Abstract
Hypothesis
Superior canal dehiscence (SCD) repair using surgical bone wax may result in variable outcomes if large wax volumes are applied.
Background
SCD is a disorder characterized by a pathologic defect in the bony labyrinth of the superior semicircular canal (SSC), resulting in vestibular and/or auditory symptoms. Repair of SCD using bone wax can provide symptomatic relief, but surgical outcomes are variable. These observations may be associated with the inconsistency in the position and extension of intralabyrinthine bone wax during surgical repair.
Methods
A pathological model of SCD was created using cadaveric human temporal bones and a microdrill. Defects in the arcuate eminence 0.5–3.5 mm in length were repaired using surgical occlusion with bone wax. The volume of wax used in the repair was quantified. The position of bone wax was evaluated by direct visualization and imaging (computed tomography [CT]). To visualize wax on CT, specimens were repaired using radiopaque wax.
Results
Exceedingly small volumes of bone wax (3.0–5.0 mm2) reliably occluded the canal lumen. Multiple wax applications resulted in extension into the common crus and ampulla. The length of this extension was related to the number of applications.
Conclusions
Repair of SCD with bone wax occludes the bony defect completely in most cases. Wax can extend along the lumen of the superior canal beyond the limits of the dehiscence to reach the sensory neuroepithelium of the vestibular end organs. Limiting the number of wax applications is essential to avoid inadvertent injury to the delicate neurosensory systems.
Introduction
Superior canal dehiscence (SCD) describes the pathological absence of bone covering the superior semicircular canal (SSC). This bony defect is associated with a variety of signs and symptoms, including sound-(Tullio’s phenomenon) and pressure-(Hennebert’s sign) induced vertigo, autophony, pulsatile tinnitus, hyperacusis and the unusual complaint of hearing one’s eye movements.1,2 In some patients, symptoms can have a profound impact on quality of life and surgical repair of SCD can provide significant improvements.3–7
When Minor et al. first described SCD in 19981, they reported success in managing SCD symptoms by repairing the defects via middle-fossa craniotomy (MFC) approach and occluding the bony defect, a technique which is still used today.5–15 Although currently debated in the literature8–11, studies indicate that occlusion of the SSC is associated with lower recurrence of symptoms compared to resurfacing or “capping” repairs.3,4,8 However, despite the durability of SCD occlusion, variable outcomes and complications can occur.6–9
Occlusion or plugging of the bony defect has been theorized to improve SCD signs and symptoms by sealing the “third window”, and compressing the membranous labyrinth in the process.2,10 Bone wax, autologous grafting material (e.g. fascia, bone chips, cartilage) and hydroxyapatite cement have been used for SCD repair and there is currently no general consensus on the preferred material. At our center, we currently perform the majority of our SCD repairs using bone wax because we find that it forms a good seal of the defect and is relatively bio-inert, which theoretically makes it less susceptible to resorption and therefore more likely to result in a stable and durable repair.
To further our understanding of the effect of a repair, it is important to determine the position of the wax within the canal. Currently, we lack the means to monitor the precise volume of wax entering the SSC and the extent of occlusion during the wax application. Intraoperative monitoring of auditory brainstem responses (ABRs) and electrocochleagraphy (ECochG) may provide real-time information of pressure transmission to the cochlea during occlusion16, but there are no routine intraoperative vestibular measurements available to monitor vestibular function. Variability in wax position is clinically relevant as it may be associated with auditory and vestibular complications following surgery. Herein, we aim to determine the position of wax following repair of SCD under controlled conditions using a human cadaveric temporal bone model.
Material and Methods
The study (Study ID: 742367–1) was deemed exempt by the Human Studies Committee at the Massachusetts Eye and Ear Infirmary.
Creation of temporal bone model of superior semicircular canal dehiscence
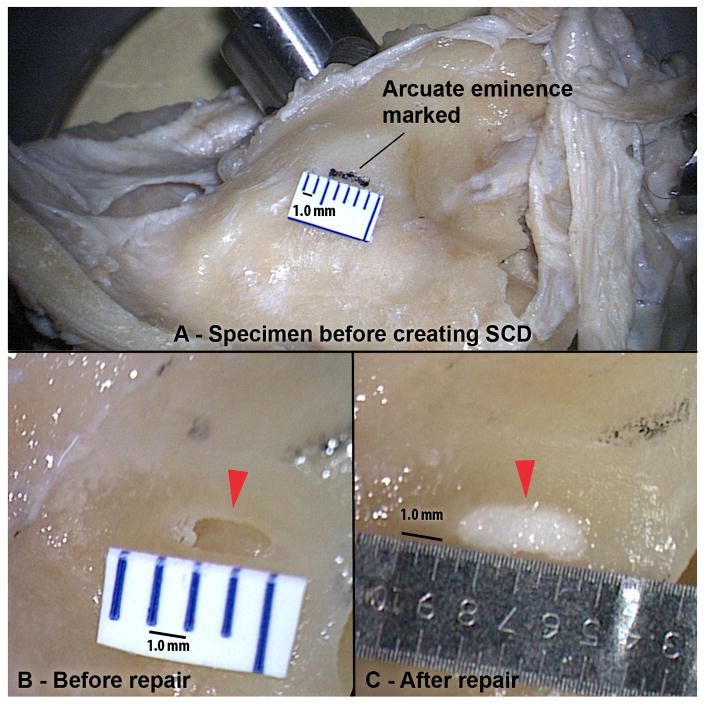
Freshly fixed human cadaveric temporal bones were used (n=9). The skull base surrounding the arcuate eminence was thinned and leveled using a large diamond burr to replicate the thin bony middle fossa floor often encountered during surgery in patients with SCD. A bony defect of the superior canal was created using a micro-drill and diamond burr at low speed (Fig. 1). SCDs are commonly elliptical in shape with uneven edges, and attempts were made to recreate this in our specimens. Dehiscence size was measured using a micrometer ruler (Microscale #23; Minitool Inc, Los Gatos, California, USA) that has an estimated error of 0.05 mm.
Figure 1. Experimental Setup.
(A) Temporal bone specimen with the arcuate eminence marked with ink before creation of SCD. (B) Before and (C) after repair of 2.0 mm SCD. Arrows mark SCD.
Repair of superior semicircular canal dehiscence
Specimens were divided into two groups based on the method used to examine the repairs – direct visualization using an operating microscope or by computed tomography (CT) imaging. For the first group of specimens, a neurotologic surgeon with experience in SCD surgery performed the repairs (n=5) to simulate the real surgical technique. The specimens were warmed to physiological temperatures in a water bath. A thermocouple (Model 43; YSI Inc, Yellow Springs, Ohio, USA) was set at 37.0 °C to keep the temperature stable. To simulate surgical conditions, the specimens were positioned at an angle similar to the anatomical orientation encountered in the MFC approach and moistened regularly with warm saline during the procedure. The surgical bone wax was manipulated to form a small spherical mass, which was perched at the tip of a small (2.0mm) Rosen knife (Fig. 2). The wax was pressed against the SCD with gentle downward motion, followed by a lateral motion to withdraw the instrument. The flat surface of a Freer-elevator or a drum elevator was then used to evenly flatten the bone wax in a similar downward and lateral movement.
Figure 2. Volume and size of wax used in repairs.
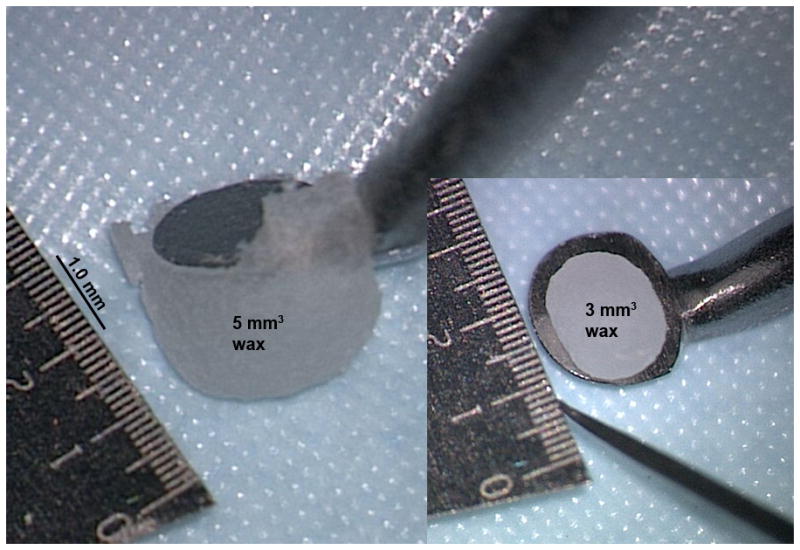
Size of wax relative to 2.0 mm Rosen knife. Wax on the left is 5 mm3. Spherical wax on right is 3 mm3.
When an SCD appeared to be completely sealed, the stability of the wax plug was tested in surgical standard fashion by irrigating the wax covering the SCD with saline. If the wax was mobile or a gap between the wax and the edge of the SCD appeared, the SCD was repaired with a further application of wax. This cycle was repeated until a “stable” repair was achieved. For the purpose of this study, an application of wax was counted each time the surgeon applies a fresh sphere of wax. The sizes of wax spheres were generally 1.5 – 2mm in diameter as per routine during surgery for SCD. Also, with each application, excess wax left on the bony surface was wipe off and discarded.
The specimens in the second group (n=4) was repaired at room temperature using a radiopaque bone wax created by adding 0.8 g of 98% barium sulfate powder (Cat. No. 764, E-Z-HD Inc, Lake Success, NY, USA) to 2.5 g of surgical bone wax (model W31.O31, Ethicon, Somerville, NJ, US). The SCD repairs were performed using the same technique as mentioned above. After the repairs, the specimens were examined using cone beam CT imaging (3D Accuitomo 170; J. Morita Corporation, Japan).
Quantifying volumes of intraluminal wax following repair of SCD
To quantify the volumes of wax used in an SCD repair, wax plugs were retrieved from repeated attempts (4 repetitions) of repairing selected SCDs. To measure the small volumes, 0.1 – 10 μL pipette tips (Pipetman diamond tips, model d10, Gilson Inc, Middleton, WI, USA) were customized to create a measuring cylinder. The wax plugs were each placed in the pipette tip, after which 5.0 mm3 of water was drawn into the pipette. Graduations were made along the pipette tip and these provided a total volume of plug and fluid, from which the volume of the plug was calculated. An average volume of wax used was calculated from the wax plugs retrieved from each SCD tested.
Visualization of wax following occlusion techniques
After repairing SCDs in human temporal bone specimens, the superior canal was fenestrated using a diamond burr at low speed both towards the ampulla and common crus so as not to disturb the repair area and to visualize how far the wax traveled during the repair. The intraluminal wax was examined using an operating microscope, which has image-recording capabilities, for evidence of partial occlusion of canal and position within the SSC including any extension along the canal. In SCDs repaired with radiopaque wax, specimens were left intact and imaged using a cone beam CT scanner. CT images (125 μm thick slices) were reformatted in Pöschl and Stenvers planes to provide longitudinal and transverse views of the SSC respectively.
Results
Dimensions of defects
Previous studies have reported average SCD lengths of 3.3 to 3.6 mm.17–20 Large dehiscences can be repaired by either occluding the SCD itself or by occluding both exposed ends of the canal. In this study, we created dehiscences of 0.5 to 3.5 mm in length that are typically repaired by occluding the SCD itself. The width of SCD measured at the widest point ranged from 0.5 to 0.9 mm.
Volume of wax plugs
Volumes of wax plugs were retrieved from representative stable repairs of 2 SCDs as performed in typical surgery. Based on 4 repeated repairs, a 3.5 mm SCD required an average of 4.0 mm3 of wax for a stable plug. Similarly, from 4 repeated repairs, a 1.5 mm SCD required an average of 3.0 mm3 of wax for a stable repair. The estimated error using our technique for measuring volume is 0.5 mm3. We also measured 2 wax spheres that are typically created before the start of each wax application. These were found to have measured volumes of 3.0 and 5.0 mm3 (Fig. 2), which is close to the calculated volume of a 2.0 mm diameter sphere. It is worth noting that when a wax sphere is pressed against a SCD, only some of the wax enters the canal. Excess wax outside the defect is discarded during an application.
Direct visualization of wax position following occlusion technique
In the first group of specimens (repaired using unaltered surgical wax at physiologic temperatures), dehiscences were made with a range of lengths: 0.6, 1.0, 2.0, 2.5 and 3.5 mm. Our method of visualizing the surgical repair under microscopy on either ends of the semicircular canal (away from the SCD) allowed us to clearly and directly determine the location and extension of the wax without disturbing the repair area. Surgical repair of the SCDs resulted in complete occlusion of SSC lumen in each specimen. Wax was observed extending beyond the anterior and posterior limit of the dehiscence in all specimens to varying degrees (Fig. 3). The length of wax extension was measured from the edge of the SCD along the longitudinal axis of the canal (Fig. 3) and was found to be unrelated to the size of the SCD (Table 1). Instead, the length of wax extension appeared to be positively related to the number of fresh wax spheres required during the repair. Based on our method of counting wax applications, the longest wax extension was seen in the specimen that required the most number of wax applications, while the shortest wax extension was seen in the specimen that needed the least.
Figure 3. Direct visualization of intraluminal wax.
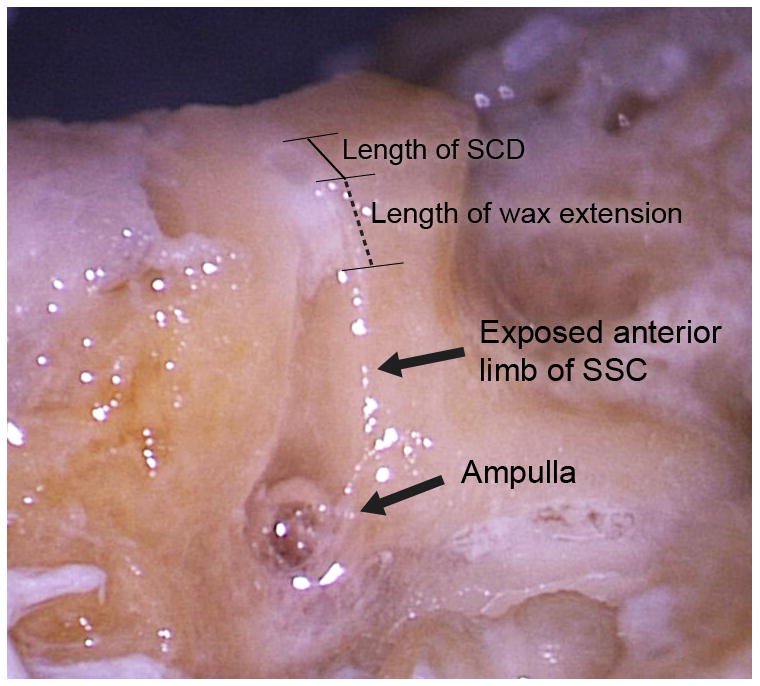
Examination of wax location within the SSC lumen by exposing anterior arm of SSC. Image demonstrates how anterior wax extension is measured.
Table 1.
Extension of bone wax and number of wax application required
| Length of SCD (mm) | Side | Number of wax application required | Anterior extension | Posterior extension | Total extension |
|---|---|---|---|---|---|
| 0.6 | L | 2 | 0.5 | 0.3 | 0.8 |
| 1.0 | L | 3 | 1.4 | 1.5 | 2.9 |
| 2.0 | R | 1 | 0.3 | 0.0 | 0.3 |
| 2.5 | L | 2 | 1.0 | 0.8 | 1.8 |
| 3.5 | R | 2 | 0.2 | 0.3 | 0.5 |
Multiple applications of unaltered surgical bone wax (5 applications) resulted in extension along the entire length of the SSC, which was apparent as the bone was thinned down for direct visualization. The posterior tip was seen at the common crus and the anterior tip of the intra-luminal wax was seen abutting the macula utriculus in the vestibule.
CT imaging of temporal bone following occlusion with radiopaque wax
In the second group of specimens, SCDs measuring 0.5, 1.0, 1.5 and 3.5 mm in length were repaired using our radiopaque wax. Imaging showed that 3 out of 4 superior canals were completely occluded by an intraluminal opacity (wax) at the canal defect. Of note, partial occlusion of the canal was observed in the smallest SCD (0.5 mm), where Stenvers CT views showed intraluminal gaps (fluid) around a region of opacity (wax) (Fig. 4). In addition, in another specimen (SCD = 1.0 mm), a fragment of wax was identified in the posterior arm of the SSC between the common crus and the wax plug (Fig. 5). In this specimen, it was noted that the initial attempt at repairing this SCD was unsuccessful, with most of the wax plug lifting off with the Rosen knife when it was withdrawn, possibly leaving a small fragment within the SSC. The second attempt was made with a fresh sphere of wax being pressed over the small fragment. This successfully sealed the specimen.
Figure 4. Partial occlusion of SSC.
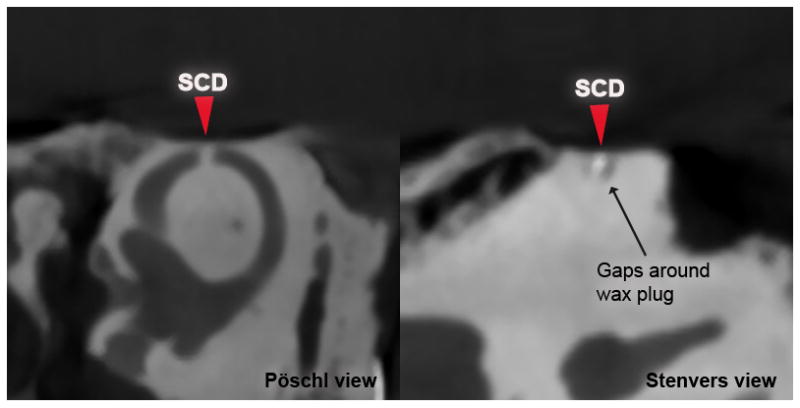
Pöschl (left) and Stenvers CT (right) views of a specimen after repair of 0.5 mm dehiscence using radiopaque wax. Arrows mark location of SCD.
Figure 5. Fragmentation of wax.
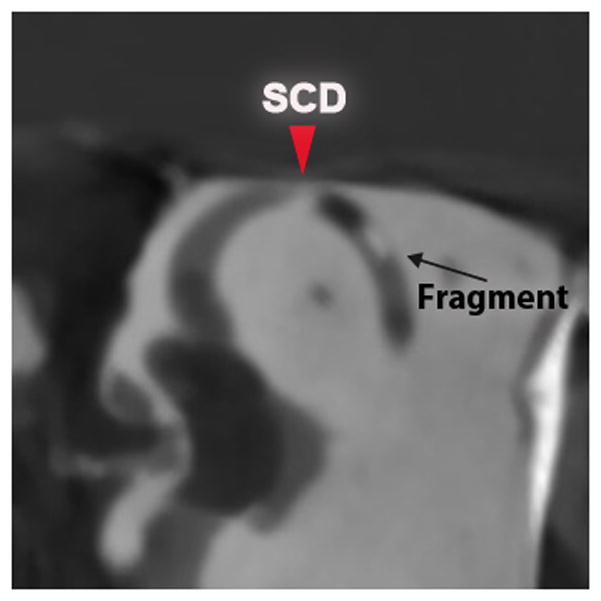
Pöschl CT views of a specimen after repair of 1.0 mm dehiscence using radiopaque wax. Arrows mark location of SCD.
Multiple application of radiopaque wax (4 applications in total) resulted in extension of wax along the long-axis of the SSC into the ampulla on CT imaging (Fig. 6). This effect of wax extending to the ampulla or common crus is consistent with findings from the direct visualization (as detailed above).
Figure 6. Multiple radiopaque wax applications.
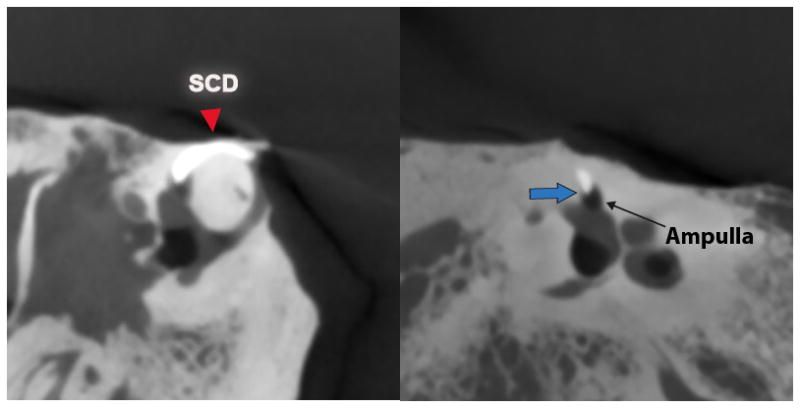
CT views after repair of 3.5 mm dehiscence using radiopaque wax. Blue arrow indicates anterior tip of wax extension in ampulla.
Discussion
The study demonstrated that an exceedingly small volume of wax was required to repair the superior canal – which may be less than surgeons anticipate. Based on the surgical experience and technique tested in our experiment, most SCD repairs required two applications of wax spheres (~2mm in diameter) to occlude the canal. This volume did not physically disrupt the ampullary structures on gross examination or extend into the crus commune.
Furthermore, both the direct visualization and imaging studies revealed that more than 2 applications of wax resulted in extension of wax along the canal towards the ampulla or common crus. Extension of wax could result in disturbing delicate vestibular structures resulting in unwanted post-operative vestibular symptoms. Given the variable outcomes of post-operative SCD patients, as well as potential for complications, these data emphasize the need for surgeons to be cognizant of and limit the amount of wax placed in the canal.
Vestibular hypofunction after SCD repair is a well-known complication after SCD repair (38–47%).7, 21 By measuring pre-and post-operative angular vestibular-ocular reflexes using search coil technique, Carey et al.22 found that after surgical SCD repair via MFC approach, patients often suffer from vestibular hypofunction which is limited to the operated SSC, but in some patients, the ipsilateral posterior canal exhibits reduced function as well. It was postulated that occlusion material might extend to the posterior canal. Our study provides further evidence for this theory and highlights potential unintended consequences of using excessive wax during SCD surgery. Indeed, investigation of wax localization demonstrated that wax extended beyond the limits of the SCD to varying degrees in our specimens. In our experimental simulation of surgery, the increase in the number of applications of wax (defined by number of wax spheres) required for a stable repair in each specimen appeared to encourage this unintended extension.
Intraoperative monitoring of vestibular function is currently not available. Wenzel et al.16 performed intraoperative ABRs and ECochG, and reported that summating potential to action potential ratio (elevated in SCD) typically decreased during SCD repair. While these changes were not found to predict post-operative audiometric outcomes, information on real-time labyrinthine pressure transmission during occlusion may provide useful feedback regarding the extent of canal occlusion during surgery.
Development of a radiopaque surgical wax for SCD repair is another potential method of monitoring wax position post-occlusion of canals with CT imaging. This would be particularly useful in the management of patients who suffer recurrence of SCD symptoms post SCD repair. Heavily T2-weighted MRI sequences could be an alternative imaging technique under these circumstances, but the resolution of current MRI scans may not be sufficient to determine subtle differences of wax versus fluid in such a small structure. Intratympanic gadolinium-based MRI studies have shown promise in sensing details such as inner-ear endolymphatic hydrops and this technique has recently been found to be safe.23 Higher labyrinthine contrast uptake may enhance the SSC sufficiently to clearly delineate intraluminal wax, but is a subject of future studies.
One might argue that using a material of defined volume (eg. fascia) might negate the extension of repair material beyond the defect, but as mentioned, the moldable property of wax may help form a hermetic seal of the defect. Brockenbrough et al.24 demonstrated that bone wax was better at preventing nystagmus in guinea pigs immediately after the repair of a semicircular canal fenestration, while fascia and fibrin glue or fascia alone took 3–5 days before nystagmus resolved. They postulated that the soft moldable wax is better able to form a perfect seal of the third window, which is critical when attempting to restore normal labyrinthine physiology – a hypothesis support by several animal25–27 and human temporal bone studies28.
Concerns regarding the inflammatory response to bone wax were raised13 following reports of mild high frequency hearing loss in 3 of 11 patients after SCD repair with bone wax in an early series3. However, similar patterns of hearing loss were also seen in a comparable proportion (25%) of patients after they underwent SCDs occlusion with autologous materials (fascia, bone dust and bone chip)14, which suggest that this complication is not specific to bone wax. Kim et al.28 repaired lateral canal fenestrations of chinchillas using bone wax, bone dust and muscle and observed inflammation around the bone wax, but also found necrotic material around the bone dust and muscle. Some inflammation seems inevitable after SCD occlusion, and autologous materials may be more susceptible to being resorbed, which has been observed in bone grafts used for SCD repairs2,15. For these reasons, we have persisted with the use of surgical bone wax for SCD repair. All patients are prescribed a short course of oral steroids postoperatively.
There are several limitations to the study. First, specimens in this study were prepared with defects of 0.5–3.5 mm in size over the arcuate eminence; however, SCD defects do vary in anatomical size, location and shape. The slope of the tegmen at the defect and its location along the canal influences the surgical approach30, which can in turn affect the orientation from which a surgeon repairs a defect. This has implications for the consequential position of wax, and volume of wax necessary to achieve a stable occlusion of the canal. Second, surgical techniques vary between surgeons, which can potentially influence the number of applications of wax required to achieve a similar outcome. Third, this study describes the position of wax immediately after surgical application, and does not account for the effects of subsequent attempts to resurface the skull base after wax occlusion (e.g. bone graft or bone cement), that may influence the final position of wax in the SSC lumen. The senior surgeon of this study does not apply any additional autologous grafts directly over the repaired arcuate eminence defect in order to avoid disturbing the wax plug and so this was not specifically examined in our model. Finally, for the CT imaging study, the small addition of barium sulfate could alter the physical properties of the radiopaque bone wax. To determine if any change in the property of wax affects SCD repair, identical repairs with unaltered surgical wax were performed and examined directly using an operating microscope, which revealed similar results.
Conclusion
Using human temporal bones allowed us to understand the location of surgically applied wax during SCD surgery in a controlled manner. We found that bone wax repair of SCDs typically results in complete occlusion of the SSC lumen. Wax used in SCD repairs can extend along the canal beyond the edges of the SCD and repeated application of wax encourages this extension. Surgical bone wax has been shown to be an effective material for SCD repairs4–6, but surgeons should be acutely aware that excessive wax applications could result in unintended extension into the ampulla, vestibule and/or common crus. Consequently, vestibular and/or auditory complications could result in patients. The results of this study provide a reference volume of wax for use in SCD repairs. Two applications of 2mm diameter bone wax spheres reliably occlude the SSC and further applications should be avoided when possible.
Acknowledgments
The authors thank Renee Mitchell for inspiring the creation the radiopaque wax and for imaging the bone specimens, Diane Jones for collecting the temporal bones specimens and Jeananne Phillips for providing ready access to the Joseph B. Nadol, Jr., MD Surgical Training Laboratory at the Massachusetts Eye and Ear Infirmary. This study was supported by R01 DC004797 and the American Otological Society.
Footnotes
Conflict of Interest: None
Disclosures: None
References
- 1.Minor LB, Solomon D, Zinreich JS, Zee DS. Sound-and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249–58. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- 2.Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005;115:1717–27. doi: 10.1097/01.mlg.0000178324.55729.b7. [DOI] [PubMed] [Google Scholar]
- 3.Mikulec AA, Poe DS, McKenna MJ. Operative management of superior semicircular canal dehiscence. Laryngoscope. 2005;115:501–7. doi: 10.1097/01.mlg.0000157844.48036.e7. [DOI] [PubMed] [Google Scholar]
- 4.Crane BT, Minor LB, Carey JP. Superior canal dehiscence plugging reduces dizziness handicap. Laryngoscope. 2008;118:1809–13. doi: 10.1097/MLG.0b013e31817f18fa. [DOI] [PubMed] [Google Scholar]
- 5.Crane BT, Lin FR, Minor LB, Carey JP. Improvement in autophony symptoms after superior canal dehiscence repair. Otol Neurotol. 2010 Jan;31(1):140–6. doi: 10.1097/mao.0b013e3181bc39ab. [DOI] [PubMed] [Google Scholar]
- 6.Goddard JC, Wilkinson EP. Outcomes following Semicircular Canal Plugging. Otolaryngol Head Neck Surg. 2014 Sep;151(3) doi: 10.1177/0194599814538233. [DOI] [PubMed] [Google Scholar]
- 7.Jung D, Lookabaugh S, Owoc M, McKenna MJ, Lee DJ. Dizziness is More Prevalent than Autophony Among Patients Who Have Undergone Repair of Superior Canal Dehiscence. Otol Neurol. 2014;36:126–32. doi: 10.1097/MAO.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 8.Vlastarakos PV, Proikas K, Tavoulari E, Kikidis D, Maragoudakis P, Nikolopoulos TP. Efficacy assessment and complications of surgical management for superior semicircular canal dehiscence: a meta-analysis of published interventional studies. Eur Arch Otorhinolaryngol. 2009;266:177–86. doi: 10.1007/s00405-008-0840-4. [DOI] [PubMed] [Google Scholar]
- 9.Mueller SA, Vibert D, Haeusler R, Raabe A, Caversaccio M. Surgical capping of superior semicircular canal dehiscence. Eur Arch Otorhinolaryngol. 2014 Jun;271(6):1369–74. doi: 10.1007/s00405-013-2533-x. [DOI] [PubMed] [Google Scholar]
- 10.Moskowitz HS, Tolle F, Carey JP. Pressure assessment of superior semicircular canal dehiscence repair techniques--a temporal bone study. Otol Neurotol. 2014 Dec;35(10):e331–6. doi: 10.1097/MAO.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 11.Shaia WT, Diaz RC. Evolution in surgical management of superior canal dehiscence syndrome. Curr Opin Otolaryngol Head Neck Surg. 2013;21:497–502. doi: 10.1097/MOO.0b013e328364b3ff. [DOI] [PubMed] [Google Scholar]
- 12.Niesten ME, McKenna MJ, Grolman W, Lee DJ. Clinical factors associated with prolonged recovery after superior canal dehiscence surgery. Otol Neurotol. 2012 Jul;33(5):824–31. doi: 10.1097/MAO.0b013e3182544c9e. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal SK, Parnes LS. Transmastoid superior semicircular canal occlusion. Otol Neurotol. 2008 Apr;29(3):363–7. doi: 10.1097/mao.0b013e3181616c9d. [DOI] [PubMed] [Google Scholar]
- 14.Ward BK, Agrawal Y, Nguyen E, et al. Hearing outcomes after surgical plugging of the superior semicircular canal by a middle cranial fossa approach. Otol Neurotol. 2012;33:1386–91. doi: 10.1097/MAO.0b013e318268d20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limb CJ, Carey JP, Srireddy S, Minor LB. Auditory function in patients with surgically treated superior semicircular canal dehiscence. Otol Neurotol. 2006;27:969–80. doi: 10.1097/01.mao.0000235376.70492.8e. [DOI] [PubMed] [Google Scholar]
- 16.Wenzel A, Ward BK, Ritzl EK, et al. Intraoperative Neuromonitoring for Superior Semicircular Canal Dehiscence and Hearing Outcomes. Otol Neurotol. 2015 Jan;36(1):139–45. doi: 10.1097/MAO.0000000000000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chien WW, Janky K, Minor LB, Carey JP. Superior canal dehiscence size: multivariate assessment of clinical impact. Otol Neurotol. 2012 Jul;33(5):810–5. doi: 10.1097/MAO.0b013e318248eac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavassolie TS, Penninger RT, Zuñiga MG, Minor LB, Carey JP. Multislice computed tomography in the diagnosis of superior canal dehiscence: how much error, and how to minimize it? Otol Neurotol. 2012 Feb;33(2):215–22. doi: 10.1097/MAO.0b013e318241c23b. [DOI] [PubMed] [Google Scholar]
- 19.Pfammatter A, Darrouzet V, Gärtner M, et al. A superior semicircular canal dehiscence syndrome multicenter study: is there an association between size and symptoms? Otol Neurotol. 2010 Apr;31(3):447–54. doi: 10.1097/MAO.0b013e3181d27740. [DOI] [PubMed] [Google Scholar]
- 20.Puram SV, Roberts DS, Niesten ME, Dilger AE, Lee DJ. Cochlear implant outcomes in patients with superior canal dehiscence. Cochlear Implants Int. 2013 Nov 25; doi: 10.1179/1754762813Y.0000000044. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal Y, Migliaccio AA, Minor LB, Carey JP. Vestibular hypofunction in the initial postoperative period after surgical treatment of superior semicircularcanal dehiscence. Otol Neurotol. 2009 Jun;30(4):502–6. doi: 10.1097/MAO.0b013e3181a32d69. [DOI] [PubMed] [Google Scholar]
- 22.Carey JP, Migliaccio AA, Minor LB. Semicircular canal function before and after surgery for superior canal dehiscence. Otol Neurotol. 2007 Apr;28(3):356–64. doi: 10.1097/01.mao.0000253284.40995.d8. [DOI] [PubMed] [Google Scholar]
- 23.Louza J, Krause E, Gürkov R. Hearing function after intratympanic application of gadolinium-based contrast agent: A long-term evaluation. Laryngoscope. 2015 Mar 30; doi: 10.1002/lary.25259. [DOI] [PubMed] [Google Scholar]
- 24.Brockenbrough JM, Marzo S, Wurster R, Young MR. Bone wax prevents nystagmus after labyrinthine fenestration in guinea pigs. Otolaryngol Head Neck Surg. 2003 May;128(5):726–31. doi: 10.1016/S0194-59980223289-1. [DOI] [PubMed] [Google Scholar]
- 25.Antonelli PJ, Bouchard KR, Kartush JM, Kubilis PS. Triple semicircular canal occlusion in the guinea pig. Otolaryngol Head Neck Surg. 1997;117:509–15. doi: 10.1016/S0194-59989770023-8. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi T, Shiga N, Hozawa K, Hashimoto S, Takasaka T. Effect on cochlear potentials of lateral semicircular canal destruction. Arch Otolaryngol Head Neck Surg. 1991;117:1292–5. doi: 10.1001/archotol.1991.01870230108018. [DOI] [PubMed] [Google Scholar]
- 27.Gjuric M, Wigand ME, Hosemann W. Selective semicircular canals of rabbits with preservation of hearing. Acta Otolaryngol. 1992;112:907–15. doi: 10.3109/00016489209137490. [DOI] [PubMed] [Google Scholar]
- 28.Pisano DV, Niesten ME, Merchant SN, Nakajima HH. The effect of superior semicircular canal dehiscence on intracochlear sound pressures. Audiol Neurotol. 2012;17(5):338–48. doi: 10.1159/000339653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim TH, Nam BH, Park CI. Histologic changes of lateral semicircular canal after transection and occlusion with various materials in chinchillas. Korean J Otolaryngol Head Neck Surg. 2002;45:318–21. [Google Scholar]
- 30.Lookabaugh S, Kelly HR, Carter MS, et al. Radiologic classification of superior canal dehiscence: implications for surgical repair. Otol Neurotol. 2015 Jan;36(1):118–25. doi: 10.1097/MAO.0000000000000523. [DOI] [PubMed] [Google Scholar]