Abstract
Children with Down syndrome (DS) have increased susceptibility to infections and a high frequency of leukemia and autoimmune disorders, suggesting that immunodeficiency and immune dysfunction are integral parts of the syndrome. A reduction in B-cell numbers has been reported, associated with moderate immunodeficiency and normal immunoglobulin levels. Here, we compared B-cell populations of 19 children with DS with those in healthy age-matched controls. We found that all steps of peripheral B-cell development are altered in DS, with a more severe defect during the later stages of B-cell development. Transitional and mature-naïve B-cell numbers are reduced by 50% whereas switched memory B cells represent 10–15% of the numbers in age-matched controls. Serum IgM levels were slightly reduced, but all other immunoglobulin isotypes were in the normal range. The frequency of switched memory B cells specific for vaccine antigens was significantly lower in affected children than in their equivalently vaccinated siblings. In vitro switched memory B cells of patients with DS have an increased ability to differentiate into antibody-forming cells in response to TLR9 signals. Tailored vaccination schedules increasing the number of switched memory B cells may improve protection and reduce the risk of death from infection in DS.
Keywords: B cells, Down syndrome, IgM memory, Switched memory, TLR9, Vaccine
Introduction
Down syndrome (DS) is associated with increased susceptibility to bacterial and viral infections [1,2], and a high frequency of leukemia [3,4] and autoimmune disorders, such as celiac disease [5–7], type I diabetes [8,9], and acquired hypothyroidism [10,11]. In spite of a dramatic improvement of life expectancy over the last half-century [12], the risk of premature mortality remains high in DS mainly because of respiratory infections and leukemia [13]. These features already suggested in the 1970s that immunodeficiency is an integral part of the syndrome [14–17]. Indeed, this has been supported by the findings that the thymus, even in newborns, is small and has an abnormal structure [18], the absolute number of circulating lymphocytes is decreased and antibody responses to vaccine antigens such as oral polio, acellular pertussis, tetanus, hepatitis B, and influenza A are low [19–21]. The absolute number of T lymphocytes has been reported to be low at all ages [22] or decreased during the first 2 years of age approaching normal levels thereafter [23].
A decreased number of circulating B cells is a constant finding in DS [24] with variable levels of serum immunoglobulin [25]. Discrepancies between the results of aspects of these studies may derive from the small number of subjects included, differences in the age groups considered, poor matching between DS and controls, inclusion of institutionalized subjects with DS and, in particular, from the use of old and poorly reliable phenotyping techniques that have subsequently greatly improved over recent years driven by the remarkable advances in flow cytometric methods.
The most common symptoms reported in children with DS are infections of the respiratory tract suggesting a B-cell defect. Diseases related to T-cell deficiency, such as infection with intracellular microorganism, fungi, and opportunistic pathogens are rare [26]. In order to verify whether a defined B-cell defect may be associated with DS, we studied in detail the B-cell compartment of 19 children with DS and compared them with 19 age-matched controls (CTR).
In the peripheral blood, four major populations of B cells can be identified: transitional B cells, representing recent BM emigrants, mature-naive B cells, IgM memory B cells, and switched memory B cells. The cells that we conventionally call IgM memory B cells also express IgD on their surface, but a minor population of IgM-only memory B cells has also been described [27]. The latter is very small in both CTR and DS children (less than 1% of all memory B cells) and its function was not analyzed in this study separately from that of IgM memory B cells. IgM memory, also known as marginal zone B cells, is probably generated through a T-cell-independent mechanism whereas switched memory B cells derive from the T-dependent germinal center reaction [28]. Since each of these subpopulations has a different origin and defined function, we carried out a detailed analysis of these B-cell subpopulations and measured the in vitro proliferation and immunoglobulin production of B cells isolated from peripheral blood isolated from a group of 6- to 12-year-old children with DS compared to age-matched CTR. The results of this study show that switched memory B cells are dramatically reduced in number but have an increased ability to differentiate into antibody-producing cells in vitro.
Results
Reduction of switched memory B cells in DS
Studies have shown that in DS children the absolute number of circulating lymphocytes is significantly lower than in CTR children [29,30] and that in the T-cell compartment only naïve CD4++T-cell numbers are reduced, whereas naïve CD8 T-cell and memory T-cell numbers (both CD8 and CD4) are in the normal range [23]. These observations were confirmed in the children included in our analysis (Table 1 and 2 and Supporting Information Fig. 1).
Table 1.
Clinical and demographic feature of Down's syndrome (DS) patients
| N | % | |
|---|---|---|
| Total DS patients | 19 | |
| Male | 9 | 47.4 |
| Female | 10 | 52.6 |
| Recurrent infections | 17/19 | 89.5 |
| Infections of the superior respiratory tract | 16 | 94.1 |
| Pneumonia | 2 | 11.8 |
| Gastroenteritis | 2 | 11.8 |
| Urinary tract infections | 1 | 5.9 |
| Hospitalization for infections | 8/19 | 42.1 |
| Hospitalization for gastroenteritis | 3/8 | 37.5 |
| Hospitalization because of lower respiratory tract infection | 3/8 | 37.5 |
| Hospitalization because of upper respiratory tract infection | 2/8 | 25 |
| Congenital heart disease | 13/19 | 68.4 |
| Heart surgery | 2 | 15.4 |
| Congenital heart disease with recurrent infections | 12/13 | 92.3 |
| Autoimmune diseases | 3/19 | 15.8 |
| Hypothyroidism | 2 | 66.7 |
| Celiac disease | 1 | 33.3 |
| Hematologic problems | 2/19 | 10.5 |
| Transient abnormal myelopoiesis | 1 | 5.3 |
| Polycitemia | 1 | 5.3 |
Table 2.
Immunological parameters
| DS median (range) | CTR median (range) | Mann–Whitney p-value | |
|---|---|---|---|
| Lymph | 1880 (870–3810) | 2370 (750–4570) | 0.009* |
| B cells | 170 (40–290) | 343.62 (160–940) | 0.03* |
| Transitional | 20.05 (4–49) | 37 (9–140) | 0.0003* |
| Mature | 116 (6–320) | 224 (20–731) | 0.0001* |
| Memory B | 10 (4–30) | 50 (20–110) | 0.05* |
| IgM memory | 25 (5–80) | 9 (0–30) | 0.007* |
| Switched memory | 26 (10–210) | 4 (0.1–9) | 0.01* |
| T cells (CD3) | 1405 (490–2830) | 1915.1 (1071–2915) | 0.015* |
| CD4 | 665 (260–960) | 957.9 (483–1818) | 0.005* |
| CD8 | 581 (226–826) | 660 (210–1860) | 0.18 |
| CD4-RA | (107–850) | 554.31 (53–1434) | 0.002* |
| CD4-RO | 338.21 (100–525) | 328.8 (150–432) | 0.9 |
| CD8-RA | 363.34 (99–1180) | 432.435 (173–758) | 0.6 |
| CD8-RO | 213.77 (20–853) | 186 (68–500) | 0.9 |
| Serum IgM | 55 (30.7–110.8) | 108.7 (38–187) | 0.001* |
| Serum IgA | 120.2 (11.4–278.2) | 107.1 (82.6–227.9) | 0.4 |
| Serum IgG | 1088 (667–1558) | 1193 (583–1517) | 0.5 |
| Salivary IgA | 63.9 (21.6–194.4) | 63 (21.6–121.5) | 0.9 |
Absolute cell numbers are expressed as cells/mm3. The range is mentioned between brackets.
Immunoglobulin levels are measured in mg/dl. The range is mentioned between brackets.
*significant difference.
The three major subsets of CD19+ B cells in the peripheral blood were identified in our study by staining with antibodies to CD24, CD38, CD27, and IgM (Fig.1A). Transitional B cells express high levels of CD24 and CD38 (trans), the mature-naive population (mature) is CD24+ CD38+ CD27− and memory B cells are CD24++ CD38− and express CD27. IgM is detected on the surface of IgM memory B cells (IgM). Switched memory B cells (switched) are IgM−. All B-cell populations in the peripheral blood (naïve, transitional, switched memory, and memory B cells) were significantly reduced in DS children (Fig.1A and B).
Figure 1.
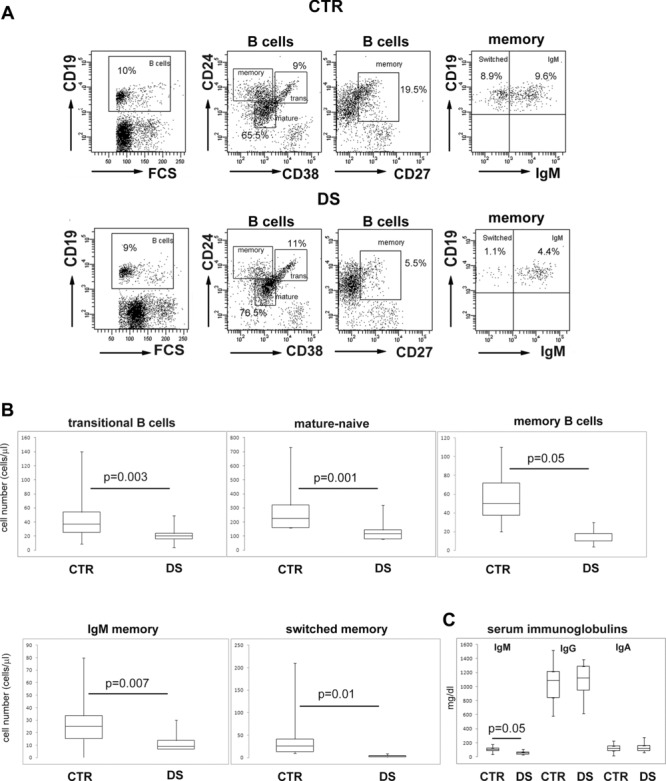
Reduction of switched memory B-cell numbers. (A) Flow cytometric analysis of B-cell populations in the peripheral blood of healthy control (CTR, top row), and Down syndrome (DS, lower row) children. Plots showing gating selection of CD19+ B cells and identification of the transitional, mature-naive and memory populations are shown. Memory B cells express CD27. IgM (IgM+) and switched (IgM−) memory B cells were identified by the expression of IgM on gated CD27+ B cells. One representative example of the analysis performed on all 19 DS and 19 CTR children is shown. (B) Absolute numbers of transitional, mature-naive, memory, IgM, and switched memory B-cell populations were determined by flow cytometry. (C) Total serum IgM, IgG, and IgA levels in CTR and DS children were measured by ELISA. (B, C) Data are shown as median + IQR of 19 CTR and 19 DS donors. Statistical significance was calculated by the Mann–Whitney U-test.
B-cell development in the periphery results in the differentiation of short-lived transitional B cells into either mature/naïve B cells or IgM memory B cells. The germinal center reaction is absolutely required for the generation of switched memory B cells from mature/naïve B cells activated by antigen in the lymphoid follicle. Circulating transitional B-cell numbers in DS children were roughly half the levels seen in CTR children (Table 2, Fig.1B), suggesting reduced BM production in DS children. The number of mature/naïve B cells was in DS children also 50% of that seen in the CTR children. A further restriction of the B-cell compartment in DS was observed at the memory stage with the number of IgM memory B cells being 30% of those seen in the CTR children; however, the most important alteration was observed in the switched memory pool. The median number of switched memory B cells in DS children was 14% of that observed in the CTR group (Fig.1B, Table 2). The alterations of B-cell numbers were a constant finding in affected children and, for this reason, all differences between the DS and CTR groups were highly significant.
A B-cell phenotype characterized by an extreme reduction of switched memory B cells is observed in patients with common variable immune deficiency (CVID) [31]. In contrast to CVID, however, immunoglobulin levels are not dramatically reduced in DS [29]. In our patients, IgG and IgA levels were comparable to those of the CTR group and only IgM was significantly reduced, but nonetheless remained in the age-normal range (31–208 mg/dL; Table 2, Fig.1C).
Normal CpG-induced proliferative response and immunoglobulin production
As memory B-cell numbers appear to be particularly reduced in DS, we measured their function by the stimulation in vitro with the TLR9 ligand CpG which induces memory B-cell proliferation and differentiation into plasma cells. In DS children, after 7 days of such stimulation, the frequency of cells that had proliferated was comparable to the frequency observed in the CTR group (Fig.2A and B) as demonstrated by the proportion of cells that had a reduced 5-chloromethylfluorescein diacetate (CMFDA) staining (Fig.2A). In culture after CpG stimulation, plasma cells, identified by the high expression of CD27 and CD38, can be either IgM+ (IgM plasma cells) or IgM− (switched plasma cells). We found that the frequency of total plasma cells was comparable in the CTR and DS groups (Fig.2B). In particular, switched plasma cell numbers (Fig.2A) were not significantly reduced in the DS as compared with the CTR group (Fig.2C). In the culture supernatants of stimulated cells, IgM, IgA, and IgG antibodies were all reduced but never significantly (Fig.2D). The observation that both proliferation and antibody production were not significantly reduced in DS children was unexpected because memory B-cell and, in particular, switched memory B-cell numbers are dramatically diminished in the peripheral blood of such children (Fig.1).
Figure 2.
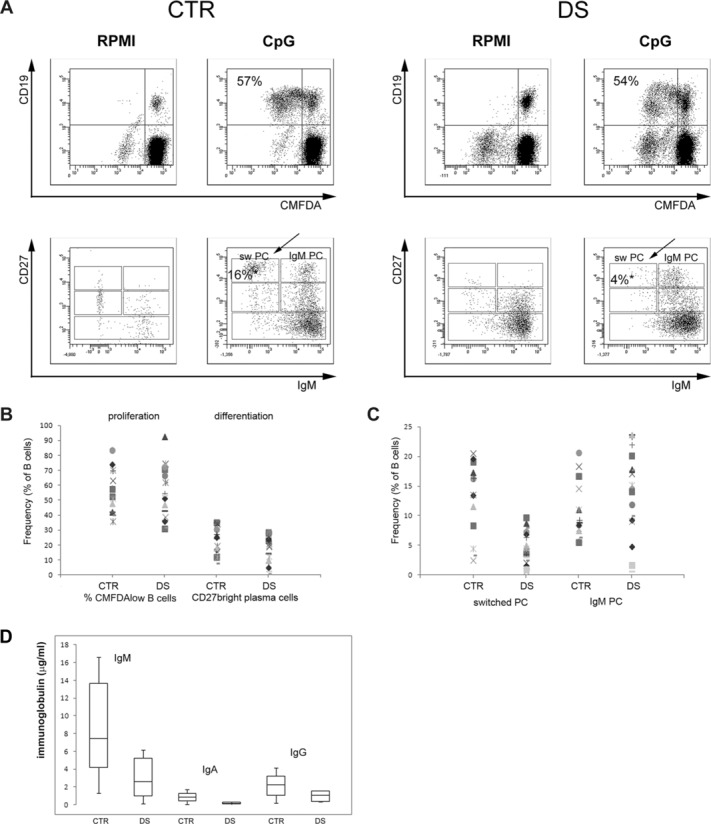
In vitro proliferation and differentiation of B-cell populations. (A) PBMCs isolated from CTR and DS children were cultured for 7 days without (RPMI) or with CpG (CpG). Proliferation was measured by the loss of CMFDA fluorescence in daughter cells and expressed as percentage of proliferating (CMFDAlow) CD19+ B cells by cytofluorimetry (top). Plasma cells of IgM and switched isotype were identified by cytofluorimetric analysis, CD27 versus IgM staining of cells of CD19+ cells is shown (bottom). CD27+++ IgM− B cells are switched plasma cells (sw PC, indicated by the arrow). The plot shows one representative example of the analysis performed on all 19 DS and 19 CTR children. (B) Frequency of proliferating B cells for each of the 19 CTR and 19 DS children. Differentiation is expressed as frequency of total CD27+++ CD38+++ plasma cells obtained in culture. Proliferation was measured as indicated in (A). (C) Frequency of switched and IgM plasma cells detected in culture for each CTR and DS child measured as indicated in (A). (D) The concentration of IgM, IgG, and IgA was measured in the supernatants of CpG-stimulated cultures collected at day 7. Data are shown as median + IQR of 19 DS and 19 CTR children. (B–D) Statistical significance was calculated by the Mann–Whitney U-test.
We performed a more detailed analysis of the data evaluating the proliferation and differentiation of gated CD27+ B cells (see Supporting Information Fig. 2 for the gating strategy) that were either IgM+ or IgM−. We performed the analysis in nine CTR and nine DS children. In the IgM memory pool, we confirmed that there was no statistical difference in the frequency of memory B cells that had proliferated (Fig.3A, % divided cells). IgM memory B cells of DS children, however, had a higher proliferation index as compared with that in the CTR children and most of the IgM memory B cells of DS children were at the third cycle of cell division after 7 days in culture with CpG. In contrast, most of the IgM memory B cells of the CTR group had only divided twice. Differentiation into IgM plasma cells was also increased in DS (p = 0.0006): whereas in the CTR group only around 20% of the CD27+IgM+ population was composed of CD38+++ plasma cells, plasma cells constituted 80% of the CD27+IgM− B cells in DS. In the CD27+ IgM− population (Fig.3B), the frequency of divided cells was higher in the DS group, although in this case statistical significance was not reached. Switched memory B cells proliferated at equal rates in the CTRs and DS groups, but switched plasma cells were present at an increased frequency in the CD27+ population of DS children (p = 0.0187).
Figure 3.
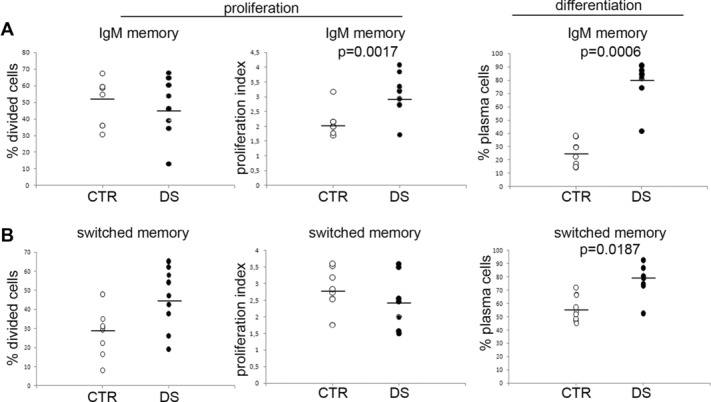
Increased response to CpG of B cells of DS children. Cells from a subgroup of nine DS and nine CTR children from whom a sufficient number of cells were available were labeled with CMFDA, cultured with CpG for 7 days, stained and analyzed by flow cytometry. The number of CD27+ IgM+ or IgM− cells that had proliferated (% divided cells), and the proliferation index (number of cycles/divided cells) were determined using FlowJo. The number of plasma cells (CD27+++CD38+++ was calculated from the standard cytofluorimetric analysis of IgM+or IgM−CD27+cells (see also Supporting Information Fig. 2). (A, B) The percentage of cells that had proliferated in culture, the proliferation index, and the percentage of plasma cells of (A) IgM isotype and (B) switched isotypes (CD27posIgMneg B cells) are shown. Each symbol represents an individual donor and bars represent means. Statistical significance was calculated by the Mann–Whitney U-test.
Thus, in response to CpG, both IgM and switched memory B cells of DS children show an increased proliferative response and have an increased ability to differentiate into plasma cells as compared to the equivalent cells in the CTR children.
Increased generation of CpG-induced antibody-producing cells
In order to confirm the data on plasma cell formation obtained in bulk culture after 7 days, we measured the ability of individual memory B cells from both DS and CTR children to generate antibody-producing cells as detected and enumerated by ELISPOT. The methodology established in the laboratory requires a shorter CpG stimulation time than the proliferation test (5 instead than 7 days) described above.
We stimulated peripheral blood mononuclear cells (PBMCs) isolated from 18 DS and 16 CTR children with CpG for 5 days and then counted the antibody-producing cells of IgM and switched isotypes. In each experiment, 2 × 106 PBMCs were plated and stimulated with CpG. Based on the cytofluorimetic analysis at day 0, we calculated the number of IgM and switched memory B cells seeded in each well for each individual. In the cultures from DS children, IgM memory B cells were around 47% (median value) of the values of the CTR cultures (p = 0.02, Fig.4A). Switched memory B cells were 17% of the values of the CTR group (p < 0.001, Fig.4A). At day 5, IgM, IgA, and IgG spots were counted. The number of IgM and switched (IgG+IgA) spots was significantly lower in the cultures from DS as compared with those from CTR children (1.8- and twofold lower, respectively, Fig.4B). We calculated how many antibody-producing cells each seeded memory B cell was able to generate, by dividing the number of spots obtained at day 5 by the number of memory B cells plated at day 0. In Figure4C, the ratio between the number of IgM spots and IgM memory B cells is shown for CTR (white columns) and DS children (black columns). The median ratio value was 0.3 in the CTR and 0.2 in the DS. This indicates that in healthy children one in three IgM memory B cells generates one plasma cell after 5 days of CpG stimulation whereas in DS children one in two IgM memory B cells produces plasma cells that can be detected by ELISPOT. Figure4C shows that the ability to form IgM plasma cells in vitro is increased in DS children, but the difference is not statistically significant at day 5. The difference is, however, significant in the switched memory populations (Fig.4D). Each switched memory B cell gives rise to one plasma cell in the CTR group, but 2.5 plasma cells are generated by each switched memory B cells in DS children (p = 0.02). Thus, switched memory B cells of DS children show an increased capacity to differentiate into antibody-secreting cells in response to TLR9 signals also at day 5 (Fig.4D).
Figure 4.
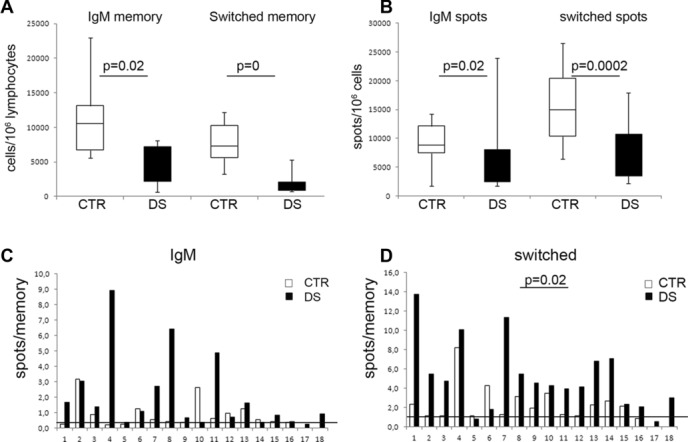
Increased differentiation potential of switched memory B cells of DS children. (A) Number of IgM and switched memory B cells seeded at day 0 for CTR (white columns) and DS (black columns) children contained in 106 PBMCs. PBMCs of DS children contain significantly less memory B cells. The numbers were calculated based on the flow cytometric analysis performed on day 0. (B) Number of spots obtained at day 5 from 106 cells of CTR (white columns) and DS (black) children, secreting either IgM or IgG as determined by ELISPOT using isotype-specific antibodies. Fewer antibody-producing cells are generated in DS children. (C) Ratio between the number of IgM spots obtained at day 5 and of IgM memory B cells seeded at day 0 (CTR, white; DS, black). Cells numbers were determined as in A and the number of IgM-secreting cells was calculated by ELISPOT. The line indicates the median value of the ratio calculated in CTR. Eleven of the 18 patients with DS have a higher ratio than the median of CTR. Statistical significance was calculated by the Mann–Whitney U-test. The difference between the two groups is not significant. (D) Ratio between the number of switched (IgG + IgA) spots and of switched memory B cells plated at day 0 (cell numbers were calculated as described in A). The line shows the median value of the ratio in CTR. A total of 16/18 DS patients have a higher ratio than the median of CTR. Statistical significance was calculated by the Mann–Whitney U-test. The difference between the two groups is significant.
Reduction of specific memory B cells
Vaccinations exert a protective effect by generating long-lived plasma cells that continuously produce specific antibodies, acting as a first-line defense in case of infection. Switched memory B cells are also induced by vaccine injection and are indispensable for the recall response. In order to measure the ability of children with DS to generate and maintain their specific memory pool, we studied a group of seven DS children that had received a complete cycle of the pneumococcal glyconjugated vaccine (three injections) 3–5 years before this study was performed. Each DS child was directly compared to his CTR sibling of roughly the same age who had also received three doses of vaccine 3–5 years before, meaning that we compared children living in the same environment and with a comparable immunization history (Supporting Information Table 1).
We found that, with one exception, all DS children analyzed had a reduced number of B cells producing IgG against pneumococcal polysaccharides in comparison with their own siblings (Fig.5A). When we compared the seven DS children to the seven CTR children, we found that the difference between the two groups was significant (p = 0.04). In contrast, the number of B cells producing IgM specific for pneumococcal polysaccharides in DS children was comparable (p = 0.8) to that of the CTR group (Fig.5B) confirming that the immune defect of DS children significantly affects the adaptive immune system and, in particular, one of its most important products, i.e. antigen-specific switched memory B cells. Serum IgG antibodies specific for pneumococcal polysaccharides were not reduced in the DS children suggesting that the other products of the germinal center reaction, long-lived plasma cells, are efficiently generated and persist in both DS and CTRs (Fig.5C).
Figure 5.
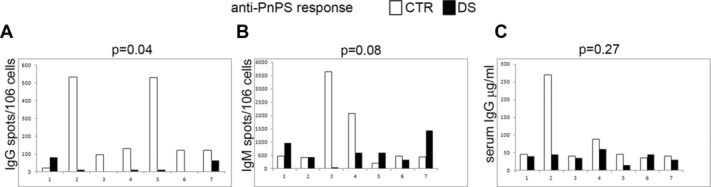
Reduced number of specific memory B cells in vaccinated DS children. Seven pairs of siblings, each with one CTR (white column) and one DS (black column) child, were vaccinated with a glyconjugated vaccine and their anti-PnPS responses were monitored by (A and B) ELISPOT and (C) ELISA. (A, B) The number of (A) switched memory and (B) IgM memory B cells secreting anti-PnPS antibodies was determined by ELISPOT on PnPS-coated plates developed with isotype-specific antibodies. (C) Serum anti-PnPS IgG levels were measured by ELISA. Statistical significance was calculated using the Mann–Whitney U-test to compare the seven CTR to the seven DS children.
Discussion
The results of this study fully confirm that subjects with DS are affected with a primary immunodeficiency (PID). The number of circulating lymphocytes, both T and B cells, was significantly lower in DS as compared with CTR children, as reported in the literature [29,30]. In the present study, we show that the most striking and consistent defect in DS is the reduced number of switched memory B cells that, however, have an increased capacity to differentiate into antibody-producing cells.
DS PID has been suggested to be reminiscent of CVID [31] mainly because of the B-cell anomalies. In fact, DS children suffer from recurrent respiratory tract infections and have an increased risk of severe sepsis [32]. However, these features are much more severe in CVID than in DS children who, in contrast with CVID patients, have normal IgG and IgA levels and respond to vaccinations although with a variable but generally reduced specific antibody production [33].
Our data show that trisomy 21 affects the differentiation of B cells at different developmental stages and checkpoints. B cells originate in the BM from stem cells and develop following a tightly regulated pathway. They leave the BM at the transitional B-cell stage, when they are still relatively immature and short-lived. Maturation to the long-lived naive pool occurs in the periphery and is an antigen- and T-cell-independent phenomenon [34]. The size of the mature-naive pool depends on the BM input (i.e. number of transitional B cells) and on the availability of space and survival factors in the secondary lymphoid tissues [35]. In DS children, the BM produces half the number of transitional B cells generated by CTR children (Table 2 and Fig.1B). In both DS and CTR transitional B cells, in turn, effectively differentiate into mature-naive B cells. This pool in DS children is half the size of that in the CTR group (Fig.1B). We show that the pool of switched memory B cells, which embodies the past antigen experience of each individual, is severely reduced in DS. This population is generated in the germinal centers in response to infections or vaccinations. The level of serum antibodies also results from antigen-driven differentiation of B cells. In contrast to the reduction of switched memory B-cell numbers, IgG and IgA antibody levels were normal in DS children. Most serum antibodies are produced by long-lived plasma cells that have relocated to the BM following their exit from the germinal center. Memory B cells also contribute to the maintenance of the level and diversity of serum immunoglobulin by terminally differentiating into plasma cells upon exposure to innate signals [36].
We investigated the ability of memory B cells to differentiate in vitro into antibody-producing cells in response to TLR9 engagement. Both proliferation and differentiation of memory B cells in response to CpG were increased in DS children. In vitro CpG induces the proliferation of memory B cells associated with differentiation. Proliferation was measured at day 7. We show that IgM memory B cells of DS children proliferate faster than those of CTR children (Fig.3A). Differentiation was analyzed both at day 7 and at day 5. Both IgM and switched memory B cells demonstrated an increased ability to differentiate into plasma cells in DS children. At day 5 (Fig.4D), the difference between the DS and CTR groups is significant only for switched memory B cells. In contrast, at day 7 both switched and IgM memory B cells of DS children produce more plasma cells than the CTR group. This result can be understood by considering that differentiation occurs after a certain number of proliferation cycles and 2 days more in culture allow more proliferation and differentiation, i.e. plasma cells. Our results also show different kinetics of response to CpG in IgM and switched memory B cells of CTR; however, further studies are necessary to clarify this phenomenon. As the functions of IgM memory/marginal zone and IgM-only memory B cells cannot be discriminated in our study, additional experiments are necessary to investigate whether the two populations behave the same in DS children. As reduced numbers and responses of T cells have been demonstrated in DS, we cannot exclude that the defective B-cell function is a secondary phenomenon [26]. However, although fewer T and B cells are generated in the primary lymphoid organs of children with DS, the fate of the differentiating T cells is quite different from that of B cells. The latter, indeed, show an impaired differentiation into switched memory B cells. In contrast, T cells generate normal numbers of both CD8+ and CD4+ memory T cells suggesting that they are functionally normal in vivo. Further studies are certainly needed to investigate this point.
We also compared seven pairs of siblings, one with and one without DS. All children had been vaccinated 2–3 years before this study and each couple lived in the same household ensuring a similar environmental exposure to infection in addition to the partially common genetic background. We found that, despite a normal level of specific antibodies, specific memory B cells were extremely rare in DS siblings whereas such cells persisted in normal children.
Taken as a whole, the results of the present study strongly suggest that DS children suffer from an intrinsic defect of B-cell differentiation resulting in a striking reduction of switched memory B cells which are crucial for the secondary response to pathogens, as well as for the response to vaccine antigens. The increased susceptibility to infections of DS children despite normal immunoglobulin levels confirms that switched memory B cells protect the organism against pathogen challenge, playing a function that is independent of the level of preformed serum antibodies, constantly secreted by long-lived plasma cells. In the case of reinfection, memory B cells sense the pathogen and rapidly react producing high-affinity protective antibodies not only in the serum but also locally at the site of pathogen entry [28]. The reduction of switched memory B-cell numbers represents the most likely explanation of the clinical and immunological features of DS PID (Fig.5D).
Further studies are necessary to fully explain our results. On the one hand, our findings are compatible with an alteration of the germinal center reaction resulting in the asymmetrical generation of normal numbers of long-lived plasma cells and a reduced fraction of switched memory B cells. Another possible scenario includes a normal germinal center reaction generating both long-lived plasma cells and sufficient amounts of memory B cells, followed by the exhaustion of the switched memory pool due to their activation and terminal differentiation into plasma cells (Fig.6). According to the latter hypothesis, the differentiation of memory B cells into plasma cells may contribute to the maintenance of antibody levels. Regardless of the explanation underlying these results, our study indicates that the primary and recall immune response of DS children to vaccines should be evaluated in order to design tailored immunization schedules for an effective vaccine-induced protection of DS children against infection.
Figure 6.
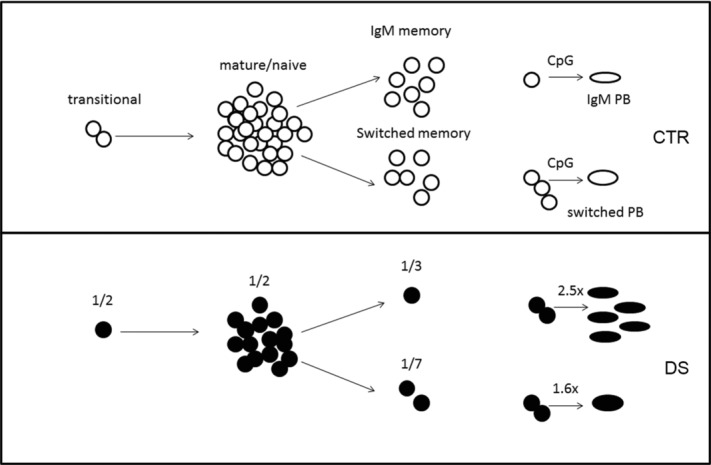
Reduced numbers of memory B cells with high terminal differentiation potential in DS children. The output of newly generated transitional B cells in the DS children (filled symbols, bottom) is half that seen in the CTR group (open symbols, top). The number of mature-naive B cells is also reduced by 50% in DS children. A further and more significant reduction of B-cell numbers is observed in the memory pool, with IgM and switched memory B-cell levels in DS comprising one-third and one-seventh of that in CTR children, respectively. In response to TLR9 signals, both IgM and switched memory B cells of DS children produce an increased number of antibody-producing cells in comparison to switched memory B cells in CTR children.
We do not know which sequences on chromosome 21 influence the immune response. High steady state levels of certain microRNAs have been recently demonstrated in the peripheral blood cells of DS children [37]. MicroRNA 125B and 155, encoded by chromosome 21, play a role in the germinal center reaction and in the development and proliferation of B and T cells [38,39]. It would be very interesting to evaluate the level of these microRNAs in the B and T cells of DS children in response to innate or adaptive stimuli and determine their effects on cell proliferation and class switching.
Materials and methods
Study population
We first enrolled 19 DS children and 19 healthy (CTR) subjects without DS living in the Rome area and not institutionalized. An additional 14 children were enrolled for the study of antigen-specific memory B cells. In this additional group, children were recruited in pairs. Each pair was composed of two siblings, one normal and one with DS. The study protocols and consent form were approved by the Ethical Committee of Ospedale Pediatrico Bambino Gesù, Rome. Written informed consent was obtained from the families of the persons included in this study according to the principles expressed in the Declaration of Helsinki.
Inclusion criteria for DS children were diagnosis of DS proven by chromosome analysis, age between 6 and 12 years, and no symptoms of infection at the time of blood sampling. CTR subjects had the same age and no signs of infection. Exclusion criteria for CTR and DS children were evidence of malignancy, chemotherapy, post chemotherapy, and immunosuppressive treatment.
The clinical history of the patients was obtained from medical records and interviews conducted by the physician. A standard questionnaire for the occurrence of infections and any related hospitalization was also used. The medical records of all participants were checked for the frequency of infections, presence of congenital heart disease, previous heart surgery, and occurrence of autoimmune diseases.
Signs and symptoms to define recurrent respiratory infection (IRR) were pneumonia (two or more episodes/year), otitis (three or more episodes/6 months or four or more episodes/year), tonsillitis (three infections/year), and rhinosinusitis (three or more episodes/6 months or four or more/year) [40–43].
Demographic characteristics and clinical history
The median age of the DS children included in this study was 8.74 years, range 6–12 years, and did not differ significantly from that of CTR (median age 8 years, range 6–12) subjects. The sex distribution was nine males and ten females in DS children and 11 males and eight females in the CTR children.
According to the clinical history, 89.5% (17/19) of DS children experienced recurrent infections of the upper respiratory tract (94.1%; 16/17) and 11.8% (2/19) suffered from recurrent pneumonia or gastroenteritis. Furthermore, eight of the 19 DS children included in the study (42.1%) had required hospitalization because of infection. Thirteen DS patients had congenital heart disease (68.4%) but only two of them (15.4%) had undergone heart surgery.
Three patients (15.8%) had autoimmune diseases, two suffered from hypothyroidism and one from celiac disease. One child had polycitemia and one had experienced transient abnormal myelopoiesis in the neonatal age (Table 1).
In the group of 14 children enrolled for the study of antigen-specific memory B cells, the mean age at analysis was 6.8 years (range 4–12), nine were males and five females. All children had received a complete course (three doses) of a glyconjugated antipneumococcal vaccine before. None of the subjects had undergone thymectomy or had autoimmune disorders (Supporting Information Table 1).
All DS patients and CTR children underwent venipuncture and 5 mL EDTA-venous blood was collected for baseline immunological evaluation and cell culture experiments. All analyses were performed blinded in regard of diagnosis.
Cell isolation and flow cytometry analysis
Heparinized PBMCs were isolated by Ficoll Paque™ Plus (Amersham Pharmacia Biotech) density-gradient centrifugation, counted and stained with the appropriate combination of fluorescent labeled antibodies and analyzed by flow cytometry [44]. Dead cells were excluded from analysis by side/forward scatter gating. All analyses were performed on a FACSCanto (BD Biosciences) interfaced to PC FACSDiva software. A total of 50 0000 events per sample were analyzed.
B-cell proliferation and immunoglobulin production in vitro
Before stimulation, peripheral blood lymphocytes were labeled with CMFDA at a final concentration of 0.1 mg/mL (CellTrackerCMFDA; Molecular Probes, Eugene, OR, USA). The cells were cultured at 5 × 105 cells per well in 96-well plates (Becton Dickinson, San Jose, CA, USA) in complete RPMI 1640 (InvivoGen, San Diego, CA, USA), supplemented with 10% FBS (Hyclone Laboratories, Logan, UT, USA), 2% l-glutamine (Gibco BRL), 5 × 10–5 M 2-beta-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA), and 20 mg/mL gentamycin (Gibco BRL). CpG ODN (Hycult Biotechnology, The Netherlands) was added at the concentration of 2.5 mg/mL [45].
Cell proliferation and phenotype were measured on day 7 by flow cytometry. For ELISPOT cells were used at day 5 of CpG stimulation.
ELISA immunoassay
Secreted Igs were detected on day 7 by ELISA. Briefly, 96-well plates (Corning Inc., Corning, NY, USA) were coated overnight with purified goat anti-human IgA plus IgG, plus IgM (Jackson ImmunoResearch Laboratories). After washing with PBS containing 0.05% Tween and blocking with PBS containing 1% gelatin (1 h, room temperature), plates were incubated for 1 h at 37°C with the supernatants of the cultured cells. After washing, plates were incubated for 1 h with peroxidase-conjugated fragment goat anti-human IgA or IgG or IgM antibodies (Jackson ImmunoResearch Laboratories). The assay was developed with O-phenylendiamine tablets (Sigma-Aldrich) as a chromogenic substrate. Absorbance at 405 nm was measured, and Ig concentrations were calculated by interpolation with the standard curve [45].
Specific anti-PnPS IgG serum concentrations were measured using VaccZymeTM Anti-PCP IgG Enzyme Immunoassay kit from Binding Site (The Binding Site) according to the manufacturer's instructions. For anti-PCP, IgG concentrations were calculated from the standard curves in milligrams per liter.
ELISPOT
Ninety-six-well plates (MultiScreen-HA, Milipore) were coated overnight with AffiniPure F(ab’)2 Fragment Goat anti-human IgA+IgG+IgM (H+L; Jackson Immuno Research Laboratories) or PneumoVax® (Sanofi Pasteur MSD) containing Streptococcus pneumoniae polysaccharides of the following serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F. After washing with sterile PBS/0.05% Tween20, plates were blocked for 1 h at 37°C with PBS/gelatin 1%.
PBMCs stimulated for 5 days, as described before, were collected, counted, and seeded in the precoated plates. Plates were left at 37°C, 2% CO2 for 4–6 h to allow antibody secretion. A total of 1/2 serial dilutions were done starting in the first well with 5 × 104 cells for detection of total IgM and IgG and IgA. A total of 2 × 105 cells were seeded in the first dilution well for the detection of specific anti-PnPS Igs. After incubation, plates were washed with dH2O/0.05% Tween20 (once) and PBS/0.05% Tween20 (two times) and incubated overnight with either anti-IgM HRPO (1:1000), anti-IgG HRPO (1:2000), or anti-IgA (1:2000; Jackson Immuno Research Laboratories) diluted in PBS + gelatin (1 + 0.05%) Tween20 (Sigma). After washing twice as before, TMB substrate (ready to use from Mabtech-ELISpot plus for human IgG kit, Mabtech AB) was used according to the manufacturer's instructions. Plates were left at room temperature to allow the blue color to develop and the reaction was stopped with dH2O. Plates were left to dry before counting with an ELISCAN (A-EL-VIS, Germany) [44].
Statistical analysis
Comparison between immunological values in the CTR and DS children was performed using the Mann–Whitney U-test. p Values lower than 0.05 were considered statistically significant.
Acknowledgments
This work was supported by a grant of the Italian Ministry of Health to RC.
Glossary
- CMFDA
5-Chloromethylfluorescein diacetate
- CVID
common variable immune deficiency
- DS
Down syndrome
- PBMC
peripheral blood mononuclear cell
- PID
primary immunodeficiency
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Fig. 1. T cell populations in the peripheral blood of CTR and DS children.
Fig. 2. Gating strategy for the analysis of proliferation and differentiation in response to CpG.
Table 1. Siblings included in the study, demographic and vaccine information
References
- 1.Ram G, Chinen J. Infections and immunodeficiency in Down syndrome. Clin. Experim. Immunol. 2011;164:9–16. doi: 10.1111/j.1365-2249.2011.04335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloemers BLP, Broers CJM, Bont L, Weijerman ME, Gemke RJBJ, Marceline van Furth A. Increased risk of respiratory tract infections in children with Down syndrome: the consequence of an altered immune system. Microb. Infection. 2010;12:799–808. doi: 10.1016/j.micinf.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down's syndrome. Lancet. 2000;355:165–169. doi: 10.1016/S0140-6736(99)05264-2. [DOI] [PubMed] [Google Scholar]
- 4.Hasle H. Pattern of malignant disorders in individuals with Down's Syndrome. Lancet Oncol. 2001;2:429–436. doi: 10.1016/S1470-2045(00)00435-6. [DOI] [PubMed] [Google Scholar]
- 5.Bonamico M, Mariani P, Danesi HM, Crisogianni M, Failla P, Gemme G, Quartino AR, et al. Prevalence and clinical picture of celiac disease in Italian Down syndrome patients: a multicenter study. J. Pediatr. Gastroenterol. Nutr. 2001;33:139–143. doi: 10.1097/00005176-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez-Albisua I, Storm W, Wäscher I, Stern M. How frequent is coeliac disease in Down syndrome? Eur. J. Pediatr. 2002;161:683–684. doi: 10.1007/s00431-002-1078-6. [DOI] [PubMed] [Google Scholar]
- 7.Book L, Hart A, Black J, Feolo M, Zone JJ, Neuhausen SL. Prevalence and clinical characteristics of celiac disease in Down's syndrome in a U.S. study. Am. J. Med. Gen. 2001;98:70–74. [PubMed] [Google Scholar]
- 8.Van Goor JC, Massa GG, Hirasing R. Increased incidence and prevalence of diabetes mellitus in Down's syndrome. Arch. Dis. Child. 1997;77:186. doi: 10.1136/adc.77.2.183g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anwar AJ, Walker JD, Frier BM. Type 1 diabetes mellitus and Down's Syndrome: prevalence, management and diabetic complications. Diabet. Med. 1998;15:160–163. doi: 10.1002/(SICI)1096-9136(199802)15:2<160::AID-DIA537>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 10.Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D. Cancers and immune related diseases associated with Down's syndrome: a record linkage study. Arch. Dis. Child. 2004;89:1014–1017. doi: 10.1136/adc.2003.046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King K, O'Gorman C, Gallagher S. Thyroid dysfunction in children with Down syndrome: a literature review. Ir. J. Med. Sci. 2014;183:1–6. doi: 10.1007/s11845-013-0994-y. [DOI] [PubMed] [Google Scholar]
- 12.Irving C, Basu A, Richmond S, Burn J, Wren C. Twenty-year trends in prevalence and survival of Down syndrome. Eur. J. Hum. Genet. 2008;16:1336–1340. doi: 10.1038/ejhg.2008.122. [DOI] [PubMed] [Google Scholar]
- 13.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359:1019–1025. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
- 14.Burgio GR, Ugazio AG, Nespoli L, Marcioni AF, Bottelli AM, Pasquali F. Derangements of immunoglobulin levels, phytohemagglutinin responsiveness and T and B cell markers in Down's syndrome at different ages. Eur. J. Immunol. 1975;5:600–603. doi: 10.1002/eji.1830050904. [DOI] [PubMed] [Google Scholar]
- 15.Burgio GR, Lanzavecchia A, Maccario R, Vitiello A, Plebani A, Ugazio AG. Immunodeficiency in Down's syndrome: T-lymphocyte subset imbalance in trisomic children. Clin. Exp. Immunol. 1978;33:298–301. [PMC free article] [PubMed] [Google Scholar]
- 16.Levin S, Nir E, Mogilner BM. T system immune-deficiency in Down's syndrome. Pediatrics. 1975;56:123–126. [PubMed] [Google Scholar]
- 17.Levin S, Schlesinger M, Handzel Z, Hahn T, Altmam Y, Czernobilsky B, Boss J. Thymic deficiency in Down's syndrome. Pediatrics. 1979;63:80–87. [PubMed] [Google Scholar]
- 18.Ugazio AG, Maccario R, Notarangelo LD, Burgio GR. Immunology of Down syndrome: a review. Am. J. Med. Genet. Suppl. 1990;7:204–212. doi: 10.1002/ajmg.1320370742. [DOI] [PubMed] [Google Scholar]
- 19.Avanzini MA, Monafo V, De Amici M, Maccario R, Burgio GR, Plebani A, Ugazio AG, et al. Humoral immunodeficiencies in Down Syndrome; serum IgG subclass and antibody response to hepatitis B vaccine. Am. J. Med. Genet. Suppl. 1990;7:231–233. doi: 10.1002/ajmg.1320370746. [DOI] [PubMed] [Google Scholar]
- 20.Costa-Carvalho BT, Martinez RM, Dias AT, Kubo CA, Barros-Nunes P, Leiva L, Solé D, et al. Antibody response to pneumococcal capsular polysaccharide vaccine in Down syndrome patients. Braz. J. Med. Biol. Res. 2006;39:1587–1592. doi: 10.1590/s0100-879x2006001200010. [DOI] [PubMed] [Google Scholar]
- 21.Kusters MA, Bok VL, Bolz WE, Huijskens EG, Peeters MF, de Vries E. Influenza A/H1N1 vaccination response is inadequate in Down Syndrome children when the latest cut-off values are used. Pediatr. Infect. Dis. J. 2012;31:1284–1285. doi: 10.1097/INF.0b013e3182737410. [DOI] [PubMed] [Google Scholar]
- 22.Guazzarotti L, Trabattoni D, Castelletti E, Boldrighini B, Piacentini L, Duca P, Beretta S, et al. T lymphocyte maturation is impaired in healthy young individuals carrying trisomy 21 (Down syndrome) Am. J. Intellect. Dev. Disabil. 2009;114:100–109. doi: 10.1352/2009.114.100-109. [DOI] [PubMed] [Google Scholar]
- 23.Kusters MA, Gemen EF, Verstegen RH, Wever PC, de Vries E. Both normal memory counts and decreased naïve cells favor intrinsic defect over early senescence of Down Syndrome T lymphocites. Pediatr. Res. 2010;67:557–562. doi: 10.1203/PDR.0b013e3181d4eca3. [DOI] [PubMed] [Google Scholar]
- 24.Cetiner S, Demirhan O, Inal TC, Tastemir D, Sertdemir Y. Analysis of peripheral blood T-cell subsets, natural killer cells and serum levels of cytokines in children with Down syndrome. Int. J. Immunogenet. 2010;37:233–237. doi: 10.1111/j.1744-313X.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- 25.Broers CJ, Gemke RJ, Weijerman ME, van derSluijs KF, van Furth AM. Increased pro-inflammatory cytokine production in Down syndrome children upon stimulation with live influenza A virus. J. Clin. Immunol. 2012;32:323–329. doi: 10.1007/s10875-011-9625-4. [DOI] [PubMed] [Google Scholar]
- 26.Verstegen RH, Kusters MA, Gemen EF, de Vries E. Down Syndrome B-lymphocyte subpopulations, intrinsic defect or decresead T-lymphocyte help. Pediatr. Res. 2010;67:563–569. doi: 10.1203/PDR.0b013e3181d4ecc1. [DOI] [PubMed] [Google Scholar]
- 27.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capolunghi F, Rosado MM, Sinibaldi M, Aranburu A, Carsetti R. Why do we need IgM memory B cells. Immunol. Lett. 2013;152:114–120. doi: 10.1016/j.imlet.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Kusters MA, Verstegen RH, Gemen EF, de Vries E. Intrinsic defect of the immune system in children with Down syndrome: a review. Clin. Exp. Immunol. 2009;156:189–193. doi: 10.1111/j.1365-2249.2009.03890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Hingh YC, van der Vossen PW, Gemen EF, Mulder AB, Hop WC, Brus F, de Vries E. Intrinsic abnormalities of lymphocyte counts in children with Down syndrome. J. Pediatr. 2005;147:744–747. doi: 10.1016/j.jpeds.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Verstegen RH, Borte S, Bok LA, Van Zwieten PH, Von Döbeln U, Hammarström L, de Vries E. Impact of Down syndrome on the performance of neonatal screening assays for severe primary immunodeficiency diseases. J. Allergy Clin. Immunol. 2014;133:1208–1211. doi: 10.1016/j.jaci.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Garrison MM, Jeffries H, Christakis DA. Risk of death for children with Down syndrome and sepsis. J. Pediatr. 2005;147:748–752. doi: 10.1016/j.jpeds.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 33.Joshi AY, Abraham RS, Snyder MR, Boyce TG. Immune evaluation and vaccine responses in Down Syndrome: evidence of immunodeficiency. Vaccine. 2001;29:5040–5046. doi: 10.1016/j.vaccine.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol. Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 35.Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu. Rev. Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- 36.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Li W, Liu X, Chen H, Tan K, Chen Y, Tu Z, et al. Identification of dysregulated microRNAs in lymphocytes from children with Down syndrome. Gene. 2013;530:278–286. doi: 10.1016/j.gene.2013.07.055. [DOI] [PubMed] [Google Scholar]
- 38.Gururajan M, Haga CL, Das S, Leu CM, Hodson D, Josson S, Turner M, et al. MicroRNA 125b inhibition of B cell differentiation in germinal centers. Int. Immunol. 2010;22:583–592. doi: 10.1093/intimm/dxq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willimott S, Wagner SD. miR-125b and miR-155 contribute to BCL2 repression and proliferation in response to CD40 ligand (CD154) in human leukemic B-cells. J. Biol. Chem. 2012;287:2608–2617. doi: 10.1074/jbc.M111.285718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Martino M, Ballotti S. The child with recurrent respiratory infections: normal or not. Pediatr. Allergy Immunol. 2007;18:13–18. doi: 10.1111/j.1399-3038.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- 41.Principi N, Esposito S. New insights into pediatric rhinosinusitis. Pediatr. Allergy Immunol. 2007;18:7–9. doi: 10.1111/j.1399-3038.2007.00623.x. [DOI] [PubMed] [Google Scholar]
- 42.Revai K, Dobbs LA, Nair S, Patel JA, Grady JJ, Chonmaitree T. Incidence of acute otitis media and sinusitis complicating upper respiratory tract infection: the effect of age. Pediatrics. 2007;119:1408–1412. doi: 10.1542/peds.2006-2881. [DOI] [PubMed] [Google Scholar]
- 43.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosado MM, Gesualdo F, Marcellini V, Di Sabatino A, Corazza GR, Smacchia MP, Nobili B, et al. Preserved antibody levels and loss of memory B cells against pneumococcus and tetanus after splenectomy: tailoring better vaccination strategies. Eur. J. Immunol. 2013;43:2659–2670. doi: 10.1002/eji.201343577. [DOI] [PubMed] [Google Scholar]
- 45.Rosado MM, Scarsella M, Cascioli S, Giorda E, Carsetti R. Evaluating B-cells: from bone marrow precursors to antibody-producing cells. Methods Mol. Biol. 2013;1032:45–57. doi: 10.1007/978-1-62703-496-8_4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1. T cell populations in the peripheral blood of CTR and DS children.
Fig. 2. Gating strategy for the analysis of proliferation and differentiation in response to CpG.
Table 1. Siblings included in the study, demographic and vaccine information


