Abstract
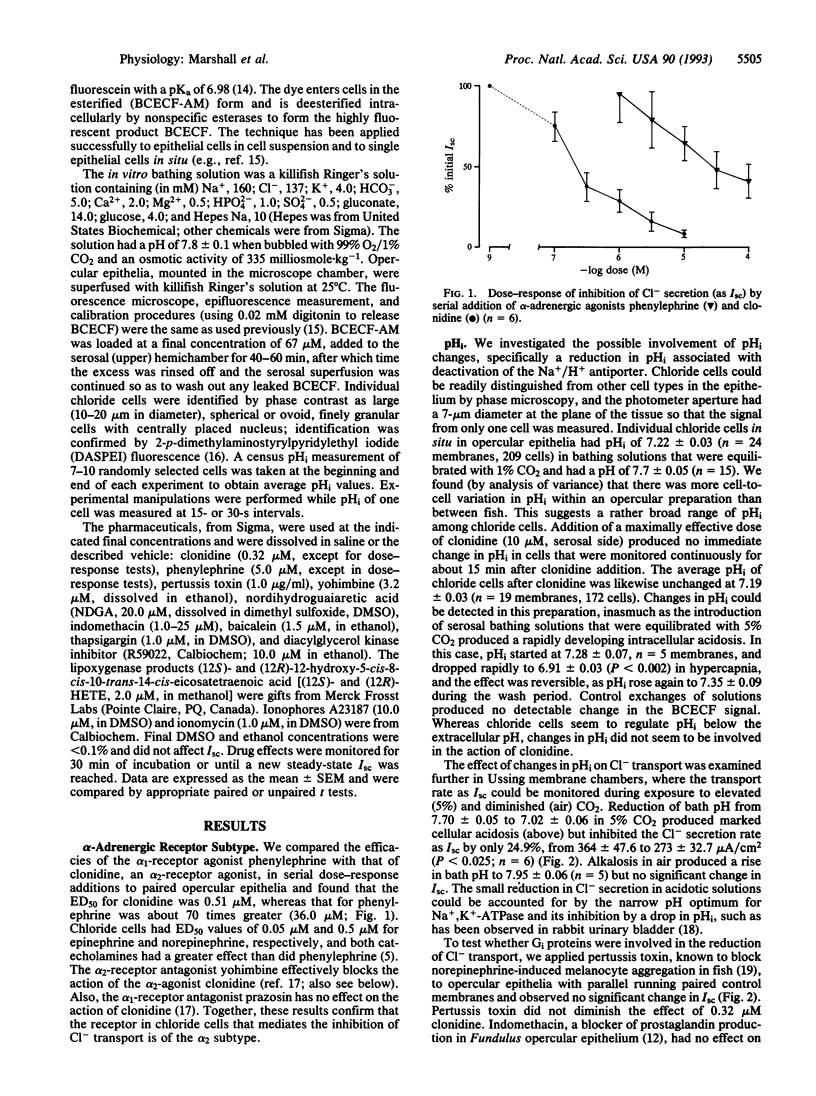
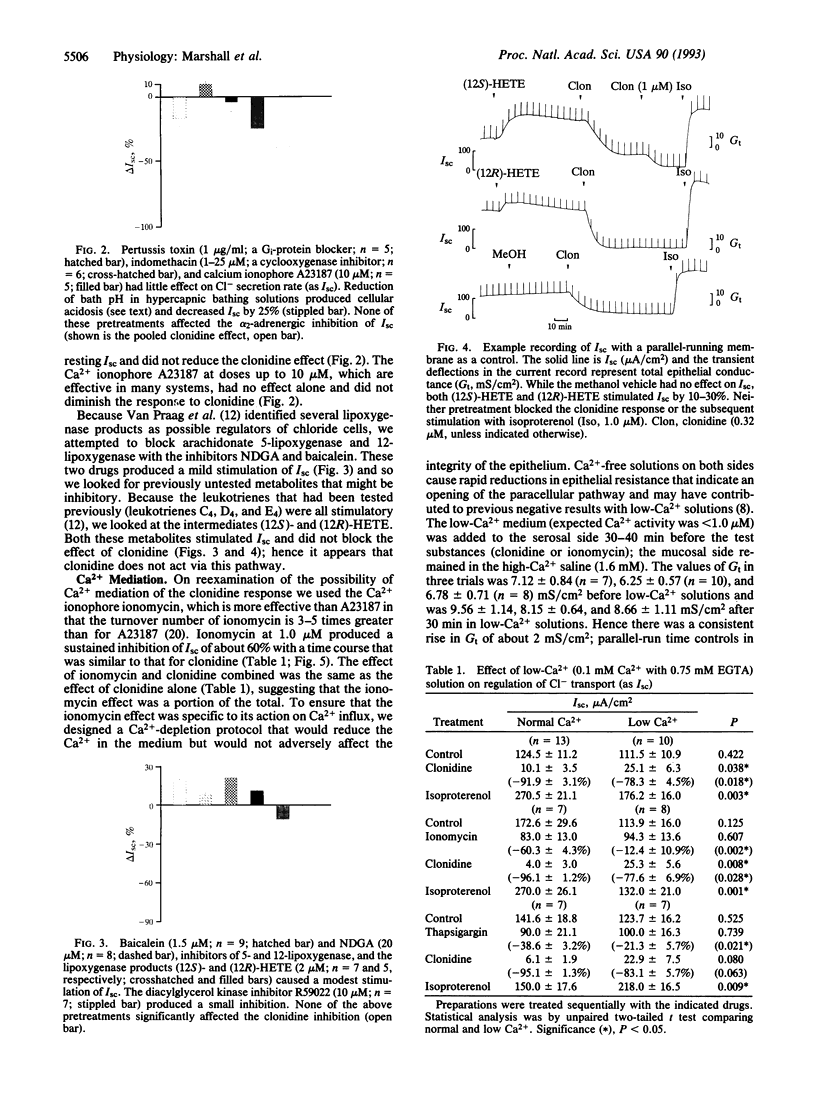
We isolated the opercular epithelium of sea-water killifish (Fundulus heteroclitus) to study the mediation of catecholamine inhibition of Cl- secretion. The receptors are alpha 2-adrenergic, as they have a high affinity for the alpha 2-adrenergic agonist clonidine over phenylephrine and clonidine action is blocked by yohimbine. Pertussis toxin and indomethacin did not block the clonidine effect; hence inhibitory guanine nucleotide-binding proteins (Gi proteins) and prostaglandins (respectively) are not involved. Intracellular pH (pHi) of single chloride cells was measured microspectrofluorometrically and resting pHi was 7.22 +/- 0.03. However, pHi was unaffected by clonidine; hence pHi and Na+/H+ exchange are not involved. The lipoxygenase inhibitors nordihydroguaiaretic acid and baicalein and the lipoxygenase products (12S)- and (12R)-12-hydroxyeicosatetraenoic acid stimulated Cl- secretion. Protein kinase C is an unlikely site of action because the diacylglycerol kinase inhibitor R59022 had no effect alone and did not block the clonidine effect. Ionomycin (1 microM) in normal but not low-Ca2+ solutions mimicked the action of clonidine and both inhibitions were reversible by isoproterenol. Thapsigargin, a releaser of intracellular Ca2+, inhibited Cl- secretion and this effect was reduced in low-Ca2+ solutions. Low-Ca2+ solutions also blunted but did not block entirely the clonidine response, indicating that the primary Ca2+ release was from intracellular stores. Whereas alpha 1-adrenergic receptors commonly act via the Ca2+/inositol trisphosphate pathway, to our knowledge this is the first report of a Ca(2+)-mediated alpha 2-adrenergic response in a nonmammalian vertebrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron B. M., Siegel B. W. Alpha 2-adrenergic and muscarinic cholinergic receptors have opposing actions on cyclic AMP levels in SK-N-SH human neuroblastoma cells. J Neurochem. 1989 Aug;53(2):602–609. doi: 10.1111/j.1471-4159.1989.tb07376.x. [DOI] [PubMed] [Google Scholar]
- Eaton D. C., Hamilton K. L., Johnson K. E. Intracellular acidosis blocks the basolateral Na-K pump in rabbit urinary bladder. Am J Physiol. 1984 Dec;247(6 Pt 2):F946–F954. doi: 10.1152/ajprenal.1984.247.6.F946. [DOI] [PubMed] [Google Scholar]
- Gesek F. A., Strandhoy J. W. Dual interactions between alpha 2-adrenoceptor agonists and the proximal Na(+)-H+ exchanger. Am J Physiol. 1990 Mar;258(3 Pt 2):F636–F642. doi: 10.1152/ajprenal.1990.258.3.F636. [DOI] [PubMed] [Google Scholar]
- Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985 Jul;65(3):760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- Guan Y. Y., Kwan C. Y., Daniel E. E. Does in vitro activation of postsynaptic alpha 2-adrenoceptor utilize intracellular Ca2+ for contraction in dog saphenous vein? Can J Physiol Pharmacol. 1989 Sep;67(9):1086–1091. doi: 10.1139/y89-171. [DOI] [PubMed] [Google Scholar]
- Karlsson J. O., Andersson R. G., Askelöf P., Elwing H., Granström M., Grundström N., Lundström I., Ohman L. The melanophore aggregating response of isolated fish scales: a very rapid and sensitive diagnosis of whooping cough. FEMS Microbiol Lett. 1991 Aug 1;66(2):169–175. doi: 10.1111/j.1574-6968.1991.tb04860.x. [DOI] [PubMed] [Google Scholar]
- Kauffman R. F., Taylor R. W., Pfeiffer D. R. Cation transport and specificity of ionomycin. Comparison with ionophore A23187 in rat liver mitochondria. J Biol Chem. 1980 Apr 10;255(7):2735–2739. [PubMed] [Google Scholar]
- Klyce S. D., Crosson C. E. Transport processes across the rabbit corneal epithelium: a review. Curr Eye Res. 1985 Apr;4(4):323–331. doi: 10.3109/02713688509025145. [DOI] [PubMed] [Google Scholar]
- Marshall W. S., Bryson S. E. Intracellular pH regulation in trout urinary bladder epithelium: Na(+)-H+(NH4+) exchange. Am J Physiol. 1991 Sep;261(3 Pt 2):R652–R658. doi: 10.1152/ajpregu.1991.261.3.R652. [DOI] [PubMed] [Google Scholar]
- Marshall W. S. Independent Na+ and Cl- active transport by urinary bladder epithelium of brook trout. Am J Physiol. 1986 Feb;250(2 Pt 2):R227–R234. doi: 10.1152/ajpregu.1986.250.2.R227. [DOI] [PubMed] [Google Scholar]
- Marshall W. S., Nishioka R. S. Relation of mitochondria-rich chloride cells to active chloride transport in the skin of a marine teleost. J Exp Zool. 1980 Nov;214(2):147–156. doi: 10.1002/jez.1402140204. [DOI] [PubMed] [Google Scholar]
- May S. A., Degnan K. J. Converging adrenergic and cholinergic mechanisms in the inhibition of Cl secretion in fish opercular epithelium. J Comp Physiol B. 1985;156(2):183–189. doi: 10.1007/BF00695772. [DOI] [PubMed] [Google Scholar]
- May S. A., Degnan K. J. cAMP-mediated regulation of chloride secretion by the opercular epithelium. Am J Physiol. 1984 May;246(5 Pt 2):R741–R746. doi: 10.1152/ajpregu.1984.246.5.R741. [DOI] [PubMed] [Google Scholar]
- Middleton J. P., Albers F. J., Dennis V. W., Raymond J. R. Thapsigargin demonstrates calcium-dependent regulation of phosphate uptake in HeLa cells. Am J Physiol. 1990 Oct;259(4 Pt 2):F727–F731. doi: 10.1152/ajprenal.1990.259.4.F727. [DOI] [PubMed] [Google Scholar]
- Negulescu P. A., Machen T. E. Intracellular Ca regulation during secretagogue stimulation of the parietal cell. Am J Physiol. 1988 Jan;254(1 Pt 1):C130–C140. doi: 10.1152/ajpcell.1988.254.1.C130. [DOI] [PubMed] [Google Scholar]
- Nilsson M., Hansson E., Rönnbäck L. Adrenergic and 5-HT2 receptors on the same astroglial cell. A microspectrofluorimetric study on cytosolic Ca2+ responses in single cells in primary culture. Brain Res Dev Brain Res. 1991 Nov 19;63(1-2):33–41. doi: 10.1016/0165-3806(91)90064-p. [DOI] [PubMed] [Google Scholar]
- Paradiso A. M., Tsien R. Y., Machen T. E. Na+-H+ exchange in gastric glands as measured with a cytoplasmic-trapped, fluorescent pH indicator. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7436–7440. doi: 10.1073/pnas.81.23.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt J. D., Connolly T. M., Cragoe E. J., Limbird L. E. Evidence that Na+/H+ exchange regulates receptor-mediated phospholipase A2 activation in human platelets. J Biol Chem. 1986 Jul 5;261(19):8667–8673. [PubMed] [Google Scholar]
- Tabcharani J. A., Low W., Elie D., Hanrahan J. W. Low-conductance chloride channel activated by cAMP in the epithelial cell line T84. FEBS Lett. 1990 Sep 17;270(1-2):157–164. doi: 10.1016/0014-5793(90)81257-o. [DOI] [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Van Praag D., Farber S. J., Minkin E., Primor N. Production of eicosanoids by the killifish gills and opercular epithelia and their effect on active transport of ions. Gen Comp Endocrinol. 1987 Jul;67(1):50–57. doi: 10.1016/0016-6480(87)90204-8. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Inhibition of chloride secretion by furosemide in canine tracheal epithelium. J Membr Biol. 1983;71(3):219–226. doi: 10.1007/BF01875463. [DOI] [PubMed] [Google Scholar]


