Abstract
Soil microbes play critical roles in regulating terrestrial carbon (C) cycle and its feedback to climate change. However, it is still unclear how the soil microbial community and abundance respond to future climate change scenarios. In this meta-analysis, we synthesized the responses of microbial community and abundance to experimental warming from 64 published field studies. Our results showed that warming significantly increased soil microbial abundance by 7.6% on average. When grouped by vegetation or soil types, tundras and histosols had the strongest microbial responses to warming with increased microbial, fungal, and bacterial abundances by 15.0%, 9.5% and 37.0% in tundra, and 16.5%, 13.2% and 13.3% in histosols, respectively. We found significant negative relationships of the response ratios of microbial, fungal and bacterial abundances with the mean annual temperature, indicating that warming had stronger effects in colder than warmer regions. Moreover, the response ratios of microbial abundance to warming were positively correlated with those of soil respiration. Our findings therefore indicate that the large quantities of C stored in colder regions are likely to be more vulnerable to climate warming than the soil C stored in other warmer regions.
An important ongoing endeavor in current global change ecology and biology is incorporating microbial responses into Earth system models (ESMs), as microbes have been highlighted as one of main unknowns controlling the fate and turnover of soil organic matter (SOM)1,2,3. Modeling studies predicted that climate warming would stimulate microbial decomposition of SOM, representing an important positive feedback loop4,5. Although short-term field experiment documented an initial increase of soil respiration (SR) to climate warming6, there were still large uncertainties in the strength and magnitude of the effects of warming on ecosystem carbon (C) cycles7,8. Such large uncertainties might be mainly due to the response of the soil microbial activities1, since warming-induced changes in the soil microbial abundance had the potential to either accentuate9,10 or mitigate7,11 warming-induced C losses substantially. However, it remains unclear, especially in broad-scales evaluation of how microbial abundance respond to global climate change.
The effects of warming on soil microorganisms and the causal mechanisms linking the two are varied with climate regions and ecosystem types. Firstly, warming significantly enhanced microbial abundance in alpine regions and subarctic regions12,13, whereas the effects of warming on microbial abundance in temperate regions were often neutral or negative11,14. Possible explanations were associated with the microbial temperature sensitivity and microbial adaptation strategies15, although these explanations were still highly disputed16. On the other hand, warming often decreased microbial abundance in the temperate forest17,18, while enhanced it in the tundra or peat-land12,13,14. These inconsistent responses were mainly attributed to the soil substrate availabilities and soil properties19,20. These types of divergent responses greatly hampered our understanding of how microbial abundance responds to warming and what are underlined mechanisms. Therefore, a synthesis that aims to examine the broad-scale responses of microbial abundance to warming among ecosystem types and climate regions is necessary.
Two recent meta-analyses concluded that changes in microbial abundance were significantly correlated with changes in SR following ecosystem disturbances or N additions21,22. However, to our knowledge, it remains unclear whether there are some links between the responses of microbial abundance and SR to warming in large scales. To advance the predictive ability in regard to microbial abundance and associated SR under warming scenarios, we conducted a meta-analysis on the responses of microbial abundance to warming. First, we hypothesized that warming could have positive effects on microbial abundance. Second, we hypothesized that the responses of microbial abundance to warming would vary with climate regions and ecosystem types. Finally, we hypothesized that the responses of microbial abundance could be tightly coupled with changes in SR. We also tested above hypotheses separately for fungi, bacteria and Archaea.
Results
Microbes
Across all studies, soil microbial abundances significantly increased following warming by an average of 7.6%, and there was no publication bias (Fig. 1, Table 1, Supplementary Table 1). When grouped by measurements, warming significantly enhanced microbial abundance by 8.5% and 7.0% as measured by total amount of phospholipid fatty acids (PLFA) and chloroform fumigation (CF), respectively. Within vegetation types, warming significantly increased microbial abundance in tundras by 15.0% and grasslands by 8.3%. Regarding to soil types, warming significantly increased microbial abundance in histosols by 16.5%. With reference to warming methods, warming by infrared-heaters and open top chambers (OTC) significantly increased microbial abundance by 4.6% and 15.1%, respectively, while warming by heating-cables decreased it by 16.7%. When grouped by warming time, diurnal and day warming significantly enhanced microbial abundances by 8.7% and 18.1%, respectively. In assessment of warming season, all-year warming treatments significantly enhanced microbial abundances by 7.5%. Significant between-groups heterogeneities were found when grouped by soil types, warming methods, and warming time.
Figure 1. Warming effects on microbial abundance.
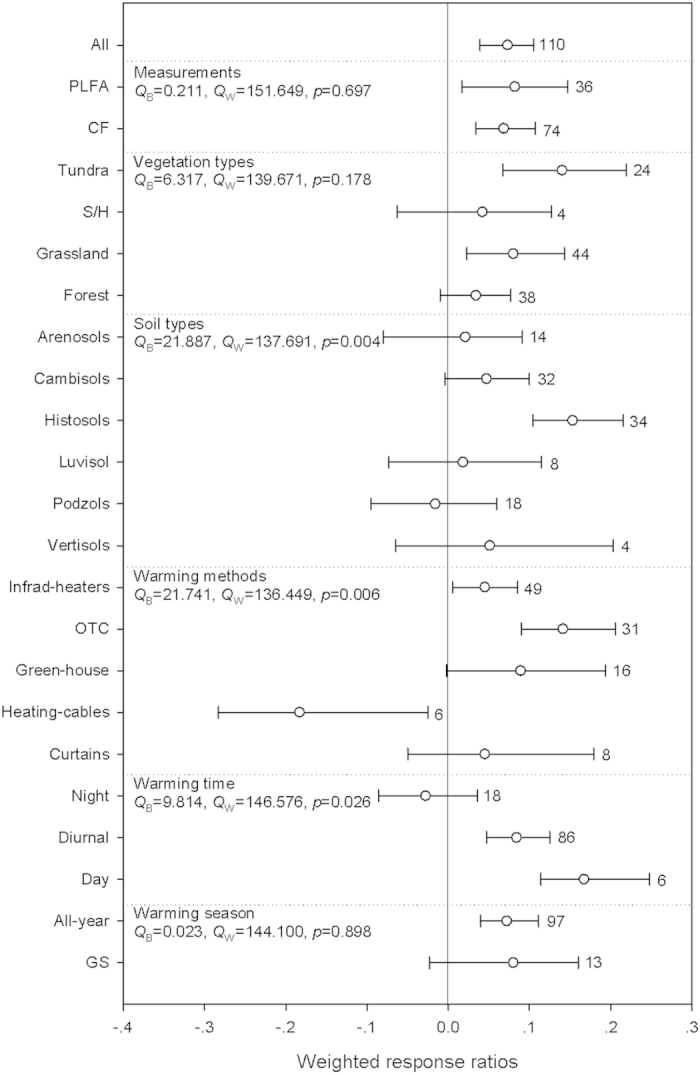
Error bars represented bootstrap 95% confidence intervals (CIs). The effect of warming was considered significant if the CI of the effect size did not cover zero. The sample size for each variable was shown next to the CI. The definition of QB and Qw can be found in materials and methods section, the p values indicate the difference within groups (Qw). The vertical solid line was drawn at mean effect size = 0. CF: chloroform fumigation, S/H: shrubland/heathland, GS: growing season, NGS: non-growing season. More information about the the percentage change for each variable and the weighted responses ratios in different warming magnitude for each variable can be found in supplemetary Table 1.
Table 1. Microbial abundance and publication bias to experimental warming.
| Group | n | RR++ | Bootstrap CI | Kendall’s tau rank | Spearman rank |
|---|---|---|---|---|---|
| Microbes* | 110 | 0.073 | 0.0389~0.1045 | 0.70018 | 0.71076 |
| Fungi | 120 | 0.0298 | −0.0265~0.085 | 0.93648 | 0.96853 |
| Bacteria | 82 | 0.0113 | −0.03~0.0531 | 0.98518 | 0.99266 |
| Archaea | 17 | −0.0754 | −0.2991~0.1258 | 0.81467 | 0.80809 |
| Specific microbial groups | |||||
| Gram positive bacteria | 39 | −0.0053 | −0.063~0.0593 | 0.02997 | 0.03449 |
| Gram negative bacteria | 35 | 0.0232 | −0.0549~0.1107 | 0.18435 | 0.27081 |
| Actinomycetes* | 14 | 0.1045 | 0.019~0.2012 | 0.58964 | 0.60127 |
| Arbuscular mycorrhizal fungi | 30 | 0.0276 | -0.086~0.1307 | 0.90306 | 0.84389 |
| Saprotrophic fungi * | 5 | 0.1909 | 0.1124~0.26 | n.a. | n.a. |
| Bacteria: Fungi* | 85 | 0.069 | 0.0078~0.1221 | 0.75148 | 0.8572 |
| GN: GP | 41 | −0.0291 | −0.0676~0.0146 | 0.65982 | 0.612 |
*Significant warming effect on group (p < 0.05). Boldface for the Kendall’s tau rank and Spearman rank indicate significant publication bias at p < 0.05. n: the number of studies included for the meta-analysis; RR++: weighted response ratio; CI: bootstrap confidence interval; GN: gram negative bacteria; GP: gram positive bacteria. See Fig. 1 for other information.
There was significant negative relationship between the response ration (RR) of microbial abundance and warming magnitude. But, there was no relationship between the RR of microbial abundance and warming duration, mean annual precipitation (MAP), latitude or elevation, even when grouped by different warming magnitudes (Supplementary Figs 1–3). In addition, warming also significantly increased the abundance of Actinomycetes, Saprotrophic fungi, and the ratio of Bacteria: Fungi (Table 1).
Fungi
Fungal abundances did not significantly respond to warming (Fig. 2, Supplementary Table 2). Regarding to vegetation types, warming significantly increased fungal abundances in Tundras by 9.5%. Within soil types, warming significantly increased fungal abundance in histosols by 13.2%, while warning decreased it in podzols by 20.9%. With respect to warming methods, warming by OTC and curtains significantly increased fungal abundances by 13.8% and 30.7%, respectively. The between-groups heterogeneity was not significant excepted when grouped by soil types (p = 0.040).
Figure 2. Warming effects on fungal abundance.
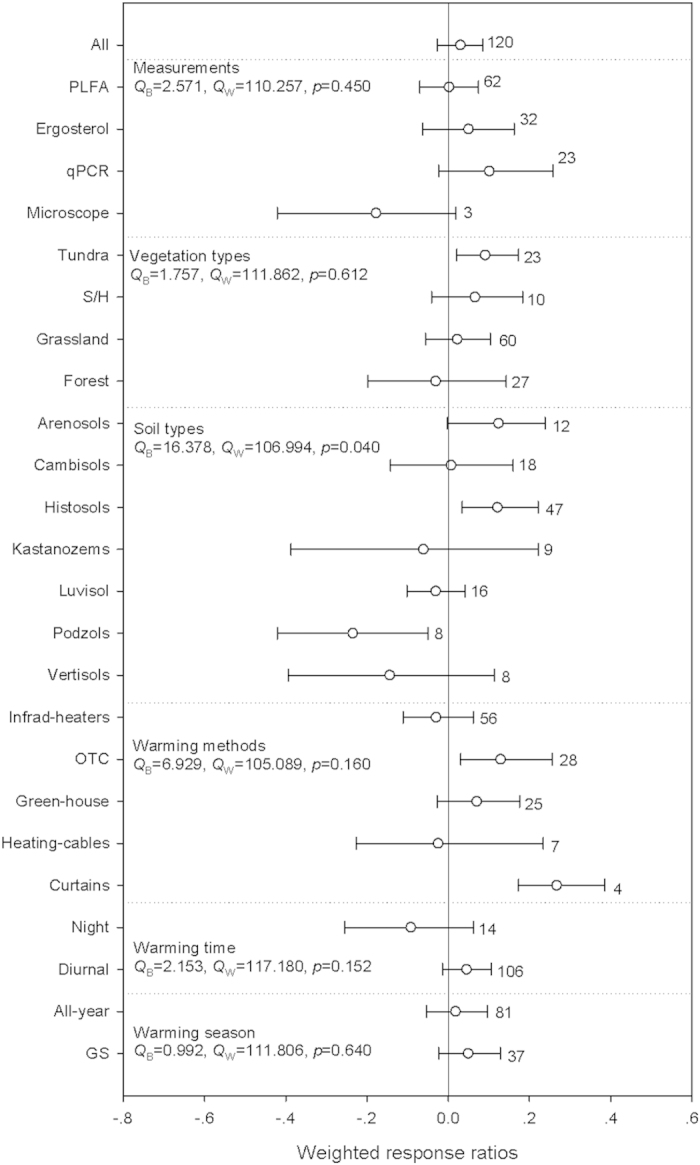
See Fig. 1 for detailed information. More information about the the percentage change for each variable and the weighted responses ratios in different warming magnitude for each variable can be found in supplemetary table 2.
There was a significant negative relationship between the RR of fungal abundance and warming magnitude and MAP. The relationship for MAP did not hold true when the results from the highest MAP site were excluded, but this relationship for MAP still hold true under medium warming magnitude. In addition, we did not find a significant relationship between the RR of fungal abundance and warming duration, latitude and elevation, even when grouped by different warming magnitudes (Supplementary Figs. 1–3).
Bacteria
Warming had no effect on overall bacterial abundance (Fig. 3, Supplementary Table 3), and no significant between-groups heterogeneity was found for the various measurement methods. The response of bacterial abundance differed among the vegetation types (p = 0.001), warming significantly increased bacterial abundance in tundras by 37.0%, but warming decreased it in forests by 9.3%. Regarding to vegetation types, warming significantly increased bacterial abundance in histosols by 13.3%, but decreased it in podzols by 14.3%, and there was significant between-groups heterogeneity for soil types. With reference to warming methods (p = 0.004), warming by OTC and curtains, significantly increased bacterial abundance by 15.5% and 21.8%, respectively, while warming by heating-cables significantly decreased it by 6.2%.
Figure 3. Warming effects on bacterial abundance.
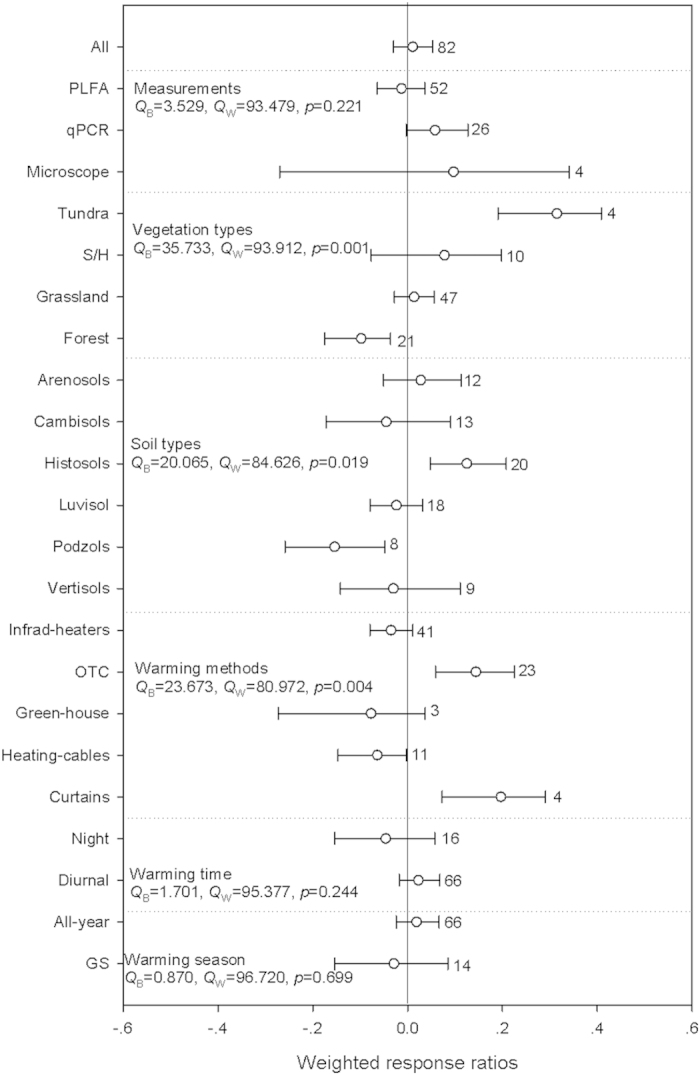
See Fig. 1 for detailed information. More information about the the percentage change for each variable and the weighted responses ratios in different warming magnitude for each variable can be found in supplemetary table 3.
There was a significant negative relationship between the RR of bacterial abundance and warming magnitudes. But, there was no relationship between the RR of bacterial abundance and warming duration, MAP, latitude or elevation, even when grouped by different warming magnitudes. When these analysis were grouped by warming magnitudes, significant relationship was only found for MAP under low warming magnitude (Supplementary Figs 1–3).
Archaea
Warming had no significant effect on Archaeal abundance (Fig. 4, Supplementary Table 4). We did not detect any significant difference between-groups heterogeneities in the analyses of Archaea abundance. In addition, there were no significant relationships between the RR of Achaea abundance and mean annual temperature (MAT), MAP, warming magnitude, warming duration, latitude and elevation, even when grouped by different warming magnitudes (Supplementary Figs 1–3).
Figure 4. Warming effects on Archaea abundance.
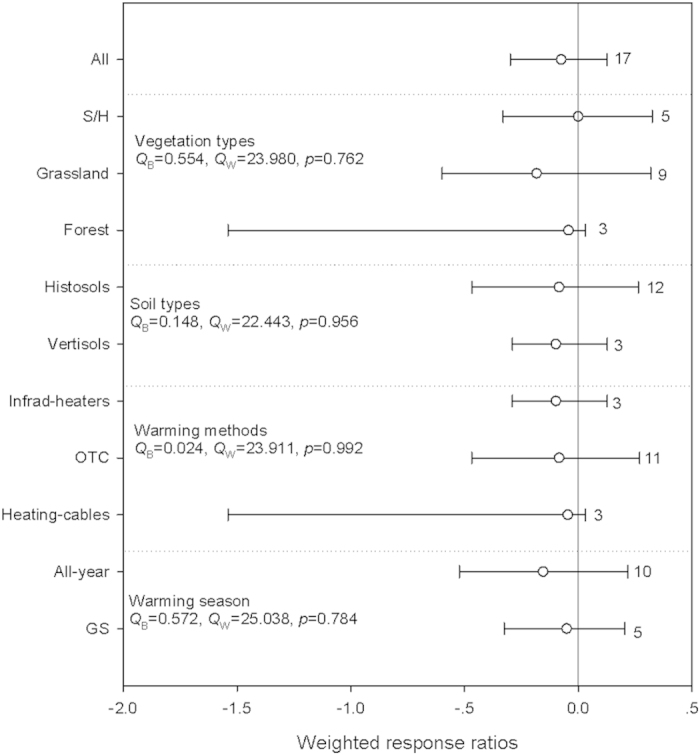
See Fig. 1 for detailed information. More information about the the percentage change for each variable and the weighted responses ratios in different warming magnitude for each variable can be found in supplemetary table 4.
Temperature sensitivity
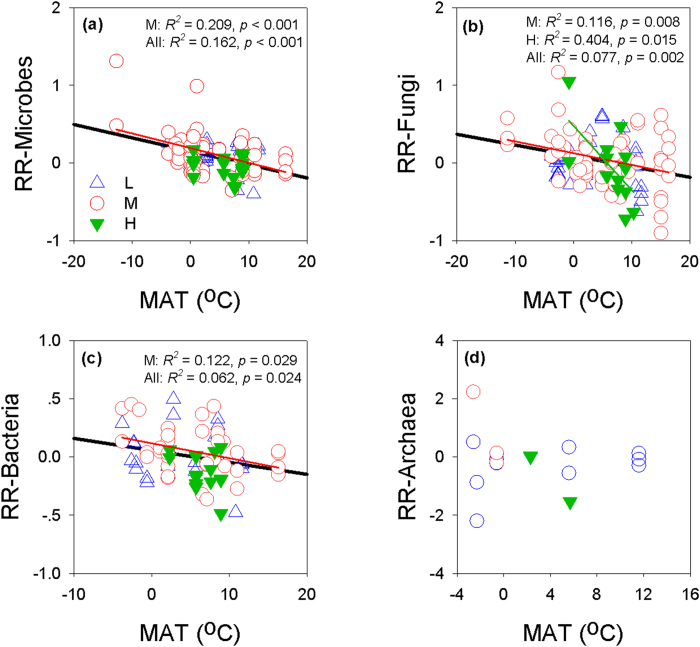
We found significant negative relationships between the RRs of microbial, fungal and bacterial abundances and MAT (Fig. 5 and Supplementary Fig. 2). For microbial and fungal abundances, these negative relationships still held true when the results from the coldest site, or the warmest site, or both sites were excluded. For bacterial abundance, this relationship remained significant when the results from the warmest region were excluded, but not when the results from coldest region were excluded. When grouped by warming magnitudes, significant relationships were found between the RRs of microbial, fungal and bacterial abundances and MAT for medium warming magnitude, and for fungal abundance under high warming magnitude. In addition, we did not detect any relationships between warming magnitude and MAT (Supplementary Fig. 4)
Figure 5. Relationships between the response ratios (RR) of the abundance of microbes (a), fungi (b), bacteria (c), and Archaea (d) and mean annual temperature (MAT).
L: low warming magnitude; M: medium warming magnitude; H: high warming magnitude; the black solid line show the overall relationship between the MAT and RR of microbial abundances to warming.
Soil respiration
A subset of studies included in this meta-analysis reported the effects of warming on SR in addition to the effects of warming on microbial, fungal and bacterial abundance. We found significant positive relationships between the RR of total microbial abundance and SR, this relationship still hold true when grouped by vegetation types and SR measurement methods. When these analyses were grouped by warming magnitudes, significant relationships were found for low and medium warming magnitudes, but not for the high warming magnitude (Fig. 6 and Supplementary Fig. 5).
Figure 6. Relationships between the response ratios (RR) of total microbial abundance and RR of soil respiration (SR).
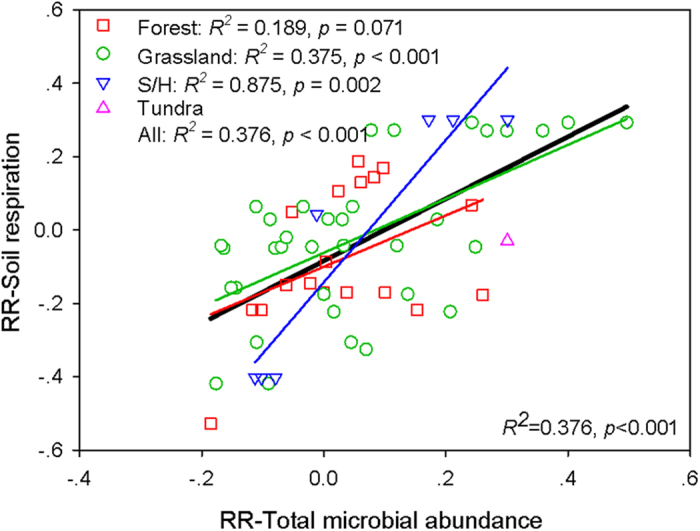
S/H: shrubland/heathland, the black solid line show the overall relationship between the RR of soil respiration and RR of total microbial abundance.
Discussion
Warming on microbial abundances
Our meta-analysis showed that warming had positive effect on microbial abundance and this effect tended to be stronger in colder regions (Figs 1 and 5 and Supplementary Fig. 2). A recent incubation study that involved the samples from wide-range of MAT gradients also showed that the stronger warming effects on microbes occurred in colder regions15. Microbes can adapt to low temperatures at the cellular level by reducing metabolic activities or by becoming dormant19, but in either cases, there would be competitive advantages to rapidly respond to rising temperatures23,24. This thermal sensitivity of microbial abundance suggest that climate warming would have non-uniform effects on microbial associated ecosystem functions, and this information should be incorporated into ESMs.
We also detected significant positive relationships between the RRs of dissolved organic carbon (DOC), soil labile nitrogen (SLN), soil total nitrogen (STN) and RR of microbial abundance, as well as significant negative relationships between the RRs of microbial, fungal, and bacterial abundances and substrate C: N ratio (Supplementary Fig. 6). This substrate-regulated the effects of warming on microbial abundances has been reported elsewhere15,20,25. Indeed, colder regions historically accumulate large amounts of SOM due to the thermodynamic constraints imposed by low temperatures26,27. The richer substrate in these regions would facilitate the stronger warming effects on microbial abundance in these colder regions when the temperature increased. This was mainly due to the warming-induced SOM decomposition in these regions would provide more substrates to further fuel microbial metabolic activities26,27.
It should be noted that warming magnitude may affect the relationships between the RR of microbial abundances and MAT (Fig. 5, Supplementary Figs 1 to 3, 5 and 6). Thus, one may reasonably concern that if more lower warming magnitude treatments occurred in colder regions, and then our key conclusion would be weakened. We therefore did a further regression to test how warming magnitudes were distributed along MAT, fortunately, we did not find any clear relationship between warming magnitude and MAT (Supplementary Fig. 4). These results suggest that despite warming magnitude may affect the relationships between the RR of microbial abundance and MAT, our key conclusion of stronger warming effects on microbial abundances in colder regions still hold true.
Archaea often were extremophiles living in harsh environments, and thus the Archaea abundance tended to be resistant to climate warming1,28,29. In addition, a subset of selected papers also showed that warming had significant effects on Actinomycetes and saprotrophic fungal abundance, but had no effects on others (Table 1). Changes in specific microbial groups could have significant implications for total microbial abundance3,30,31. Our knowledge of microbial abundance would benefit greatly from better understanding of soil specific microbial groups.
Microbial abundance and soil respiration
In support of our hypothesis, we found significant positive relationships between the RRs of SR and total microbial abundance (Fig. 6). These relationships were independent of vegetation types and SR measurement methods (Supplementary Fig. 5). Warming significantly stimulated microbial abundance as well as it synchronously accelerated decomposition32. This was further supported by our regression analysis that there were significant or marginally significant positive relationships between the RR of total microbial abundance and the RRs of phosphatase, glucosidase, phenol oxidase and N-Acetylglucosamine concentrations (Supplementary Fig. 6). Enhanced soil extracellular enzymes concentrations were expected to accelerate the degradation of litter and SOM, and that would in turn stimulate SR33,34. Consistently, three recent meta-analyses also demonstrated that warming significantly enhanced SR globally32,35,36. Our results therefore imply that the vast amounts of C accumulated in colder regions could be particular vulnerable to climate warming.
In addition, when grouped by warming magnitudes, this kind of significant positive relationship was not found for high warming magnitudes (Supplementary Fig. 5). This was partly due to the high warming magnitude induced water limitation may constrain the responses of microbial abundance and SR, or this was partly due to the publication bias (only 8 groups of data from the high warming magnitudes). Our results suggest that warming magnitude may affect the observed positive relationship between the RR of total microbial abundances and RR of SR.
Vegetation and soil types
Warming had positive effects on microbial, bacterial and fungal abundances in tundras and histosols (Figs 1, 2, 3). The preliminary explanation should be attributed to the low MAT in tundras (-2.4 °C) and histosols (3.1 °C) in the current study, which were consistent with the discussion above. Secondly, litter produced from plants in the tundras were decomposed faster than litter of woody and evergreen plants37,38, and histosols stored at least one third of terrestrial SOM39,40. These properties could eventually help bringing relative higher soil substrate availability and thus facilitate the positive microbial responses39. Our results therefore indicate that these ecosystems and warming-induced ecosystem shifting in this way could be potential C emissions hotspots in the future warming scenarios41,42.
Warming also significantly enhanced microbial abundance in grasslands, while decreased bacterial abundance in forests (Figs 1 and 3). This could be related to the relatively higher allocation of plant productivity to belowground in grasslands will bring more SOM inputs to the soils compared with other biomes43,44,45. We also detected significant negative responses of fungal and bacterial abundance in podzols (Figs 2 and 3), which soils were characterized by low nutrient concentrations, low soil-water holding capacities, and low pH. It is thus likely that the effects of warming on microbial abundance in podzols are constrained by these factors. When re-analyzed these differential responses in different warming magnitudes, we found that these negative responses were mainly resulted form the relative higher warming magnitudes with significant negative effects on fungal and bacterial abundances (Supplementary Tables 1 to 3). For example, 90.5% and 87.5% of bacterial abundances in forests and podzols were from the relative higher warming magnitudes. These results suggest that warming magnitudes may affect these differential responses of microbial abundances to warming among vegetation and soil types.
Warming protocols
We found significant negative relationships between warming magnitudes and RRs of microbial, fungal and bacterial abundances (Supplementary Fig. 1). Higher warming magnitudes were often with greater reductions in soil moisture, and thus had negative effects on soil microbial abundance (Supplementary Fig. 8). Soil warming by heating-cables were often with higher warming magnitudes and more pronounced reductions in soil moisture35. Whereas, OTC and curtains were usually associated with lower warming magnitudes and smaller negative effects on soil moisture. Our results indicate that warming magnitudes and warming methods should be considered when evaluating the effects of warming on microbial abundance.
No clear relationships were observed between the RR of microbial abundance and warming duration. One should interpret this with caution since only 11% of the observations involved warming treatments lasting more than 10 years. If warming were extended, neutral or negative responses might well be possible due to the depletion of substrate availability and limitation of soil moisture7,41. Therefore, our syntheses emphasize the necessity for long warming duration experiments. Diurnal and day warming had positive effects on microbial abundance, but not for night warming (Fig. 1). One possible consideration was that day warming promoted the C allocation to belowground, while night warming led to limitations of these substrates46,47.
In conclusion, warming had stronger impacts on microbial abundance in colder than warm regions, and there was a significant positive relationship between the RRs of SR and total microbial abundance. These results indicate that the large quantities of C stocked in colder regions could be more vulnerable than currently projected, and models should take the thermal sensitivity of microbial abundance into consideration when they are used for projecting future climate-carbon cycle feedbacks.
Methods
Source of data
We searched journal articles published before 2015 using the Web of Science (http://apps.webofknowledge.com/) and China Knowledge Resource Integrated Database (http://www.cnki.net/). Briefly, the following keywords and combinations were used for the searching: (1) “climate change” or “warming” or “temperature”; (2) “biota” or “microbe” or “microbial” or “fungi” or “bacterial” or “Archaea”; and (3) “terrestrial” or “soil” or “land”.
Based on the methods for meta-analysis48, studies were selected according to the following criteria: (1) all results were from field experiments. Specifically, we limited our data collections to studies ≥1 yr; (2) control and warming treatments had to be made at the same experimental sites. This allowed us to exclude variations induced by microclimate or vegetation or soil types; (3) data collection was limited to results in which means, stand deviations (SDs), and replicate numbers were reported. If standard errors (SEs) were reported, the following equation was used to calculate SD
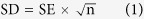 |
where n was the replicate numbers; (4) warming protocols (warming methods, warming magnitude, warming season, warming time, and warming duration) had to be clearly described or accessible from the cited articles; (5) if more than one field manipulation experiment were reported in the same article but with different environmental variables or vegetation types or soil types (e.g. experiments conducted under various geographical location or microclimate), each was regarded as an independent study; (6) if multiple measurements were measured in the same year, we only chose the last set of measurements; and (7) if the results were reported from different soil layers, we only included the results from the uppermost soil layer.
Data acquisition
In total, 64 published papers were selected from 45 study sites (Supplementary Fig. 9). For each selected paper, we recorded microbial, fungal, bacterial, and Archaea abundance or biomass. Meanwhile, we also recorded study site, latitude, longitude, elevation, MAT, MAP, vegetation types, soil types (http://www.fao.org), warming methods (infrared-heaters, OTC, green house, heating-cables, and curtains), warming time (day, night, and diurnal), warming season (all-year and growing season) from the selected papers or cited papers. When possible, DOC, SLN, STN, substrate C: N, soil extracellular enzymes, soil moisture, and SR were also recorded. We defined SR as the amount of soil CO2 release measured by soil chambers in the field studies or during laboratory incubations. This method has been successfully used in two previous meta-analyses to evaluate the responses of microbial abundance and SR to other global climate change factors21,22. If data were presented graphically, we used Engauge Digitizer 4.1 (http://digitizer.sourceforge.net) to digitize the data. For some of environmental variables that could not be acquired from the selected papers and the cited papers, we extracted these data from a global data base (http://www.worldclim.org/) using location information (latitude and longitude). If critical information could not be directly acquired from the selected articles or cited articles, the authors were contacted.
Microbial measurements
In the current meta-analysis, multiple types of microbial measurements were considered. Total microbial biomass or abundance were determined by CF49, or PLFA50. Fungal abundance was measured by microscopy, fungi PLFA, concentrations of ergosterol, or quantitative polymerase chain reaction analysis (qPCR). For bacteria, microscopy, bacteria PLFA, and qPCR were adopted, while for Archaea, the results were obtained by qPCR.
Data analysis
Meta-analysis approach was used to determine the significance of microbial responses to a variety of experimental warming treatments21,22,32,48,51. For each study, the response ratio (RR) was calculated as below:
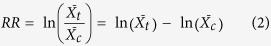 |
where 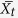 and
and 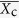 were means of warming and control treatments, respectively. The distribution of the RRs calculated in this way was typically nearly normal and the biases were minor48. The variance within each study was calculated using the means, replicate numbers, and SDs of both warming and control treatments. Details concerning the methods for calculating the variance can be found in32,48,51.
were means of warming and control treatments, respectively. The distribution of the RRs calculated in this way was typically nearly normal and the biases were minor48. The variance within each study was calculated using the means, replicate numbers, and SDs of both warming and control treatments. Details concerning the methods for calculating the variance can be found in32,48,51.
We used the MetaWin software (Sinauer AsSOMiates Inc., Sunderland, MA, USA) to calculate overall weighted response ration (RR++) and 95% bootstrap confidence intervals (CIs) for the all dataset and the grouped dataset. Significant responses (p < 0.05) were determined if CIs of RR++ did not overlap with 0. Warming-induced changes for a certain categorical group were calculated by
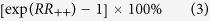 |
Random effects models in the meta-analysis were used to compare differences among groups in the ways similar to the analysis of variance framework. We sequentially compared RR++ among measurements, vegetation types, soil types, and warming protocols. A linear regression analysis was adopted to examine the relationships between the RRs of microbial, fungal, bacterial, and Archaea abundances and MAT, MAP, warming magnitude, warming duration, latitude, elevation, RRs of soil moisture, RRs of enzymatic concentrations, and RRs of SR. To test the impacts induced by warming magnitudes, we also did regression analysis for each similar warming magnitudes for all of the above regression analysis, respectively. The warming magnitude classification are consistent with previous meta-analysis32, in which the warming magnitude are grouped by low warming magnitude (<1 °C), medium warming magnitude (1~3 °C), and high warming magnitude (>3 °C).
Total heterogeneity (QT) was divided into within groups (QW) and between-group (QB) heterogeneities. For each categorical group, significant between-group difference was determined at p < 0.05. Due to the preference for publishing larger effects than smaller ones, we used Kendall’s tau rank and Spearman’s rank correlation to infer publication bias21,52,53. All the methods in the present meta-analysis have been successfully used in numerous previous studies21,51,53.
Additional Information
How to cite this article: Chen, J. et al. Stronger warming effects on microbial abundances in colder regions. Sci. Rep. 5, 18032; doi: 10.1038/srep18032 (2015).
Supplementary Material
Acknowledgments
We thanked the authors whose work was included in this meta-analysis. This study was supported by the Ministry of Science & Technology (2012BAH31B03), and the State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment of Chinese Academy of Sciences (SKLLQG1303). The authors gratefully acknowledged financial support from China Scholarship Council (award for one year’s study abroad at the University of Oklahoma). Contributions from Dr. Luo’s Eco-lab was financially supported by US Department of Energy, Terrestrial Ecosystem Sciences grant DE SC0008270 and US National Science Foundation (NSF) grant DBI 0850290, EPS0919466, DEB 0743778, DEB 0840964, and EF 1137293.
Footnotes
The authors declare no competing financial interests.
Author Contributions J.C., Y.L. and J.C. designed the research; J.C., M.L., J.L. and Z.S. performed the research and analyzed the data; J.C., L.J., S.S., J.X. and X.Z. wrote the paper.
References
- Zhou J. Z. et al. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat Clim Change 2, 106–110 (2012). [Google Scholar]
- Perveen N. et al. Priming effect and microbial diversity in ecosystem functioning and response to global change: a modeling approach using the SYMPHONY model. Global Change Biol 20, 1174–1190 (2014). [DOI] [PubMed] [Google Scholar]
- Creamer C. A. et al. Microbial community structure mediates response of soil C decomposition to litter addition and warming. Soil Biology and Biochemistry 80, 175–188 (2015). [Google Scholar]
- Friedlingstein P. et al. Climate-carbon cycle feedback analysis: Results from the C4MIP model intercomparison. J Climate 19, 3337–3353 (2006). [Google Scholar]
- Heimann M. & Reichstein M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451, 289–292 (2008). [DOI] [PubMed] [Google Scholar]
- Shao P., Zeng X., Moore D. J. & Zeng X. Soil microbial respiration from observations and Earth System Models. Environ Res Lett 8, 034034, doi: 10.1088/1748-9326/8/3/034034 (2013). [DOI] [Google Scholar]
- Luo Y., Wan S., Hui D. & Wallace L. L. Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413, 622–625 (2001). [DOI] [PubMed] [Google Scholar]
- Melillo J. et al. Soil warming and carbon-cycle feedbacks to the climate system. Science 298, 2173–2176 (2002). [DOI] [PubMed] [Google Scholar]
- Nie M. et al. Positive climate feedbacks of soil microbial communities in a semi-arid grassland. Ecol Lett 16, 234–241 (2013). [DOI] [PubMed] [Google Scholar]
- Hartley I. P., Hopkins D. W., Garnett M. H., Sommerkorn M. & Wookey P. A. Soil microbial respiration in arctic soil does not acclimate to temperature. Ecol Lett 11, 1092–1100 (2008). [DOI] [PubMed] [Google Scholar]
- Bradford M. A. et al. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett 11, 1316–1327 (2008). [DOI] [PubMed] [Google Scholar]
- Jonasson S., Michelsen A., Schmidt I. K. & Nielsen E. V. Responses in microbes and plants to changed temperature, nutrient, and light regimes in the arctic. Ecology 80, 1828–1843 (1999). [Google Scholar]
- Rinnan R., Michelsen A. & Baath E. Fungi Benefit from Two Decades of Increased Nutrient Availability in Tundra Heath Soil. Plos One 8, e56532, doi: 10.1371/journal.pone.0056532 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousk J., Smith A. R. & Jones D. L. Investigating the long-term legacy of drought and warming on the soil microbial community across five European shrubland ecosystems. Global Change Biol 19, 3872–3884 (2013). [DOI] [PubMed] [Google Scholar]
- Karhu K. et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 513, 81–84 (2014). [DOI] [PubMed] [Google Scholar]
- Schindlbacher A., Schnecker J., Takriti M., Borken W. & Wanek W. Microbial physiology and soil CO2 efflux after 9 years of soil warming in a temperate forest–no indications for thermal adaptations. Global Change Biol, doi: 10.1111/gcb.12996 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhala P. et al. Transplantation of organic surface horizons of boreal soils into warmer regions alters microbiology but not the temperature sensitivity of decomposition. Global Change Biol 17, 538–550 (2011). [Google Scholar]
- Frey S. D., Drijber R., Smith H. & Melillo J. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol Biochem 40, 2904–2907 (2008). [Google Scholar]
- Rousk J. & Bååth E. Growth of saprotrophic fungi and bacteria in soil. Fems Microbiol Ecol 78, 17–30 (2011). [DOI] [PubMed] [Google Scholar]
- Feng X. & Simpson M. J. Temperature and substrate controls on microbial phospholipid fatty acid composition during incubation of grassland soils contrasting in organic matter quality. Soil Biology and Biochemistry 41, 804–812 (2009). [Google Scholar]
- Treseder K. K. Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11, 1111–1120 (2008). [DOI] [PubMed] [Google Scholar]
- Holden S. R. & Treseder K. K. A meta-analysis of soil microbial biomass responses to forest disturbances. Front Microbiol 4, doi: 10.3389/Fmicb.2013.00163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietikåinen J., Pettersson M. & Bååth E. Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. Fems Microbiol Ecol 52, 49–58 (2005). [DOI] [PubMed] [Google Scholar]
- Rinnan R., Rousk J., Yergeau E., Kowalchuk G. A. & Baath E. Temperature adaptation of soil bacterial communities along an Antarctic climate gradient: predicting responses to climate warming. Global Change Biol 15, 2615–2625 (2009). [Google Scholar]
- Paungfoo-Lonhienne C. et al. Nitrogen fertilizer dose alters fungal communities in sugarcane soil and rhizosphere. Sci Rep-Uk 5, doi: 10.1038/srep08678 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuur E. A. et al. Vulnerability of permafrost carbon to climate change: Implications for the global carbon cycle. Bioscience 58, 701–714 (2008). [Google Scholar]
- Mackelprang R. et al. Metagenomic analysis of a permafrost microbial community reveals a rapid response to thaw. Nature 480, 368–371 (2011). [DOI] [PubMed] [Google Scholar]
- Yergeau E. et al. Shifts in soil microorganisms in response to warming are consistent across a range of Antarctic environments. Isme J 6, 692–702 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J. et al. Archaeal abundance in post-mortem ruminal digesta may help predict methane emissions from beef cattle. Sci Rep-Uk 4, doi: 10.1038/srep05892 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle D. A. & Zackrisson O. Effects of species and functional group loss on island ecosystem properties. Nature 435, 806–810 (2005). [DOI] [PubMed] [Google Scholar]
- Yue H. et al. The microbe-mediated mechanisms affecting topsoil carbon stock in Tibetan grasslands. The ISME journal, doi: 10.1038/ismej.2015.19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M. et al. Responses of ecosystem carbon cycle to experimental warming: a meta-analysis. Ecology 94, 726–738 (2013). [DOI] [PubMed] [Google Scholar]
- Allison S. D. & Treseder K. K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Global Change Biol 14, 2898–2909 (2008). [Google Scholar]
- Burns R. G. et al. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biology and Biochemistry 58, 216–234 (2013). [Google Scholar]
- Rustad L. E. et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126, 543–562 (2001). [DOI] [PubMed] [Google Scholar]
- Wang X. et al. Soil respiration under climate warming: differential response of heterotrophic and autotrophic respiration. Global Change Biol 20, 3229–3237 (2014). [DOI] [PubMed] [Google Scholar]
- Cornwell W. K. et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11, 1065–1071 (2008). [DOI] [PubMed] [Google Scholar]
- Pochikalov A. & Karelin D. A field study of tundra plant litter decomposition rate via mass loss and carbon dioxide emission: The role of biotic and abiotic controls, biotope, season of year, and spatial-temporal scale. Biology Bulletin Reviews 5, 1–16 (2015). [PubMed] [Google Scholar]
- Sistla S. A. et al. Long-term warming restructures Arctic tundra without changing net soil carbon storage. Nature 497, 615–618 (2013). [DOI] [PubMed] [Google Scholar]
- Bouskill N., Riley W. & Tang J. Meta-analysis of high-latitude nitrogen-addition and warming studies implies ecological mechanisms overlooked by land models. Biogeosciences 11, 6969–6983 (2014). [Google Scholar]
- Dorrepaal E. et al. Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 460, 616–619 (2009). [Google Scholar]
- Vicca S. et al. No signs of thermal acclimation of heterotrophic respiration from peat soils exposed to different water levels. Soil Biology and Biochemistry 41, 2014–2016 (2009). [Google Scholar]
- Chen J., Shi W. & Cao J. Effects of Grazing on Ecosystem CO2 Exchange in a Meadow Grassland on the Tibetan Plateau During the Growing Season. Environ Manage 55, 347–359 (2015). [DOI] [PubMed] [Google Scholar]
- Scurlock J. M., Johnson K. & Olson R. J. Estimating net primary productivity from grassland biomass dynamics measurements. Global Change Biol 8, 736–753 (2002). [Google Scholar]
- Litton C. M., Raich J. W. & Ryan M. G. Carbon allocation in forest ecosystems. Global Change Biol 13, 2089–2109 (2007). [Google Scholar]
- Peng S. et al. Asymmetric effects of daytime and night-time warming on Northern Hemisphere vegetation. Nature 501, 88–92 (2013). [DOI] [PubMed] [Google Scholar]
- Atkin O. K. & Tjoelker M. G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in plant science 8, 343–351 (2003). [DOI] [PubMed] [Google Scholar]
- Hedges L. V., Gurevitch J. & Curtis P. S. The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156 (1999). [Google Scholar]
- Vance E., Brookes P. & Jenkinson D. An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry 19, 703–707 (1987). [Google Scholar]
- Frostegård Å. & Bååth E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fert Soils 22, 59–65 (1996). [Google Scholar]
- Luo Y., Hui D. & Zhang D. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology 87, 53–63 (2006). [DOI] [PubMed] [Google Scholar]
- Begg C. B. Publication bias. The handbook of research synthesis 25, 299–409 (1994). [Google Scholar]
- Rosenberg M. S., Adams D. C. & Gurevitch J. MetaWin: statistical software for meta-analysis. (Sinauer Associates Sunderland, Massachusetts, USA, 2000). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.