. A prolonged decrease in plasma glucose concentrations impairs cerebral function and can cause brain damage and even death (Mitrakou et al., 1991). In humans, under fasting conditions the brain accounts for ~50% of glucose utilization (Baron et al., 1988), and for 33% after an oral glucose load (Moore et al., 2012). Glucose transport into neurons is insulin independent and is mediated by glucose transporter GLUT3, which due to its low Km for glucose is saturated under most physiological conditions (Burant and Bell, 1992). It is well established that blood glucose levels can be sensed by the central nervous system (reviewed in (Routh et al., 2004). In response to low concentrations of glucose, the ventromedial hypothalamus (VMH) controls a counter regulatory response by promoting catecholamine and glucagon secretion (Borg et al., 1994; Borg et al., 1995). When glucose is selectively infused into the VMH to prevent local hypoglycemia in this region, the counter regulatory response is markedly reduced despite systemic hypoglycemia (Borg et al., 1997). In addition, an acute increase in central nervous system glucose levels was shown to suppress glucose production by the liver (Lam et al., 2005).
Muscle and adipose tissue
The muscle and adipose tissues are major sites for insulin-mediated glucose removal from the blood stream, and the ability of these tissues to increase their glucose uptake in response to insulin is critical for maintaining normal blood glucose levels. In humans, under fasting conditions these tissues account for ~25% of glucose utilization and for ~33% after an oral glucose load (DeFronzo, 2004; Moore et al., 2012). It is important to highlight that under hyperinsulinemic-euglycemic clamp conditions, where glucose is being infused rather than digested, the muscle contribution for glucose disposal is far greater and accounts for ~80% of glucose utilization (DeFronzo, 2004). In response to rising levels of insulin, glucose uptake in muscle and adipose tissue is facilitated by localization of the glucose transporter GLUT4 to the plasma membrane. In periods of increased glucose demand, like in exercise, glucose uptake by skeletal muscle is greatly enhanced. The muscle also releases amino acids into the blood circulation that are used by the liver as substrates for gluconeogenesis. Adipose tissue contributes to whole body glucose homeostasis by releasing free fatty acids (FFA) and glycerol that can be taken up by the liver and used as substrates for gluconeogenesis, and also by secretion of adipokines that influence insulin sensitivity in muscle and liver (Bays et al., 2004).
Pancreas
The pancreas is a major contributor to whole body glucose homeostasis, as it controls the secretion of both insulin and glucagon. Similar to the liver, the transport of glucose into the pancreas is mediated by GLUT2. Glucose is then converted by glucokinase (GK) into glucose-6-phosphate (G6P). In contrast to the liver, GK in pancreatic cells is not subjected to an inhibitory mechanism and as a consequence cellular concentrations of glucose are similar to plasma glucose levels (Matschinsky, 1996). When blood glucose levels rise, insulin is secreted from pancreatic β cells to stimulate glucose clearance in muscle and adipose tissue and to suppress hepatic glucose production. In addition, hyperinsulinemia suppresses glucagon secretion from pancreatic α cells. When blood glucose levels fall, insulin levels are suppressed and glucagon is secreted in response to catecholamines and glucocorticoids to promote hepatic glucose production and maintain glucose homeostasis.
Gut
Similar to its effect on glucose uptake by the muscle, the route of glucose administration also affects the magnitude of insulin secretion. Oral glucose administration leads to a more robust glucose-stimulated insulin secretion compared to intravenous glucose infusion (Drucker, 1998). This increase is attributed to gut secreted hormones called incretins, specifically glucagon-like peptide-1 (GLP-1), which promotes insulin secretion from pancreatic β cells, inhibits gastric emptying and inhibits glucagon secretion (Drucker, 1998), thus lowering blood glucose levels.
4 Fed-fasting physiology
Maintaining relatively constant blood glucose levels is essential for the survival of the organism. This is specifically challenging in periods of reduced energy supply such as after prolonged fasting or exercise. In the fed state, circulating glucose is derived primarily from dietary sources and is distributed to the brain, the muscle and fat, and the liver. After an overnight fast (postabsorptive state) when dietary glucose supply is not available, glucose is produced primarily from glycogenolysis, the release of glucose from glycogen breakdown, or from gluconeogenesis, the synthesis of glucose from noncarbohydrates precursors (i.e., pyruvate, lactate, glycerol and amino acids). The low glucose levels are sensed by pancreatic α cells and the adrenal medulla that secrete glucagon and catecholamines, respectively. An increase in blood concentrations of these hormones promotes HGP in the fasted state and during exercise. Under overnight fasting conditions, the contribution of glycogenolysis and gluconeogenesis to overall glucose production is approximately equal. Glycogen content in the liver is limited and is largely depleted after an overnight fast. Therefore, gluconeogenesis becomes the predominant source of glucose after prolonged fasting.
After digestion of a meal, in the fed state, glucose levels are sensed by pancreatic β cells that secrete insulin while secretion of glucagon from pancreatic α cells is suppressed. The increase in circulating insulin strongly suppresses HGP and promotes energy storage by increasing glycogenesis and lipogenesis. Tracer studies performed in dogs and humans have characterized the response of the liver to an increase in circulating glucagon or insulin.
Short-term fasting
After an overnight fasting, glucagon secretion by pancreatic α cells is markedly increased. Studies performed in dogs assessed the effect of glucagon on hepatic glucose output under these conditions. In these experiments, insulin was kept constant at basal levels and glucagon was infused to result in a four-fold increase in its plasma levels. As a result, hepatic glucose output was increased almost four-fold compared to control. The increase in hepatic glucose output was primarily the result of an increase in glycogenolysis, as gluconeogenesis was hardly changed. Glucagon deficiency on the other hand, led to decreased hepatic glucose output primarily due to decreased glycogenolysis (Cherrington, 1999).
Catecholamines are also known to regulate hepatic glucose output in the fasted state. In the conscious fasted dog, infusion of epinephrine via peripheral vein, while insulin and glucagon are held constant, increases hepatic glucose output by inducing both glycogenolysis and gluconeogenesis. Within two hours, the glycogenolysis rate returns to basal levels while gluconeogenesis remains active. Infusion of norepinephrine via portal vein leads to an increase in hepatic glucose output attributed solely to an increase in glycogenolysis (Cherrington, 1999).
Refeeding
After digestion of a meal, insulin plasma levels are increased. As in the case of glucagon, studies in dogs assessed the effect of increased insulin levels on hepatic glucose output after an overnight fast. In these experiments, glucagon was kept constant at basal levels and insulin was infused to result in a four-fold increase in its plasma levels. As a result, hepatic glucose output was rapidly reduced primarily due to a decline in the rate of glycogenolysis. On the other hand, complete removal of insulin markedly augments glucose production, emphasizing the importance of basal insulin in restraining glucose production by the liver(Cherrington, 1999). Studies in humans have found that physiological hyperinsulinemia suppresses gluconeogenesis by ~20% while completely blocking glycogenolysis (Gastaldelli et al., 2001).
5 Molecular mechanisms that control hepatic glucose production
The molecular mechanisms determining whether the liver acts as a glucose producing organ or a glucose storage organ can be grossly divided into two categories: acute and long term. While the acute effects are brought about primarily by changes in metabolite flux controlled by protein modifications or allosteric effectors, the long term effects are brought about primarily by changes in the mRNA expression level of key enzymes in the glycolysis/gluconeogenesis pathways. Both the short and long term effects on net hepatic glucose output are subject to hormonal regulation by the opposing hormones insulin and glucagon.
5.1 Metabolite flux in the control of HGP
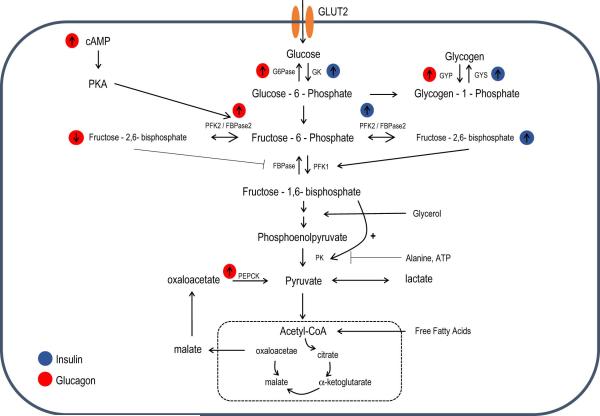
A key mode of control over hepatic glucose production is that of net glycolytic/gluconeogenic flux. By regulating the activity of key glycolytic or gluconeogenic enzymes, the liver can switch from net hepatic glucose storage to glucose output. Out of the 10 reactions in glycolysis, 7 are reversible and can be used both for glycolysis and gluconeogenesis. The remaining three reactions, consisting of the conversion of glucose-6-phosphate (G6P) to glucose, the conversion of fructose 1,6-bisphosphate (F-1,6-P2) to fructose-6-phosphate (F6P) and the conversion of pyruvate to phosphenolpyruvate (PEP), are unique to gluconeogenesis and are catalyzed by specific enzymes. If these opposite reactions were to act at the same rate, a futile cycle would occur resulting in wasteful energy expenditure. Regulation of these gluconeogenic enzymes and their glycolytic counterparts by either allosteric effectors, at the gene expression level or by covalent modifications is therefore a mechanism by which net flux toward gluconeogenesis or glycolysis is achieved in the liver (Figure 1).
Figure 1. Metabolite flux in the control of HGP.
A schematic overview of key enzymes and metabolites involved in the regulation of gluconeogenesis and hepatic glucose output (HGP). Flux through these metabolites is tightly regulated to control net gluconeogenesis or glycolysis. How insulin (blue) or glucagon (red) affect these key enzymes and metabolites is highlighted.
Glucose/Glucose-6-phosphate cycle
The transport of glucose into hepatocytes is facilitated by the GLUT2 transporter. Glucose is then phosphorylated by glucokinase (GK), a liver specific hexokinase, to generate G6P. In its phosphorylated form, glucose can no longer be exported to the circulation and is retained in hepatocytes. Unlike other hexokinases, GK is not inhibited by its product but is rather retained in the nucleus by binding to glucokinase regulatory protein (GKRP). After glucose enters the cell, GK is released to the cytoplasm where it phosphorylates glucose. GK activity in hepatocytes is also regulated by its mRNA expression (Iynedjian, 2009). The opposing gluconeogenic enzyme is glucose-6-phosphatase (G6Pase), which is located in the endoplasmic reticulum (ER) and catalyzes G6P hydrolysis. G6P is a branch point substrate that can either be metabolized through glycolysis to produce pyruvate or alternatively stored as glycogen. The final step in glycogen synthesis is catalyzed by glycogen synthase, while the first step in the breakdown of glycogen is catalyzed by glycogen phosphorylase. Similarly as in glycolysis/gluconeogenesis, the activity of these enzymes is subjected to allosteric and hormonal regulation. Phosphorylation of glycogen synthase, primarily by glycogen synthase 3 (GSK-3), inhibits its activity while phosphorylation of glycogen phosphorylase stimulates the enzyme's activity. Insulin, by activating Akt, results in phosphorylation and inactivation of GSK-3 and a subsequent activation of glycogen synthase. Glucagon, via protein kinase A (PKA)-mediated phosphorylation, activates glycogen phosphorylase while inhibiting glycogen synthase.
Fructose-6-P/Fructose -1,6-bisphosphate flux
The F-6-P/F-1,6-P2 substrate cycle is a major determinant of net glycolytic or gluconeogenic flux. The conversion of fructose-6-phosphate (F-6-P) to fructose-1,6-bisphosphate (F-1,6-P2), catalyzed by phosphofructokinase 1 (PFK1), is the first committed step in glycolysis and together with its opposing gluconeogenic enzyme, fructose 1,6-bisphoshpatase (FBPase), these enzymes control net glycolytic or gluconeogenic flux. Accordingly, pharmacological inhibition of FBPase results in a reduction in gluconeogenesis and ameliorates both fasting and postprandial hyperglycemia (van Poelje et al., 2011). The activity of these enzymes is modulated by hormones and nutritional states. Physiological conditions that favor glucose production, like fasting, will result in activation of FBPase to promote gluconeogenesis, while conditions that favor glucose storage, like feeding, will activate PFK1 to promote glycolysis (Clark et al., 1974). Both PFK1 and FBPase are subject to PKA phosphorylation, and it was originally thought that this modification controls the activity of these enzymes. However, in vitro phosphorylation by PKA has little effect on the activity of these enzymes (Claus et al., 1980; Meek and Nimmo, 1984). It was later found that the activity of both enzymes is regulated by another allosteric effector, F-2,6-P, which is subject to hormonal and nutritional regulation (Van Schaftingen et al., 1980a, b). High levels of F-2,6-P, as seen in conditions that favor glucose utilization, strongly activate PFK1 and inhibit FBPase thus favoring glycolysis, whereas low levels release FBPase inhibition and tilt the balance toward gluconeogenesis. A unique bifunctional enzyme with both PFK1 and FBPase activity that catalyzes both synthesis and hydrolysis of F-2,6-P controls the levels of this metabolite in the cell. PKA-dependent phosphorylation of the bifunctional enzyme inhibits its kinase activity and stimulates its BPase activity while dephosphorylation by protein phosphatase 2A induces its BPase activity (Pilkis et al., 1995). In addition, F-6-phosphate is a noncompetitive inhibitor of the BPase activity of the bifunctional enzyme, and in conditions where its levels are high (like after a glucose load) F-2,6-P levels increase to promote glycolysis (Van Schaftingen, 1987). Glucagon, by increasing cAMP levels in the cell and activating PKA, stimulates the bifunctional enzyme BPase activity by increasing its phosphorylation. Insulin suppresses its BPase activity by decreasing its phosphorylation, even when cAMP levels are unchanged (Garrison and Wagner, 1982; Pilkis et al., 1983). The regulation of F-2,6-P is thus a mechanism by which net glycolysis and gluconeogenesis levels are modulated.
Phosphoenolpyruvate/pyruvate cycle
The final step in glycolysis, the conversion of PEP to pyruvate is catalyzed by pyruvate kinase (PK), while the opposing gluconeogenic reaction, the conversion of oxaloacetate (generated by oxidation of cytosolic malate by malate dehydrogenase) to PEP is catalyzed by phosphoenolpyruvate carboxykinase (PEPCK). Glucagon and increased levels of cAMP strongly inhibit PK activity, blocking glycolytic flux, by increasing its PKA-mediated phosphorylation. Insulin, by reducing cAMP levels, releases the phosphorylation-mediated inhibition and activates PK to promote glycolytic flux (Engstrom, 1978; Feliu et al., 1976). PK is also subjected to allosteric activation by fructose-1,6-BP and inhibition by alanine and ATP (Flory et al., 1974). Fructose-1,6-BP, in addition to allosterically activating PK, also inhibits cAMP-mediated phosphorylation of PK, providing another level of control of PK activity (Pilkis and Granner, 1992). Glucagon also lowers hepatic fructose-1,6-BP to further increase the inhibition mediated by PKA.
5.2 Transcriptional regulation of HGP
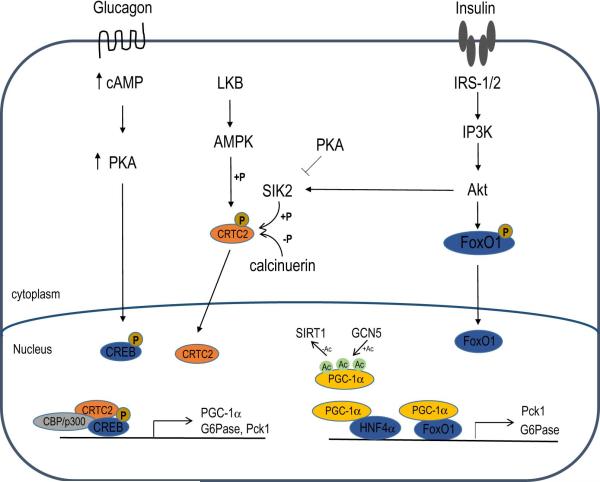
HGP and gluconeogenesis in particular are largely controlled by transcriptional regulation of key rate limiting enzymes in the gluconeogenic pathway, specifically PEPCK and G-6-Pase. Several transcription factors and coactivators have been identified to control the expression of these enzymes, and these transcription regulators are controlled by hormonal signals that regulate the response of the liver to the fed or fasted state. Numerous studies have demonstrated that suppression or induction of PEPCK and G-6-Pase can affect HGP both in cultured primary hepatocytes and in vivo. Mice with whole body knockout of PEPCK die 3 days after birth, but liver-specific knockout mice are viable (She et al., 2000). Surprisingly, these mice are euglycemic but develop hepatic steatosis after fasting (She et al., 2000). Tracer studies have shown that although hepatic glucose production in these mice is markedly reduced, supporting the importance of PEPCK in hepatic gluconeogenesis, endogenous gluconeogenesis is not changed probably due to compensation by the kidney and intestine which also express gluconeogenic enzymes (She et al., 2003). Similarly, mice with liver-specific knockout of G-6-Pase demonstrate normal fasting blood glucose due to compensation from extra-hepatic tissues (Mutel et al., 2011). These studies demonstrate that while the liver is the major source of endogenous glucose production, when HGP is impaired glucose homeostasis is maintained through compensation by extra-hepatic tissues (Figure 2).
Figure 2. Transcriptional regulation of hepatic glucose output (HGP).
The transcriptional programs controlling the expression of gluconeogenic genes in hepatocytes are regulated by insulin and glucagon. Insulin, by activating Akt, leads to phosphorylation and nuclear exclusion of FoxO1 and subsequent suppression of gluconeogenic genes. Glucagon, by increasing intracellular cAMP levels, activates PKA and results in activation of CREB and subsequent activation of gluconeogenic genes.
Insulin signaling in the liver
In the liver, insulin inhibits glucose production and stimulates storage of glucose as glycogen as well as lipid synthesis. The insulin receptor consists of two α and two β subunits, in which the β subunit possess a tyrosine kinase catalytic activity that is allosterically inhibited by the α subunit. Upon binding of insulin to the α subunit the allosteric inhibition is released and autophosphrylation of the β subunit occurs. The insulin receptor substrates (IRS1 and IRS2) are subsequently phosphorylated and associated with PI3K via its p85 regulatory subunit which leads to its activation and subsequent phosphorylation of PDK1 and Akt (Saltiel and Kahn, 2001). The importance of proper liver responsiveness to insulin is highlighted in liver-specific insulin receptor KO mice (LIRKO). These mice have elevated blood glucose levels in the fed state, are severely glucose intolerant and hyperinsulinemic (Michael et al., 2000). It was known that insulin suppresses the expression of key gluconeogenic enzymes both in vivo and in isolated hepatocytes, but the effector that mediates this response was not known. The identification of the forkhead transcription factor daf-16 as a downstream effector of insulin signaling in C. elegans highlighted the importance of FoxO1 as the insulin-mediated transcription factor that integrates insulin signaling and hepatic glucose production (Accili and Arden, 2004). Indeed, the effects of insulin at the mRNA level in the liver are primarily mediated by the canonical IRS/PI3K/Akt/FoxO1 pathway. Upon activation, Akt can phosphorylate many cellular targets, but most relevant to hepatic glucose production is FoxO1. Phosphorylation of FoxO1 leads to its proteasomal degradation and nuclear exclusion. When dephosphorylated, Foxo1 translocates to the nucleus where it induces transcription of the gluconeogenic genes Pck1 (encoding the enzyme PEPCK) and G-6-Pase. The activity of FoxO1 is also controlled by its acetylation state. Increased FoxO1 acetylation by p300/CBP inhibits its activity by decreasing its ability to bind to its target genes (Daitoku et al., 2004; Matsuzaki et al., 2005). Liver-specific knockout of FoxO1 reduces hepatic glucose output by decreasing gluconeogenesis and glycogenolysis (Matsumoto et al., 2007). Deletion of all FoxO genes, FoxO1, FoxO3a and FoxO4, leads to severe fasting hypoglycemia (Haeusler et al., 2010). In addition, liver-specific knockout of Akt1 and Akt2 also impairs insulin-mediated suppression of the gluconeogenic program, supporting the hypothesis that Akt is essential in maintaining glucose homeostasis (Lu et al., 2012). Surprisingly, after liver-specific knock out of Akt1, Akt2 and FoxO1, insulin can still suppress HGP, suggesting the presence of an Akt/FoxO-independent mechanism (Lu et al., 2012).
Peroxisome proliferator-activated receptor γ coactivator-1a (PGC-1α)
PGC-1α was originally discovered as a PPARγ coactivator that strongly induces adaptive thermogenesis in brown adipose tissue (Puigserver et al., 1998). Upon fasting, the mRNA levels of PGC-1α in the liver are dramatically elevated, and under these conditions PGC-1α coactivates FoxO1 to further increase transcription of gluconeogenic genes and hepatic glucose production (Puigserver et al., 2003). In addition to FoxO1, PGC-1α was also shown to co-activate the liver-enriched nuclear receptor hepatocyte nuclear factor 4α (HNF4α) (Yoon et al., 2001). Studies performed in cultured primary hepatocytes and in cell lines identified that PGC-1α strongly activates the PEPCK promoter via functional interaction with HNF4α. This interaction is mediated by the LXXLL motif in PGC-1α that is also known to mediate its binding to other nuclear hormone receptors. This interaction between PGC-1α and HNF-4α strongly induces expression of gluconeogenic genes and promotes hepatic glucose output (Yoon et al., 2001). In the absence of HNF-4α, PGC-1α can no longer induce the expression of gluconeogenic genes (Rhee et al., 2003).
The activity of PGC-1α is strongly affected by its acetylation state. While deacetylation potently activates the transcriptional activity of PGC-1α, hyperacetylation inhibits its activity. Several factors that modulate the acetylation state of PGC-1α and subsequently its transcriptional activity have been identified. In response to fasting, the deacetylase SIRT1 is induced and was shown to bind and deacetylate PGC-1α on specific lysine residues (Rodgers et al., 2005). The deacetylation of PGC-1α induced by SIRT1 is sufficient to elevate the expression of gluconeogenic genes in the liver, but not mitochondrial genes whose expression is also controlled by PGC-1α (Rodgers et al., 2005).
The strong effect of the PGC-1α acetylation state on its transcriptional activity is further demonstrated by the ability of the histone acetyl transferase general control of amino acid synthesis 5 (GCN5) to hyperacetylate PGC-1α and suppress its activity in the liver (Lerin et al., 2006). Additional studies support the importance of GCN5 in regulating the activity of PGC-1α in the liver. SIRT6, a nuclear histone deacetylase, surprisingly increases the acetylation of PGC-1α and this was shown to be a result of SIRT6-mediated deacetylation of GCN5 that leads to its activation and subsequent inhibition of hepatic glucose production (Dominy et al., 2012). In a diabetic mouse model, SIRT6 levels are reduced and ectopic expression of SIRT6 normalizes glycemia (Dominy et al., 2012). Recently, it was demonstrated that GCN5 is a novel target of the cyclin D1/CDK4 complex and that insulin signaling activates the cyclin D1/CDK4 complex by enhancing cyclin D1 protein stability (Lee et al., 2014). As a result, GCN5 activity is enhanced owing to CDK4-mediated phosphorylation, and hepatic glucose production is suppressed (Lee et al., 2014).
Phosphorylation of PGC-1α was also shown to affect its transcriptional activity. As part of the insulin signaling pathway, Cdc-like kinase 2 (Clk2) is induced and becomes more active after refeeding (Rodgers et al., 2010). Clk2 then phosphorylates PGC-1α, leading to repression of gluconeogenic gene expression and HGP (Rodgers et al., 2010). In addition, activation of S6 kinase upon refeeding leads to phosphorylation of PGC-1α in its arginine/serine rich domain (RS) resulting in impaired ability of PGC-1α to induce gluconeogenic genes and promote HGP (Lustig et al., 2011). Interestingly, this modification only affects the ability of PGC-1α to coactivate HNF-4α but not its ability to coactivate other nuclear receptors like PPARα and ERRα (Lustig et al., 2011). Collectively, these studies demonstrate that inhibition of PGC-1α in the liver is a useful way to potentially inhibit gluconeogenesis and HGP. However, its inhibition in adipose and muscle tissues might have negative effects on whole body energy expenditure. The specificity toward gluconeogenic genes described in the above studies raises the possibility of finding novel drugs that will only inhibit PGC-1α activity in the liver while leaving its activity in other tissues intact.
Glucagon signaling in the liver
In response to fasting, the pancreas secretes glucagon. Studies performed in mice identified the importance of the cAMP response element binding protein (CREB) transcription factor in mediating the transcriptional adaptive response of hepatocytes to glucagon (Altarejos and Montminy, 2011). Binding of glucagon to its cell surface receptor triggers the activation of adenylate cyclase that catalyzes the production of cAMP in hepatocytes. The increased levels of cAMP promote the release of the catalytic subunit of PKA and its nuclear localization. PKA phosphorylates CREB at Ser133 and the phosphorylation of CREB stimulates its activity by promoting binding to the histone acetyl transferases CBP/p300 (Herzig et al., 2001; Quinn and Granner, 1990). To gain full capacity of CREB to induce its target genes, binding to CREB-regulated transcriptional coactivator 2 (CRTC2) is required. CRTC2 is subjected to phosphorylation by the salt inducible kinase 2 (SIK2) which promotes its retention in the cytoplasm, and dephosphorylation by calcineurin which promotes its nuclear translocation. Increases in cAMP and Ca2+ trigger PKA-mediated inhibition of SIK2 and calcineurin-mediated dephosphorylation, respectively, which ultimately lead to nuclear translocation of CRTC2 (Screaton et al., 2004). CRTC2 then associates with CREB and potentiates its transcriptional activity toward specific genes. In the liver, CREB binds the promoter regions of the gluconeogenic genes Pck1 and G-6-Pase, and increased levels of circulating glucagon induce the transcriptional activity of CREB toward these genes (Herzig et al., 2001). A dominant negative CREB inhibitor leads to fasting hypoglycemia due to reduced hepatic glucose production and reduced expression of gluconeogenic genes (Herzig et al., 2001). CREB also controls the gluconeogenic program by increasing the expression of PGC-1α providing another level of control of the expression of gluconeogenic genes (Herzig et al., 2001). Interestingly, overexpression of PGC-1α in the presence of the dominant negative inhibitor of CREB restores normal glycemia, emphasizing the importance of PGC-1α in CREB-mediated control of glucose homeostasis (Herzig et al., 2001).
Glucocorticoids
Glucocorticoids are released by the adrenal medulla in response to stress and promote HGP by binding to the glucocorticoid receptor (GR) resulting in transcriptional activation of Pck1 (Wynshaw-Boris et al., 1986). The activity of GR is further enhanced by binding to PGC-1α (Herzig et al., 2001; Knutti et al., 2000). Liver-specific knockout of GR leads to fasting hypoglycemia (Opherk et al., 2004) and knockdown of GR in liver and fat reduces HGP in rats (Watts et al., 2005). Glucocorticoids also promote protein degradation in skeletal muscle and lipolysis in adipose tissue (Hasselgren, 1999; Peckett et al., 2011), leading to an increase in gluconeogenic substrates available to the liver. Another pro-diabetic effect of glucocorticoids is the suppression of insulin secretion from β cells in the pancreas (Lambillotte et al., 1997).
AMP activated protein kinase (AMPK)
AMP-activated protein kinase (AMPK) is a cellular sensor of low energy states that is activated when the AMP/ATP ratio is high. AMPK is activated by LKB1-mediated phosphorylation and can also be activated by Ca2+-dependent phosphorylation mediated by CaMKKβ. In the liver, AMPK phosphorylates CRTC2 leading to its retention in the cytoplasm and suppression of gluconeogenic genes (Koo et al., 2005). In addition, inactivation of LKB1 leads to nuclear accumulation of CRTC2 (Shaw et al., 2005).
5.3 Indirect effects of insulin on hepatic glucose output
Insulin can suppress hepatic glucose output indirectly by affecting extra-hepatic tissues. In the pancreas, insulin secretion from β cells suppresses the secretion of glucagon from α cells primarily by altering α cells’ membrane potential (Quesada et al., 2008). Another indirect mechanism by which insulin suppresses HGP is by reducing gluconeogenic substrates supplied by adipose tissue and muscle. The release of amino acids from skeletal muscle supplies the liver with essential gluconeogenic substrates, and the inhibition of proteolysis by insulin reduces substrate flux coming from the muscle. In adipose tissue, insulin suppresses the release of glycerol, a gluconeogenesis substrate, and non-esterified fatty acids (NEFAs). NEFAs supplied to the liver lead to fatty acid oxidation and the production of ATP, NADH and acetyl-CoA, which promote gluconeogenesis by activating pyruvate carboxylase. A reduction in FFA flux to the liver was shown to be a major mechanism of the indirect control of insulin on HGP (Lewis et al., 1997; Rebrin et al., 1995; Sindelar et al., 1997). Insulin action in the brain was also shown to control HGP. Injection of insulin to the third ventricle of the hypothalamus reduces HGP independent of systemic levels of insulin and glucagon, while suppression of insulin signaling in the hypothalamus impairs insulin's ability to reduce HGP (Gelling et al., 2006; Obici et al., 2002)
6 Pathophysiology of type 2 diabetes
6.1 The liver is the major contributor to hyperglycemia observed in diabetes
T2DM has become a worldwide epidemic and is expected to affect almost one third of adult Americans by 2050 (Boyle et al., 2010). The current increase in the prevalence of T2DM is believed to be a result of increases in energy consumption and in sedentary lifestyle in combination with genetic predisposition (Chen et al., 2012). T2DM is a multifactorial disease characterized by postprandial and postabsorptive hyperglycemia, insulin resistance and insulin deficiency (Rizza, 2010). As discussed above, in the postabsorptive state the brain is responsible for ~50% of oral glucose disposal (Cherrington, 1999; Huang et al., 1980), muscle and adipose tissue for ~25% of glucose disposal (Baron et al., 1988; Cherrington, 1999) and the remainder is disposed of by the liver, RBC and kidney. In the diabetic state, the muscle becomes resistant to insulin, and glucose clearance both in the fasted and fed state is reduced (Firth et al., 1986; Gerich et al., 1990). However, the reduced glucose clearance by muscle only accounts for <10% of the total reduction in systemic glucose clearance (Gerich et al., 1990) suggesting that the reduction in systemic glucose clearance is probably the result of a decrease in brain glucose clearance (Gerich et al., 1990). In the diabetic state, the liver also becomes resistant to the actions of insulin and several studies have shown that hepatic glucose production is increased in patients with T2DM (Campbell et al., 1988; Consoli et al., 1989; DeFronzo et al., 1982; Magnusson et al., 1992). Several studies have also clearly demonstrated that the increase in HGP during fasting in the diabetic state is primarily the result of an increase in gluconeogenesis and that glycogenolysis remains unchanged (Magnusson et al., 1992). Therefore, in the diabetic state it is hepatic insulin resistance and the consequent increase in hepatic glucose production that is the major contributor to postabsorptive and postprandial hyperglycemia.
6.2 Pathophysiology of the liver in T2DM
Increased rates of hepatic glucose production in the diabetic state are a result of an imbalance in several factors affecting the different aspects involved in the regulation of HGP as discussed above. These factors include: 1) increased availability of gluconeogenic substrates that ultimately leads to increased gluconeogenesis; 2) resistance of the liver to the action of insulin leading to improper suppression of hepatic glucose output; 3) elevated levels of glucagon leading to hyperactivation of signaling pathways that are normally activated in the fasted state when glucose supply is needed.
Altered glucose homeostasis in T2DM
Glucose homeostasis in diabetic patients is disturbed both in insulin secretion from pancreatic β cells and in insulin sensitivity of liver, muscle and adipocytes (insulin resistance). Insulin resistance is acquired early in the progression of T2DM, but initially glucose tolerance is normal due to a compensatory increase in insulin secretion. In individuals with mild fasting hyperglycemia (<140 mg/dL) insulin concentration is up to 2.5 times higher compared to non-diabetic controls (DeFronzo, 2004). When fasting hyperglycemia exceeds 140 mg/dL, pancreatic β cells can no longer maintain elevated insulin secretion, and plasma concentrations of insulin start to drop and HGP starts to rise.
Increased supply of gluconeogenic substrates
Studies in perfused diabetic rat liver have demonstrated that increased availability of gluconeogenic substrates results in increased gluconeogenesis (Exton and Park, 1967). In contrast, infusion of gluconeogenic substrates to non-diabetic animal models and humans does not result in a similar increase in gluconeogenesis (Ahlborg et al., 1976; Diamond et al., 1988; Jahoor et al., 1990). One possible explanation for this discrepancy is that autoregulatory mechanisms in the liver prevent increases in glucose production when substrates become more available. In support of this, it was shown that increased infusion of lactate to non-diabetic subjects increases glucose production from this substrate, but fails to increase total glucose production and fasting blood glucose (Jenssen et al., 1990). In T2DM, these autoregulatory mechanisms might be impaired and lead to increased gluconeogenesis and HGP when gluconeogenic substrates are more available. Several studies have demonstrated an increase in plasma levels of alanine, glycerol and lactate in diabetic subjects. A potential source for the increased availability of gluconeogenic substrates is the adipose tissue. The vast majority of diabetic patients are obese and are characterized by elevated plasma levels of free fatty acids and glycerol. Insulin is a potent antilipolytic hormone, and in T2DM adipose tissue becomes resistant to insulin, leading to increased lipolysis and release of glycerol to the circulation.
Insulin resistance
The resistance to insulin's action is characteristic of both lean and obese diabetic patients as well as diabetic rodent models (DeFronzo et al., 1985; Groop et al., 1989; Koranyi et al., 1990), and is considered the main driver of T2DM. Together with muscle and adipose tissues, the liver is a major site for insulin's action and in T2DM glucose production by the liver fails to be suppressed by insulin, leading to excessive hepatic glucose output. The development of insulin resistance is tightly correlated with obesity, although it is still not completely clear why overnutrition results in insulin resistance. Several mechanisms have been proposed to explain the pathogenesis of insulin resistance (Johnson and Olefsky, 2013; Samuel and Shulman, 2012). Tissue inflammation is an established cause of insulin resistance (Lumeng and Saltiel, 2011) and is considered a main driver of insulin resistance in adipose tissue (Johnson and Olefsky, 2013). A marked increase in hepatic macrophages is also observed during obesity, contributing to hepatic insulin resistance (Obstfeld et al., 2010). Depletion of hepatic macrophages using chemical agents improves the hepatic response to insulin, highlighting their importance in mediating insulin resistance in the liver (Lanthier et al., 2010; Neyrinck et al., 2009). Ectopic lipid accumulation is also considered a main driver of insulin resistance both in muscle and adipose tissues as well as in the liver (Samuel and Shulman, 2012). The resistance of adipose tissue and muscle to insulin leads to a subsequent increase in lipolysis and a divergence of glucose to the liver, respectively. As a result, de novo lipogenesis and gluconeogenesis in the liver are increased.
Glucagon action
As discussed above, glucagon is a powerful regulator of glucose production by the liver in the fasted state. Glucagon receptor is expressed mainly in the liver and kidney, where it can increase glucose production. Glucagon levels in the blood were found to be increased in patients with T2DM (Unger et al., 1970). Reduced expression of glucagon receptor in a diabetic mouse model (db/db) reduces both fed and fasting blood glucose (Liang et al., 2004). However, a dominant role for insulin resistance is the prevailing hypothesis to explain hyperglycemia (Lin and Accili, 2011; Raju and Cryer, 2005).
6.3 Common therapies for T2DM
How does metformin work?
The biguanide metformin was originally obtained from the Galega officinalis plant (French lilac) in the 1920s and has been used as a glucose lowering drug for centuries in Europe and in the US. Metformin is the most prescribed antidiabetic drug worldwide, and has gained further attention in recent years due to several studies attributing to metformin a role in reducing cancer incidence (Martin and Marais, 2012). It is well accepted that the primary anti-diabetic effect of metformin is lowering hepatic glucose production, mainly by lowering rates of gluconeogenesis. However, the exact mechanism of action of metformin is controversial and several mechanisms have been proposed to mediate the blood glucose lowering effect of this drug. Several studies demonstrated that metformin can inhibit complex I activity in the mitochondria (El-Mir et al., 2000; Owen et al., 2000) and impair ATP production. Metformin was originally found to activate the energy sensor AMPK (Zhou et al., 2001).I In support of this finding, liver-specific knockout of liver kinase B1 (LKB1), an upstream kinase of AMPK, abolishes the antidiabetic effect of metformin in mice fed a high-fat diet (Shaw et al., 2005). However, this hypothesis has been seriously questioned by another study demonstrating that in mice lacking both catalytic subunits of AMPK the effect of metformin is intact (Foretz et al., 2010). Another hypothesis proposed that an increase in AMP levels by metformin suppresses HGP by inhibiting cAMP production by adenylyl cyclase, thus opposing the action of glucagon (Miller et al., 2013). As a result, glucagon-mediated PKA activation is impaired leading to a reduction in glucose output (Miller et al., 2013). Most recently, a new target for metformin action has been suggested (Madiraju et al., 2014). In this study metformin was shown to inhibit the activity of mitochondrial glycerol 3-phosphate dehydrogenase, shifting the NADH/NAD+ ratio, and as a consequence reduces gluconeogenesis from glycerol and lactate (Madiraju et al., 2014). It is worth noting that the dose of metformin being used was considerably different between the studies mentioned above, and that this difference may account for some of the conflicting results as was recently suggested (He and Wondisford, 2015).
Insulin secretagogues (sulfonylurea)
The oldest drug class for lowering blood glucose levels is the sulfonylurea insulin secretagogues. The ATP sensitive potassium channels (KATP) play a central role in maintaining pancreatic β-cell membrane potential. Closure of this channel by either ATP or sulfonylurea leads to β-cell membrane depolarization which triggers opening of voltage-gated Ca2+ channels. The subsequent Ca2+ influx stimulates the exocytosis of insulin containing vesicles (Ashcroft and Rorsman, 1989). The closure of the KATP channel by sulfonylurea is mediated by binding of the drug to the SUR subunit of the KATP channel. The KATP channels are also expressed in skeletal, smooth and cardiac muscle, but in these tissues the KATP is composed of a different SUR subunit that is not inhibited by sulfonylurea, thus conferring tissue specificity of this drug (Gribble et al., 1998; Proks et al., 2002). The use of this drug is associated with modest weight gain and risk of hypoglycemia (Inzucchi et al., 2012).
Glucagon-like peptide-1 receptor agonists
The GLP-1 receptor agonists mimic the effects of endogenous GLP-1 resulting in increased glucose-dependent insulin secretion, suppression of glucagon release and gastric emptying, thus lowering blood glucose (Drucker, 1998; Holst, 2007). These drugs cannot be administered orally and are currently injected making their use more complicated. GLP-1 is rapidly metabolized and inactivated by the enzyme dipeptidyl peptidase-4 (DPP-4) (Holst, 2007). Inhibitors of DPP-4 increase circulating concentrations of GLP-1 and are also used as antidiabetic drugs (Deacon, 2011).
7 Conclusions
Glucose homeostasis in the liver is tightly controlled by nutritional and hormonal cues that regulate hepatic glucose production to meet whole body energy demands. The activity of numerous enzymes, transcription factors and coactivators is regulated by these nutritional and hormonal cues to control HGP both in the postprandial and postabsorptive states. Alteration in the mechanisms that control HGP can lead to hyperglycemia and to the development of T2DM. Although acquired late in the progression of T2DM, excessive HGP is a major contributor to the pathogenesis of this disease, and suppression of HGP by metformin is currently the gold standard for clinical treatment of T2DM. It is largely accepted that in the diabetic state the chronically elevated HGP is a result of increased gluconeogenesis, rather than glycogenolysis. Therefore, inhibition of gluconeogenesis is an important target for the development of antidiabetic drugs, and indeed the clinical effect of metformin appears to be due in large part to its ability to suppress gluconeogenesis. Despite the low cost and effectiveness of metformin usage, combination therapy with additional drugs may enhance its durability and effectiveness.
Acknowledgements
This work was supported by NIH grants (R24 DK080261-06, RO1 DK081418, RO1 DK089883, RO1 DK069966) and American Diabetes Association grant to P.P. K.S is supported by the American Heart Association Postdoctoral Fellowship. A.K.R is supported by NIDDK grant F32DK102293-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117(4):421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- Ahlborg G, Hagenfeldt L, Wahren J. Influence of lactate infusion on glucose and FFA metabolism in man. Scandinavian journal of clinical and laboratory investigation. 1976;36(2):193–201. doi: 10.1080/00365517609055248. [DOI] [PubMed] [Google Scholar]
- Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nature reviews. Molecular cell biology. 2011;12(3):141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Progress in biophysics and molecular biology. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. The American journal of physiology. 1988;255(6 Pt 1):E769–774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. The Journal of clinical endocrinology and metabolism. 2004;89(2):463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. The Journal of clinical investigation. 1997;99(2):361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest. 1994;93(4):1677–1682. doi: 10.1172/JCI117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes. 1995;44(2):180–184. doi: 10.2337/diab.44.2.180. [DOI] [PubMed] [Google Scholar]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population health metrics. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burant CF, Bell GI. Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry. 1992;31(42):10414–10420. doi: 10.1021/bi00157a032. [DOI] [PubMed] [Google Scholar]
- Campbell PJ, Mandarino LJ, Gerich JE. Quantification of the relative impairment in actions of insulin on hepatic glucose production and peripheral glucose uptake in non-insulin-dependent diabetes mellitus. Metabolism. 1988;37(1):15–21. doi: 10.1016/0026-0495(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nature reviews. Endocrinology. 2012;8(4):228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48(5):1198–1214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- Clark MG, Kneer NM, Bosch AL, Lardy HA. The fructose 1,6-diphosphatasephosphofructokinase substrate cycle. A site of regulation of hepatic gluconeogenesis by glucagon. The Journal of biological chemistry. 1974;249(18):5695–5703. [PubMed] [Google Scholar]
- Claus TH, Schlumpf JR, el-Maghrabi MR, Pilkis J, Pilkis SJ. Mechanism of action of glucagon on hepatocyte phosphofructokinase activity. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(11):6501–6505. doi: 10.1073/pnas.77.11.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli A, Nurjhan N, Capani F, Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989;38(5):550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(27):10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes, obesity & metabolism. 2011;13(1):7–18. doi: 10.1111/j.1463-1326.2010.01306.x. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. The Medical clinics of North America. 2004;88(4):787–835. dix. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Simonson D, Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982;23(4):313–319. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- Diamond MP, Rollings RC, Steiner KE, Williams PE, Lacy WW, Cherrington AD. Effect of alanine concentration independent of changes in insulin and glucagon on alanine and glucose homeostasis in the conscious dog. Metabolism. 1988;37(1):28–33. doi: 10.1016/0026-0495(88)90025-x. [DOI] [PubMed] [Google Scholar]
- Dominy JE, Jr., Lee Y, Jedrychowski MP, Chim H, Jurczak MJ, Camporez JP, Ruan HB, Feldman J, Pierce K, Mostoslavsky R, Denu JM, Clish CB, Yang X, Shulman GI, Gygi SP, Puigserver P. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Molecular cell. 2012;48(6):900–913. doi: 10.1016/j.molcel.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47(2):159–169. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. The Journal of biological chemistry. 2000;275(1):223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- Engstrom L. The regulation of liver pyruvate kinase by phosphorylation--dephosphorylation. Current topics in cellular regulation. 1978;13:28–51. [PubMed] [Google Scholar]
- Exton JH, Park CR. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. The Journal of biological chemistry. 1967;242(11):2622–2636. [PubMed] [Google Scholar]
- Feliu JE, Hue L, Hers HG. Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes. Proceedings of the National Academy of Sciences of the United States of America. 1976;73(8):2762–2766. doi: 10.1073/pnas.73.8.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth RG, Bell PM, Marsh HM, Hansen I, Rizza RA. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J Clin Invest. 1986;77(5):1525–1532. doi: 10.1172/JCI112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory W, Peczon BD, Koeppe RE, Spivey HO. Kinetic properties of rat liver pyruvate kinase at cellular concentrations of enzyme, substrates and modifiers. The Biochemical journal. 1974;141(1):127–131. doi: 10.1042/bj1410127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, xE, brard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. The Journal of clinical investigation. 2010;120(7):2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison JC, Wagner JD. Glucagon and the Ca2+-linked hormones angiotensin II, norepinephrine, and vasopressin stimulate the phosphorylation of distinct substrates in intact hepatocytes. The Journal of biological chemistry. 1982;257(21):13135–13143. [PubMed] [Google Scholar]
- Gastaldelli A, Toschi E, Pettiti M, Frascerra S, Quiñones-Galvan A, Sironi AM, Natali A, Ferrannini E. Effect of physiological hyperinsulinemia on gluconeogenesis in nondiabetic subjects and in type 2 diabetic patients. Diabetes. 2001;50(8):1807–1812. doi: 10.2337/diabetes.50.8.1807. [DOI] [PubMed] [Google Scholar]
- Gelling RW, Morton GJ, Morrison CD, Niswender KD, Myers MG, Jr., Rhodes CJ, Schwartz MW. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab. 2006;3(1):67–73. doi: 10.1016/j.cmet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabetic medicine : a journal of the British Diabetic Association. 2010;27(2):136–142. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich JE, Mitrakou A, Kelley D, Mandarino L, Nurjhan N, Reilly J, Jenssen T, Veneman T, Consoli A. Contribution of impaired muscle glucose clearance to reduced postabsorptive systemic glucose clearance in NIDDM. Diabetes. 1990;39(2):211–216. doi: 10.2337/diab.39.2.211. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Tucker SJ, Seino S, Ashcroft FM. Tissue specificity of sulfonylureas: studies on cloned cardiac and beta-cell K(ATP) channels. Diabetes. 1998;47(9):1412–1418. doi: 10.2337/diabetes.47.9.1412. [DOI] [PubMed] [Google Scholar]
- Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84(1):205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler RA, Kaestner KH, Accili D. FoxOs function synergistically to promote glucose production. The Journal of biological chemistry. 2010;285(46):35245–35248. doi: 10.1074/jbc.C110.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselgren PO. Glucocorticoids and muscle catabolism. Current opinion in clinical nutrition and metabolic care. 1999;2(3):201–205. doi: 10.1097/00075197-199905000-00002. [DOI] [PubMed] [Google Scholar]
- He L, Wondisford FE. Metformin action: concentrations matter. Cell Metab. 2015;21(2):159–162. doi: 10.1016/j.cmet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413(6852):179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The Physiology of Glucagon-like Peptide 1. 2007 doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Huang SC, Phelps ME, Hoffman EJ, Sideris K, Selin CJ, Kuhl DE. Noninvasive determination of local cerebral metabolic rate of glucose in man. The American journal of physiology. 1980;238(1):E69–82. doi: 10.1152/ajpendo.1980.238.1.E69. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- Iynedjian PB. Molecular physiology of mammalian glucokinase. Cellular and molecular life sciences : CMLS. 2009;66(1):27–42. doi: 10.1007/s00018-008-8322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoor F, Peters EJ, Wolfe RR. The relationship between gluconeogenic substrate supply and glucose production in humans. The American journal of physiology. 1990;258(2 Pt 1):E288–296. doi: 10.1152/ajpendo.1990.258.2.E288. [DOI] [PubMed] [Google Scholar]
- Jenssen T, Nurjhan N, Consoli A, Gerich JE. Failure of substrate-induced gluconeogenesis to increase overall glucose appearance in normal humans. Demonstration of hepatic autoregulation without a change in plasma glucose concentration. J Clin Invest. 1990;86(2):489–497. doi: 10.1172/JCI114735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152(4):673–684. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- Knutti D, Kaul A, Kralli A. A tissue-specific coactivator of steroid receptors, identified in a functional genetic screen. Molecular and Cellular Biology. 2000;20(7):2411–2422. doi: 10.1128/mcb.20.7.2411-2422.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437(7062):1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Koranyi L, James D, Mueckler M, Permutt MA. Glucose transporter levels in spontaneously obese (db/db) insulin-resistant mice. Journal of Clinical Investigation. 1990;85(3):962–967. doi: 10.1172/JCI114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309(5736):943–947. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. Journal of Clinical Investigation. 1997;99(3):414–423. doi: 10.1172/JCI119175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanthier N, Molendi-Coste O, Horsmans Y, van Rooijen N, Cani PD, Leclercq IA. Kupffer cell activation is a causal factor for hepatic insulin resistance. American journal of physiology. Gastrointestinal and liver physiology. 2010;298(1):G107–116. doi: 10.1152/ajpgi.00391.2009. [DOI] [PubMed] [Google Scholar]
- Lee Y, Dominy JE, Choi YJ, Jurczak M, Tolliday N, Camporez JP, Chim H, Lim JH, Ruan HB, Yang X, Vazquez F, Sicinski P, Shulman GI, Puigserver P. Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression. Nature. 2014;510(7506):547–551. doi: 10.1038/nature13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006;3(6):429–438. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Lewis GF, Vranic M, Harley P, Giacca A. Fatty acids mediate the acute extrahepatic effects of insulin on hepatic glucose production in humans. Diabetes. 1997;46(7):1111–1119. doi: 10.2337/diab.46.7.1111. [DOI] [PubMed] [Google Scholar]
- Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, She P, Jetton TL, Demarest KT. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes. 2004;53(2):410–417. doi: 10.2337/diabetes.53.2.410. [DOI] [PubMed] [Google Scholar]
- Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14(1):9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, Ahima RS, Ueki K, Kahn CR, Birnbaum MJ. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18(3):388–395. doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig Y, Ruas JL, Estall JL, Lo JC, Devarakonda S, Laznik D, Choi JH, Ono H, Olsen JV, Spiegelman BM. Separation of the gluconeogenic and mitochondrial functions of PGC-1{alpha} through S6 kinase. Genes & development. 2011;25(12):1232–1244. doi: 10.1101/gad.2054711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, Cline GW, Samuel VT, Kibbey RG, Shulman GI. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90(4):1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Marais R. Metformin: a diabetes drug for cancer, or a cancer drug for diabetics? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(21):2698–2700. doi: 10.1200/JCO.2012.42.1677. [DOI] [PubMed] [Google Scholar]
- Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45(2):223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6(3):208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(32):11278–11283. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek DW, Nimmo HG. Effects of phosphorylation on the kinetic properties of rat liver fructose-1,6-bisphosphatase. The Biochemical journal. 1984;222(1):125–130. doi: 10.1042/bj2220125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Molecular cell. 2000;6(1):87–97. [PubMed] [Google Scholar]
- Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494(7436):256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrakou A, Ryan C, Veneman T, Mokan M, Jenssen T, Kiss I, Durrant J, Cryer P, Gerich J. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. The American journal of physiology. 1991;260(1 Pt 1):E67–74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr. 2012;3(3):286–294. doi: 10.3945/an.112.002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutel E, Gautier-Stein A, Abdul-Wahed A, Amigo-Correig M, Zitoun C, Stefanutti A, Houberdon I, Tourette JA, Mithieux G, Rajas F. Control of blood glucose in the absence of hepatic glucose production during prolonged fasting in mice: induction of renal and intestinal gluconeogenesis by glucagon. Diabetes. 2011;60(12):3121–3131. doi: 10.2337/db11-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyrinck AM, Cani PD, Dewulf EM, De Backer F, Bindels LB, Delzenne NM. Critical role of Kupffer cells in the management of diet-induced diabetes and obesity. Biochemical and biophysical research communications. 2009;385(3):351–356. doi: 10.1016/j.bbrc.2009.05.070. [DOI] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8(12):1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, Ferrante AW., Jr. C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes. 2010;59(4):916–925. doi: 10.2337/db09-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opherk C, Tronche F, Kellendonk C, Kohlmuller D, Schulze A, Schmid W, Schutz G. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol Endocrinol. 2004;18(6):1346–1353. doi: 10.1210/me.2003-0283. [DOI] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. The Biochemical journal. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011;60(11):1500–1510. doi: 10.1016/j.metabol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Pilkis SJ, Chrisman TD, El-Maghrabi MR, Colosia A, Fox E, Pilkis J, Claus TH. The action of insulin on hepatic fructose 2,6-bisphosphate metabolism. The Journal of biological chemistry. 1983;258(3):1495–1503. [PubMed] [Google Scholar]
- Pilkis SJ, Claus TH, Kurland IJ, Lange AJ. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a metabolic signaling enzyme. Annual review of biochemistry. 1995;64:799–835. doi: 10.1146/annurev.bi.64.070195.004055. [DOI] [PubMed] [Google Scholar]
- Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea Stimulation of Insulin Secretion. Diabetes. 2002;51(suppl 3):S368–S376. doi: 10.2337/diabetes.51.2007.s368. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423(6939):550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Quesada I, Tudurí E, Ripoll C, Nadal Á. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. Journal of Endocrinology. 2008;199(1):5–19. doi: 10.1677/JOE-08-0290. [DOI] [PubMed] [Google Scholar]
- Quinn PG, Granner DK. Cyclic AMP-dependent protein kinase regulates transcription of the phosphoenolpyruvate carboxykinase gene but not binding of nuclear factors to the cyclic AMP regulatory element. Mol Cell Biol. 1990;10(7):3357–3364. doi: 10.1128/mcb.10.7.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju B, Cryer PE. Maintenance of the postabsorptive plasma glucose concentration: insulin or insulin plus glucagon? 2005 doi: 10.1152/ajpendo.00460.2004. [DOI] [PubMed] [Google Scholar]
- Rebrin K, Steil GM, Getty L, Bergman RN. Free fatty acid as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes. 1995;44(9):1038–1045. doi: 10.2337/diab.44.9.1038. [DOI] [PubMed] [Google Scholar]
- Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza RA. Pathogenesis of Fasting and Postprandial Hyperglycemia in Type 2 Diabetes: Implications for Therapy. Diabetes. 2010;59(11):2697–2707. doi: 10.2337/db10-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza RA, Cryer PE, Haymond MW, Gerich JE. Adrenergic mechanisms of catecholamine action on glucose homeostasis in man. Metabolism. 1980;29(11 Suppl 1):1155–1163. doi: 10.1016/0026-0495(80)90025-6. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Haas W, Gygi SP, Puigserver P. Cdc2-like kinase 2 is an insulin-regulated suppressor of hepatic gluconeogenesis. Cell Metab. 2010;11(1):23–34. doi: 10.1016/j.cmet.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal Glucosensing What Do We Know After 50 Years? Diabetes. 2004 doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, 3rd, Takemori H, Okamoto M, Montminy M. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119(1):61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She P, Burgess SC, Shiota M, Flakoll P, Donahue EP, Malloy CR, Sherry AD, Magnuson MA. Mechanisms by which liver-specific PEPCK knockout mice preserve euglycemia during starvation. Diabetes. 2003;52(7):1649–1654. doi: 10.2337/diabetes.52.7.1649. [DOI] [PubMed] [Google Scholar]
- She P, Shiota M, Shelton KD, Chalkley R, Postic C, Magnuson MA. Phosphoenolpyruvate Carboxykinase Is Necessary for the Integration of Hepatic Energy Metabolism. Molecular and Cellular Biology. 2000;20(17):6508–6517. doi: 10.1128/mcb.20.17.6508-6517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindelar DK, Chu CA, Rohlie M, Neal DW, Swift LL, Cherrington AD. The role of fatty acids in mediating the effects of peripheral insulin on hepatic glucose production in the conscious dog. Diabetes. 1997;46(2):187–196. doi: 10.2337/diab.46.2.187. [DOI] [PubMed] [Google Scholar]
- Unger RH, Aguilar-Parada E, Muller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Poelje PD, Potter SC, Erion MD. Fructose-1, 6-bisphosphatase inhibitors for reducing excessive endogenous glucose production in type 2 diabetes. Handbook of experimental pharmacology. 2011;(203):279–301. doi: 10.1007/978-3-642-17214-4_12. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E. Fructose 2,6-bisphosphate. Advances in enzymology and related areas of molecular biology. 1987;59:315–395. doi: 10.1002/9780470123058.ch7. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E, Hue L, Hers HG. Control of the fructose-6-phosphate/fructose 1,6-bisphosphate cycle in isolated hepatocytes by glucose and glucagon. Role of a low-molecular-weight stimulator of phosphofructokinase. The Biochemical journal. 1980a;192(3):887–895. doi: 10.1042/bj1920887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E, Hue L, Hers HG. Fructose 2,6-bisphosphate, the probably structure of the glucose- and glucagon-sensitive stimulator of phosphofructokinase. The Biochemical journal. 1980b;192(3):897–901. doi: 10.1042/bj1920897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts LM, Manchem VP, Leedom TA, Rivard AL, McKay RA, Bao D, Neroladakis T, Monia BP, Bodenmiller DM, Cao JX, Zhang HY, Cox AL, Jacobs SJ, Michael MD, Sloop KW, Bhanot S. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes. 2005;54(6):1846–1853. doi: 10.2337/diabetes.54.6.1846. [DOI] [PubMed] [Google Scholar]
- Wynshaw-Boris A, Short JM, Loose DS, Hanson RW. Characterization of the phosphoenolpyruvate carboxykinase (GTP) promoter-regulatory region. I. Multiple hormone regulatory elements and the effects of enhancers. Journal of Biological Chemistry. 1986;261(21):9714–9720. [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413(6852):131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]