Abstract
This study investigated the performance of a new gelling fibre dressing containing silver (DURAFIBER™ Ag; Smith & Nephew, Hull, UK) in moderate to highly exuding venous leg ulcers with one or more clinical signs of infection. Fourteen patients with venous leg ulceration of median ulcer duration 12·5 weeks, recruited from three centres in South Africa, received treatment with the new dressing for a maximum of 8 weeks. Multilayer compression bandaging was used for all patients, at the majority of assessments. The objectives of this study were to assess the clinical acceptability of the dressing in terms of the following characteristics: antimicrobial properties, the progress of the wound towards healing, wear time, exudate management, conformability, patient comfort, pain on application, pain on removal and dressing integrity. The new dressing was rated as clinically acceptable for all characteristics, for all 14 patients (100%). It was easy to apply and remove; in 96·8% of removals, the dressing stayed intact on removal and could be removed in one piece. Fifty per cent of the wounds healed within the 8‐week study duration; between baseline and final assessment, the median percentage reduction in wound area was 98·2% and the median percentage reduction in devitalised tissue was 78%. Exudate levels and wound pain were significantly improved at final assessment compared to baseline assessment, and an increase in the number of patients with healthy peri‐wound skin between baseline and final assessment was observed. A reduction in bioburden and signs of clinical infection and an improvement in quality of life were observed over the 8‐week period. The average wear time was 6·4 days. This study supports the use of new dressing in the management of moderately to highly exuding venous leg ulcers with clinical signs of infection.
Keywords: Antimicrobial, Chronic wound, Dressing, Exudate, Infection
Introduction
Chronic wounds such as venous leg ulcers exert a considerable effect on the wellbeing of those who suffer with them and also impose a burden on health systems throughout the world. For example, in Europe, there are estimated to be 980 000 venous leg ulcers per year, with associated treatment costs estimated at €6·5 billion 1.
Deterioration of the patient's quality of life may occur if a wound becomes infected 2. Patients with chronic wounds are particularly susceptible to wound infection as a result of the open nature of the wound and the presence of sloughy and necrotic tissue 3. Clinical signs of infection include erythema, pain, oedema and localised heat 4, and as a further consequence, wound infections may in some cases lead to sepsis and become life‐threatening 5. Dealing with wound infection therefore presents practitioners with a significant challenge; however, the availability of topical antimicrobial products has provided a useful treatment option. In particular, the targeted use of dressings containing silver is a recognised technique for reducing bioburden and acting as an antimicrobial barrier to prevent further contamination of the wound by external micro‐organisms 6.
A new gelling fibre dressing containing silver (DURAFIBER Ag; Smith & Nephew, Hull, UK) has been developed. This product is a non‐woven antimicrobial dressing that gels on contact with wound fluid and is intended to provide a moist wound environment for use in the management of partial‐ and full‐thickness wounds. This study investigated the performance of the new dressing in moderate to highly exuding venous leg ulcers with one or more clinical signs of infection.
Methods
Study objectives
The primary objective was to assess the overall clinical acceptability of the dressing for the indication treated.
The secondary objectives were to assess detailed performance characteristics such as antimicrobial properties, the progress of the wound towards healing, wear time, exudate management, conformability, patient comfort, pain on application, pain on removal, dressing integrity and associated health‐related quality of life (HRQoL).
Inclusion/exclusion criteria
Patients were recruited from the population routinely seen by the investigator. For patients with multiple wounds, the largest wound meeting the eligibility criteria was included in the trial and was referred to as the reference wound. The following inclusion criteria were used:
Chronic wounds (category 2, 3 or 4 pressure ulcer, venous leg ulcer or grade 1 or 2 diabetic foot ulcer) that showed clinical signs of infection.
Wound area ≥2 cm2.
Wound exudate level high or moderate.
A positive tissue biopsy result at the initial assessment (≥104 cfu/g).
Patients were excluded if they were receiving topical agents at the reference wound surface, had a known sensitivity to any of the components of the new dressing, were being treated with immunosuppressive drugs or corticosteroids or had a history of poor concordance with medical treatment.
Treatment protocol
After obtaining local ethical approval, a prospective, open, multicentre clinical study was conducted in three centres in South Africa. Informed consent was obtained from all participants, and the trial was performed in accordance with the guidelines of the Declaration of Helsinki (1964) and subsequent revisions and ICH Good Clinical Practice (GCP).
A total of 14 patients were recruited from three centres; 9 patients were treated at the Kingsbury Lifehealth Care Hospital, 2 at the Constantiaberg MediClinic and 3 at the Vincent Pallotti Lifehealth Care Hospital. Patients received treatment with the new dressing for a maximum of 8 weeks, a study duration designed to allow sufficient time for the dressing performance data to be collected. Secondary dressings were used where appropriate, and dressing changes took place according to the judgement of the investigator. Patients continued on the new dressing for up to 8 weeks, or until the dressing was no longer indicated. At this point, patients continued to be treated with standard wound care for the remainder of the 8‐week study period. Other treatments such as compression therapy, pressure relief and wound debridement were carried out as appropriate throughout the treatment phase. However, topical agents were restricted from being applied to the reference wound for the duration of the study period. Any patient whose treatment with the investigational product was interrupted for 7 days or more was withdrawn from the study. During the study period, in order to minimise the possibility of confounding variables affecting the results, treatment remained consistent with routine practice. All adverse events were recorded.
Weekly assessments included assessment and measurement of the wound, a wound swab and a wound photograph. Further assessments took place at every additional dressing change. At each assessment, clinicians were asked to rate the acceptability and performance of the new dressing using a standardised questionnaire. The assessments at baseline and weeks 4 and 8 also included a tissue sample. To measure wound surface area, the wound perimeter was first traced onto a two‐layered plastic grid. The backing layer that had been in contact with the wound was then disposed of and the remaining tracing was used to measure wound area by computerised planimetry. Wound depth was measured at three locations in the reference wound using a polyurethane foam‐tipped measuring stick with a 15 cm scale consisting of 1 cm graduations. The average of the three measurements was recorded as the wound depth.
Microbiological analysis
A wound swab was taken approximately every 7 days. Sterile gauze hydrated with sterile water was used to remove excess exudate and debris from the wound. A swab pre‐moistened in sterile saline was rolled over a central 1 cm2 section of the wound (granulation tissue wherever possible) using a 360° rolling method. The position of each subsequent swab corresponded to the position of the initial swab. The swab was placed in the tube containing transport medium. Qualitative microbiological analysis for aerobic and anaerobic organisms was conducted on the swab samples. Bacterial growth was graded using the descriptors none, scanty, moderate and abundant 7.
A tissue biopsy for quantitative microbiological analysis was performed at screening and repeated at weeks 4 and 8, providing that the wound area was still > 1 cm2 ,. The ulcer was cleansed with saline and the site infiltrated with 1% lignocaine. A 3 mm punch biopsy tool was used to obtain a full‐thickness specimen of at least 100 mg in weight (to allow quantitative microbiological analysis for aerobic and anaerobic organisms). The specimen was placed in saline and transported to the laboratory immediately via courier for processing.
If the wound biopsy was missing at weeks 4 or 8, the following imputations were made:
If the wound was healed or too small to take a biopsy (and not showing signs of infection), the bacterial count was given the value zero.
If the wound was showing clinical signs of infection, or if the patient had withdrawn because the condition of the wound had worsened, the previous bacterial count was used.
Health‐related quality of life
Quality of life was evaluated at baseline and week 8 using the EQ‐5D instrument (EuroQoL Group, Rotterdam, the Netherlands). The assessment (consisting of the EQ‐5D descriptive system and the EQ visual analogue scale [VAS]) was completed by study patients prior to the initiation of treatment and again at the end of their participation in the study. The descriptive system consists of the following five dimensions: mobility, self‐care, usual activities, pain/discomfort and anxiety/depression. Respondents were asked to indicate their health state by selecting one of the three levels for each dimension: no problems, some problems or severe problems. This decision results in a single‐digit number for each dimension, which can be combined to give a five‐digit number describing the respondent's health state. The EQ VAS records patient‐rated health on a vertical VAS where the endpoints are labelled ‘Best imaginable health state’ and ‘Worst imaginable health state’.
Statistical methods
All statistical analyses were conducted in SAS Version 9.1.3 SP4. The marginal homogeneity test, stratified by patient, was used to test for reductions in bacterial load (from baseline to weeks 4 and 8), exudate level (from baseline to final assessment) and pain (from baseline to final assessment). McNemar's test was used to test for a reduction in the presence of infection from baseline to weeks 4 and 8 and for a reduction in the number of patients showing clinical signs of infection from baseline to weeks 4 and 8. Kaplan–Meier estimates of the median time to complete resolution of clinical signs of infection were calculated. The Wilcoxon signed rank test was used to test for a difference in the EQ‐5D index score between baseline and week 8. All significance tests were two sided and were conducted at the 5% significance level.
Results
Baseline characteristics
A total of 14 patients, 5 male and 9 female, were recruited, with 14 reference wounds having one or more clinical signs of infection. The first patient was enrolled into the study in November 2010 and the last patient completed the study in December 2011. All the wounds were venous leg ulcers. The baseline characteristics for the 14 patients included in the study are summarised in Table 1 and the medical conditions at baseline are summarised in Table 2.
Table 1.
Baseline characteristics
| Characteristic | Value |
|---|---|
| Age | |
| Mean | 70 years |
| Median | 71 years |
| Range | 39–93 years |
| Wound type | |
| Venous leg ulcer | 100% |
| Wound duration | |
| Mean | 33·3 weeks |
| Median | 12·5 weeks |
| Range | 2 weeks to 3 years |
| Wound area | |
| Mean | 15·9 cm2 |
| Median | 9·4 cm2 |
| Range | 2·4–74·8 cm2 |
| Recurrence of a previous ulcer | 3 ulcers (21·4%) |
Table 2.
Baseline medical conditions
| Medical condition | Current | Prior | None |
|---|---|---|---|
| Anaemia | 0 | 1 (7·1%) | 13 (92·9%) |
| Diabetes | 3 (21·4%) | 0 | 11 (78·6%) |
| Stroke (CVA) | 0 | 0 | 14 (100%) |
| Hypertension | 9 (64·3%) | 0 | 5 (35·7%) |
| Peripheral vascular disease | 3 (21·4%) | 0 | 11 (78·6%) |
| Congestive heart failure | 3 (21·4%) | 0 | 11 (78·6%) |
| Rheumatoid arthritis | 3 (21·4%) | 0 | 11 (78·6%) |
| Osteoarthritis | 3 (21·4%) | 0 | 11 (78·6%) |
| Deep vein thrombosis | 0 | 3 (21·4%) | 11 (78·6%) |
| Varicose veins | 1 (7·1%) | 5 (35·7%) | 8 (57·1%) |
| Other medical condition | 1 (7·1%) | 0 | 13 (92·9%) |
Ten patients (71·4%) were treated in a wound clinic, with one patient treated in hospital, one at home and the remaining two in other care settings. Twelve patients (85·7%) had a wound described as clinically infected. Of these, ten (83·3%) were prescribed systemic antibiotics. All wounds had at least one clinical sign of infection.
There were a total of 115 assessments, including 14 at baseline and 101 from weeks 1–8. There were 96 assessments at which the new dressing was applied. The mean number of gelling fibre dressings applied per patient throughout the treatment period was 7·8, with an average of 1·1 dressings applied at each assessment. The median duration of treatment with the new dressing was 51·5 days. Multilayer compression bandaging was used for all patients, at the majority of assessments. The reference wound was debrided at 12 (11·1%) assessments. Wound cleansing was carried out at 105 (97·2%) assessments.
Seven ulcers healed during the 8‐week study period. Of these, in four cases, the ulcer healed while using the new dressing, and in the remaining three cases, the use of the dressing had been discontinued prior to 8 weeks because the exudate level had become ‘None’ or ‘Light’ and the ulcer went on to heal during the study period. DURAFIBER Ag is not indicated in low or no exudate wounds. Six patients (42·9%) completed the 8‐week study period using the new dressing throughout and one patient discontinued early (at 46 days) because of a serious non‐device‐related adverse event.
Clinical acceptability
Clinicians were asked to indicate whether they rated the new dressing as clinically acceptable in terms of the following characteristics: overall acceptability for the indication treated, antimicrobial properties, the progress of the wound towards healing, wear time, exudate management, conformability, patient comfort, pain on application, pain on removal and dressing integrity. The new dressing was rated as clinically acceptable for all characteristics, for all 14 patients (100%).
Dressing performance characteristics
Data on the performance of the dressing were also collected at each assessment. The dressings were easy to apply at all 96 (100%) dressing changes and easy to remove at 78/95 (82·1%) removals. For most dressing changes (76·8%), no additional procedures were needed to remove the gelling fibre dressing, although sometimes (23·2% of changes) the dressing had to be soaked (usually with saline) to aid removal.
Dressing residue in the wound bed was experienced at 4/95 (4·2%) assessments. In 91/94 (96·8%) assessments, the dressing stayed intact on removal, whereas for the remaining 3 (3·2%) assessments, it had broken into two pieces. In the majority of cases, there was no pain on application or removal of the dressing (Table 3), and there was no tissue ingrowth into the dressing for 94/95 (98·9%) of assessments.
Table 3.
Pain at dressing application or removal
| Number of assessments | |||||
|---|---|---|---|---|---|
| Pain level | None | Mild | Moderate | Severe | Total |
| Pain on application | 92 (95·8%) | 2 (2·1%) | 2 (2·1%) | 0 (0%) | 96 |
| Pain on removal | 84 (88·4%) | 6 (6·3%) | 4 (4·2%) | 1 (1·1%) | 95 |
The dressing was rated as comfortable during wear at 91/96 (94·8%) assessments, and at the majority of assessments (99·0%), the dressing was observed to conform to the contours of the reference wound prior to removal. Four of the five assessments where the dressing was rated as uncomfortable related to the same patient. For 91/95 (95·8%) of the dressing changes, there was no trauma caused on dressing removal, although there was damage to wound tissue in two cases and damage to the surrounding skin in two cases. The dressing provided a cooling sensation at 14/89 (15·7%) assessments.
No maceration of the surrounding skin had occurred on removal of the gelling fibre dressing at the majority (81, 89%) of assessments, and no maceration was observed at baseline or final assessment. An increase in the number of patients with healthy peri‐wound skin between baseline and final assessment was observed (Table 4).
Table 4.
Condition of surrounding skin
| Number of patients | ||
|---|---|---|
| Condition of surrounding skin | Baseline assessment | Final assessment |
| Healthy | 5 (35·7%) | 9 (64·3%) |
| Fragile | 4 (28·6%) | 3 (21·4%) |
| Inflamed | 8 (57·1%) | 1 (7·1%) |
| Macerated | 0 | 0 |
| Dry and flaky | 2 (14·3%) | 2 (14·3%) |
The reasons for changing the dressings are summarised in Table 5.
Table 5.
Reasons for changing the dressing
| Reason for changing the dressing | Percentage of dressing changes (n = 96)a |
|---|---|
| Routine | 91·7% |
| Inadequate exudate management | 6·3% |
| Pain/discomfort from the dressing | 1·0% |
| Other | 1·0% |
Assessments where DURAFIBER Ag was applied at the previous scheduled assessment.
Clinicians were asked to compare the dressing with the antimicrobial product they usually used to treat this wound type. The results are summarised in Table 6.
Table 6.
Dressing performance characteristics
| Dressing performance | Superior | Equivalent | Inferior |
|---|---|---|---|
| Antimicrobial properties | 7 (87·5%) | 1 (12·5%) | 0 |
| Progress of wound towards healing | 1 (12·5%) | 7 (87·5%) | 0 |
| Wear time | 3 (37·5%) | 5 (62·5%) | 0 |
| Exudate management | 8 (100%) | 0 | 0 |
| Conformability | 3 (37·5%) | 5 (62·5%) | 0 |
| Patient comfort | 2 (25%) | 5 (62·5%) | 1 (12·5%) |
| Pain on application | 4 (50%) | 4 (50%) | 0 |
| Pain on removal | 3 (37·5%) | 5 (62·5%) | 0 |
| Dressing integrity | 1 (12·5%) | 7 (87·5%) | 0 |
Exudate handling
Clinicians were asked to assess the ability of the dressing to handle exudate since the previous assessment. For 91/95 (95·8%) assessments where the new dressing was used, the clinician was satisfied with the ability of the dressing to handle exudate.
Dressing wear time
The mean wear time for the new dressing averaged across all dressing changes was 6·4 days. The average wear time for each patient across all assessments where the new dressing was used was also calculated; the mean of these averages was also 6·4 days (median = 6·9 days, range 4·7–7·0 days). Calculating the values in this way avoids bias of the results towards those patients with a greater number of assessments.
Wound infection and bacterial load
Table 7 illustrates that there was significant evidence of a reduction in the presence of clinical signs of infection and the number of ulcers described by the clinician as clinically infected between baseline, week 4 and final assessment. At baseline, all patients had at least one sign of infection, whereas at the final assessment one patient (7·1%) had one sign of infection (local erythema). The median estimate of the time to complete resolution of clinical signs of infection was 29·5 days (95% confidence interval 14–49 days). Of the 12 ulcers (85·7%) described as clinically infected at the baseline assessment, all had resolved by week 4. The remaining two ulcers (14·3%) had no change in clinical infection status.
Table 7.
Signs of infection and reported wound infections
| Baseline | Week 4 | Final assessment | |
|---|---|---|---|
| Number of patients with at least one sign of infection | 14 (100%) | 4 (28·6%) | 1(7·1%) |
| P = 0·002 | P < 0·001 | ||
| Number of infected wounds | 12 (85·7%) | 0 (0%) | 1 (7·1%)a |
| P < 0·001 | P < 0·001 |
At final assessment, one wound was described as infected, although no signs of infection were present. This wound was recorded as clinically infected because of the result from the microbiological wound swab.
Table 8 shows details of the signs of infection observed at baseline and final assessment.
Table 8.
Details of clinical signs of infection
| Baseline | Final assessment | |
|---|---|---|
| Any clinical signs of infection | 14 (100%) | 1 (7·1%) |
| Wound static or deteriorating | 3 (21·4%) | 0 |
| Increased exudate/secretion levels | 9 (64·3%) | 0 |
| Increased pain | 5 (35·7%) | 0 |
| Increased temperature around the wound | 5 (35·7%) | 0 |
| Discolouration of granulation tissue | 2 (14·3%) | 0 |
| Friable granulation | 2 (14·3%) | 0 |
| Tissue necrosis | 1 (7·1%) | 0 |
| Local erythema | 6 (42·9%) | 1 (7·1%) |
| Oedema | 3 (21·4%) | 0 |
| Purulent drainage | 3 (21·4%) | 0 |
| Odour | 6 (42·9%) | 0 |
A summary of the semi‐quantitative bacterial load results, as assessed using the wound swab, is shown in Figure 1.
Figure 1.
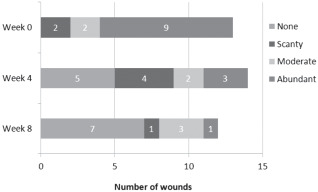
Details of changes in semi‐quantitative bacterial load.
Thirteen patients had a complete set of swab results at baseline and week 4, of which ten patients (76·9%) saw a reduction in the level of semi‐quantitative bacterial load between baseline and week 4 (P = 0·007). Two patients (15·4%) had no change and the remaining one patient (7·7%) had an increase in the bacterial load. Eleven patients had a complete set of swab results at week 8, of which the bacterial load reduced for nine (81·8%) patients between baseline and week 8 (P = 0·01). For the remaining patients, one had no change in the bacterial load and one had an increase in the bacterial load.
Table 9 shows the log10 bacterial count from the tissue biopsies at three timepoints.
Table 9.
Log10 bacterial count from tissue biopsies
| Bacterial count | ||||
|---|---|---|---|---|
| Log10 cfu/g (cfu/g) | ||||
| n | Median | Mean | Range | |
| Baseline | 14 | 7·0 (1·05 × 107) | 7·0 (3·46 × 107) | 5·6–8·4 (3·82 × 105 to 2·50 × 108) |
| Week 4 | 13 | 4·0 (1·115 × 104) | 3·2 (1·19 × 106) | 0–7·0 (0–1·00 × 107) |
| Week 8 | 13 | 0 (0) | 2·1 (8·41 × 105) | 0–7·0 (0–1·00 × 107) |
Within patients, there was significant evidence of a reduction in the log10 bacterial count between baseline and both 4 weeks (n = 13, median bacterial count reduction 3·8 log10 cfu/g, P < 0·001) and 8 weeks (n = 12, median bacterial count reduction 4·8 log10 cfu/g, P < 0·001). Table 10 shows the bacterial species identified from the biopsies.
Table 10.
Distribution of wound microflora (from tissue biopsy data)
| Timepoint(s) at which the species was identified in the biopsy (weeks) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | ||||||||||||||
| Species | 1·01 | 1·02 | 1·04 | 1·05 | 1·06 | 1·07 | 1·08 | 1·12 | 1·13 | 2·01 | 2·02 | 3·01 | 3·02 | 3·06 |
| Acinetobacter lwoffii | 0 | |||||||||||||
| Escherichia coli | 0 | 0 | ||||||||||||
| Enterococcus faecalis | 8 | 4 | 0a | 0, 4 | 4 | 8 | 4 | |||||||
| Klebsiella oxytoca | 0 | |||||||||||||
| Mixed growth | 0 | 4 | ||||||||||||
| Morganella morganii | 0 | |||||||||||||
| MRSA | 8 | 4 | ||||||||||||
| Proteus mirabilis | 0 | 4 | 0a | 0, 4 | 4 | |||||||||
| Pseudomonas aeruginosa | 0, 4 | 0 | 0 | |||||||||||
| Skin flora | 8 | 0a | 4 | 8 | ||||||||||
| Proteus penneri | 8 | |||||||||||||
| Proteus vulgaris | 0, 4 | |||||||||||||
| Serratia marcescens | 8 | 0, 4 | ||||||||||||
| Staphylococcus aureus | 0a, 4 | 0 | 0 | 0 | 0 | |||||||||
| Staphylococcus epidermis | 4 | |||||||||||||
| Staphylococcus haemolyticus | 0 | |||||||||||||
| Strep dysgalactiae ssp equisim | 0 | |||||||||||||
| Streptococcus group B | 0 | |||||||||||||
| Streptococcus pluranimalium | 4 | |||||||||||||
MRSA, Methicillin‐resistant Staphylococcus aureus.
Biopsy taken at week 1 due to insufficient biopsy size at week 0.
Other clinical observations
The median percentage reduction in wound area between baseline and final assessment was 98·2% (mean = 78·3%, range −30·2% to 100%), while the median percentage reduction in wound depth was 100% (range 50–100%). The number of wounds healed by weekly assessment and the median percentage reduction in wound area and depth between baseline and each weekly assessment are shown in Table 11. There was significant evidence of a reduction in the level of exudate between the baseline assessment and the final assessment (P < 0·001), the level of exudate for 11 (78·6%) patients decreasing during the study period. The median percentage of devitalised tissue reduced from 74% at baseline (mean = 58·8%) to 0% at final assessment (mean = 2·4%). There was also significant evidence of a reduction in the level of wound pain between the baseline assessment and the final assessment (P < 0·001). A reduction in the level of pain was observed for 12 (85·7%) patients; the remaining two (14·3%) patients had no change in the level of pain.
Table 11.
Wound healing and area and depth percentage reduction from baselinea
| Percentage reduction from baseline | |||
|---|---|---|---|
| Assessment | Cumulative number of healed wounds | Median reduction in area | Median reduction in depth |
| Week 1 | 0 (0%) | −4·7 (n = 13) | 0 (n = 12) |
| Week 2 | 0 (0%) | 28·0 (n = 13) | 0 (n = 12) |
| Week 3 | 1 (7·1%) | 38·9 (n = 13) | 33·3 (n = 12) |
| Week 4 | 3 (21·4%) | 43·5 (n = 13) | 66·7 (n = 12) |
| Week 5 | 4 (28·6%) | 56·9 (n = 14) | 91·3 (n = 12) |
| Week 6 | 4 (28·6%) | 73·8 (n = 14) | 69·4 (n = 12) |
| Week 7 | 5 (35·7%) | 91·1 (n = 14) | 85·7 (n = 12) |
| Week 8 | 7 (50%) | 98·2 (n = 14) | 100 (n = 12) |
Where missing values occurred at post‐baseline assessments, the last known observation was used in calculations.
Health‐related quality of life
The mean self‐rated health status VAS score (scale: 0–100, where 100 = best imaginable health state) at the baseline assessment was 65 (n = 12, median = 67·5, range = 20–90). The corresponding mean score 8 weeks post‐baseline was 78·9 (n = 9, median = 80, range = 60–95).
HRQoL was evaluated at both baseline and 8 weeks after the baseline assessment for 8 patients. The most noticeable differences observed related to the Usual Activities rating and the Pain/Discomfort rating. For the Usual Activities rating, five patients' ratings (62·5%) improved, the remaining three (37·5%) staying constant. For the Pain/Discomfort rating, six patients' ratings (75%) improved, the remaining two (25%) staying constant.
The median index scores at baseline and 8 weeks post‐baseline were 0·55 (n = 12) and 0·86 (n = 8), respectively. There was statistically significant evidence of an increase in the index score from baseline to 8 weeks post‐baseline, with a patient median increase of 0·41 (n = 8, P = 0·031).
Adverse events
Throughout the duration of the study, six adverse events were reported by four patients. Of these six adverse events, four (reported by two patients) were considered serious. One patient sustained a compound ankle fracture, pneumonia and septic shock. After prolonged hospitalisation, this patient subsequently died. Cellulitis was reported for a further patient. The adverse events observed during the course of the study were not related to the gelling fibre dressing and have not raised any concerns about the safety of the product in use.
Discussion
Effective wound dressings must meet a number of criteria in order to provide an optimal environment to allow wounds to heal in a timely manner. The selection of dressings that are appropriate to the needs of the patient and practitioner is an important decision that can affect patient wellbeing, clinical outcomes and resource use 8, 9. The new dressing reported in this study was rated as clinically acceptable for all 14 patients. It was easy to use, and removed as a single piece at most dressing changes, with no pain on application or removal in the majority of cases.
For exuding wounds, dressings designed to deal with exudate are essential to ensure good outcomes and effective management of resources. Although wound exudate does play a role in the wound healing process 10 and is an important part of the inflammatory response, it can also lead to deleterious effects on healing, particularly in chronic wounds 11, 12. Exudate also has the potential to influence the wellbeing of patients living with a wound – for example, the odour and appearance of exudate can lead to the patient feeling stigmatised and socially excluded 13, 14. Dressings that help to manage exudate by forming a gel on contact with wound fluid, and then retaining the fluid within the gel structure, have been widely and successfully used for managing a range of wound types including chronic wounds such as venous leg ulcers 15.
This study has demonstrated the exudate handling properties of the new dressing. In all cases where reported, clinicians observed that the exudate management capabilities of the new dressing were superior to dressings previously used for the wound.
The consequences of inadequate management of exudate can include peri‐wound maceration and an increased risk of infection, with associated increase in resources 14. Cutting and White 16 observed that maceration can complicate the healing of chronic wounds. Our study found no maceration of the surrounding skin at the majority (81, 89%) of dressing removals and no maceration at baseline or final assessment. The proportion of patients with healthy skin increased as treatment progressed.
In addition to the challenge of the management of exudate, a proportion of wounds exhibit clinical signs associated with colonisation or infection by pathogenic micro‐organisms. Recognising these signs and symptoms promptly allows antimicrobial treatment to be applied appropriately 2. There is a complex interaction between bacterial species in venous leg ulcers 17 and bacterial density at the wound surface has been shown to be a predictor of non‐healing 18. Other studies have shown associations between delays in healing correlated with the presence of bacterial species 19, 20. Diverse bacterial species can be cultured from swabs in all venous leg ulcers, whether they show signs of infection or not 21, but a recent study showed that no individual species identified by routine bacteriological culture could be used to predict healing 22.
Silver is recognised as an effective antimicrobial agent and dressings containing silver have a long history of use in wound care 6, 23. These products can provide silver topically at the wound site and have a low risk of bacterial resistance when containing a cidal dose 24. A recent consensus document recommended that silver dressings should be used to reduce bioburden and act as an antimicrobial barrier where there are signs of localised, spreading or systemic infection, or where there is a high risk of infection 6. In the group of patients that were treated with the new dressing, the presence of clinical signs of infection decreased as the study progressed, and for 81·8% of patients, the bacterial load reduced over time. Of the 85·7% of ulcers described as clinically infected at the baseline assessment, all had resolved by week 4. One was reported as infected at final assessment, although this was from the wound swab, and no signs of infection were present. This study found that in the opinion of the clinician, for the majority of performance characteristics, the new dressing was the same as or superior to existing antimicrobial products usually used for this indication.
The effect on HRQoL of a patient as treatment progresses is of interest to practitioners. There are relatively few studies of wounds that measure HRQoL, and even though this study was non‐comparative, it is interesting to observe the changes in quality of life scores. Both generic and disease‐specific assessment methods have been previously used in wound care, and both have been shown to be useful 25, 26. This study used the widely recognised EQ‐5D generic instrument, which has previously been reported as a suitable method for assessing HRQoL in people with chronic venous leg ulceration 27. When comparing HRQoL at the start and end of treatment, a statistically significant improvement in HRQoL was observed. There was also significant evidence of a reduction in the level of wound pain between the baseline assessment and the final assessment (P < 0·001). These are encouraging results, for two reasons. Firstly, although non‐comparative, it suggests that use of the new dressing, alongside existing protocols, provides a useful way of managing chronic wounds with medium to high exudate levels and clinical signs of infection. Secondly, it corroborates findings from other studies that EQ‐5D is a useful instrument for the assessment of HRQoL in patient populations such as this.
The mean wear time averaged across all dressing changes was 6·4 days. This compares favourably with typical dressing change practice, where dressings are often changed every 2–3 days. One audit of wound care practice showed an overall frequency of 2·96 times per week in community care, with 8·9% of leg and foot ulcers being changed daily 28. Another audit demonstrated a frequency of 2·17 times per week for leg and foot ulcers 29. Reducing dressing change frequency where appropriate could potentially have a dramatic impact on wound care resources. For example, for a facility treating 100 patients each week, reducing frequency from 2·5 to 1·1 times per week (as indicated by this study), with an average change time of 20 minutes, would free up 2427 hours of nursing time annually. This is equivalent to more than one full‐time health care professional. In the light of the increasing burden of wounds as a result of demographic and lifestyle changes, freeing up time in this way is increasingly important.
Limitations
This study demonstrated interesting and encouraging results, but has limitations. Firstly, although it was shown that signs of infection reduced and quality of life increased during the study period, there was no comparative control group using gold standard wound care. Secondly, the study population is limited to one wound type in a relatively small number of patients, although the study was open to other wound types. The issues surrounding chronic wounds such as exudate and wound infection apply to other chronic wound aetiologies, and therefore, the findings of this study can be seen as transferrable to other wound types. However, further work in other populations would be of benefit.
Conclusions
This study supports the use of a new gelling fibre dressing containing silver in the management of moderately to highly exuding venous leg ulcers with clinical signs of infection. The dressing was found to be clinically acceptable by the clinician for all 14 patients, and easy to apply and remove; in 96·8% of removals, the dressing stayed intact on removal and could be removed in one piece. Fifty per cent of the wounds healed within the 8‐week study duration; between baseline and final assessment, the median percentage reduction in wound area was 98·2% and the median percentage reduction in devitalised tissue was 78%. Exudate levels and wound pain were significantly improved at final assessment compared to baseline assessment. A reduction in bioburden and clinical signs of infection and an improvement in quality of life were observed over the 8‐week period. The dressing can contribute to the effective use of resources, demonstrating an average wear time of 6·4 days.
Acknowledgements
This study was funded by Smith & Nephew. AR and RS are employees of Smith & Nephew. Emma Woodmansey (Smith & Nephew Medical, Hull, UK) provided advice on the microbiological aspects of the article.
References
- 1. Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health‐care providers in Europe. J Wound Care 2009;18:154–61. [DOI] [PubMed] [Google Scholar]
- 2. Cooper R. European Wound Management Association (EWMA). Position document: identifying criteria for wound infection. London: MEP Ltd, 2005. [Google Scholar]
- 3. Wounds UK. Best practice statement: the use of topical antiseptic/antimicrobial agents in wound management. Aberdeen: Wounds UK, 2010. [Google Scholar]
- 4. Cutting KF, White R. Defined and refined: criteria for identifying wound infection revisited. Br J Community Nurs 2004;9:S6–15. [DOI] [PubMed] [Google Scholar]
- 5. Collier M. Recognition and management of wound infections. Worldwidewounds, 2004. [WWW document]. URL http://www.worldwidewounds.com [accessed on 15 June 2013]
- 6. International consensus. Appropriate use of silver dressings in wounds. An expert working group consensus. London: Wounds International, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Healy B, Freedman A. ABC of wound healing: infections. BMJ 2006;332:838–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamilton C. Speculating to accumulate: reducing the cost of wound care by appropriate dressing selection. J Wound Care 2008;17:359–63. [DOI] [PubMed] [Google Scholar]
- 9. White R. Wound dressings and other topical treatment modalities in bioburden control. J Wound Care 2011;20:431–9. [DOI] [PubMed] [Google Scholar]
- 10. Cutting KF. Wound exudate: composition and functions. Br J Community Nurs 2003;8:4–9. [DOI] [PubMed] [Google Scholar]
- 11. Spear M. Wound exudate – the good, the bad, and the ugly. Plast Surg Nurs 2012;32:77–9. [DOI] [PubMed] [Google Scholar]
- 12. Vowden K, Vowden P. Understanding exudate management and the role of exudate in the healing process. Br J Community Nurs 2003;8(11 Suppl):4–13. [DOI] [PubMed] [Google Scholar]
- 13. International consensus. Optimising wellbeing in people living with a wound. An expert working group review. London: Wounds International, 2012. [Google Scholar]
- 14. Menon J. Managing exudate associated with venous leg ulceration. Br J Community Nurs 2012;(Suppl: S6, S8, S10 passim). [DOI] [PubMed] [Google Scholar]
- 15. Barnea Y, Weiss J, Gur E. A review of the applications of the hydrofiber dressing with silver (Aquacel Ag) in wound care. Ther Clin Risk Manag 2010;6:21–7. [PMC free article] [PubMed] [Google Scholar]
- 16. Cutting KF, White RJ. Maceration of the skin and wound bed. 1: its nature and causes. J Wound Care 2002;11:275–8. [DOI] [PubMed] [Google Scholar]
- 17. Bowler PG, Davies BJ. The microbiology of infected and noninfected leg ulcers. Int J Dermatol 1999;38:573–8. [DOI] [PubMed] [Google Scholar]
- 18. Davies CE, Hill KE, Newcombe RG, Stephens P, Wilson MJ, Harding KG, Thomas DW. A prospective study of the microbiology of chronic venous leg ulcers to reevaluate the clinical predictive value of tissue biopsies and swabs. Wound Repair Regen 2007;15:17–22. [DOI] [PubMed] [Google Scholar]
- 19. Madsen SM, Westh H, Danielsen L, Rosdahl VT. Bacterial colonization and healing of venous leg ulcers. APMIS 1996;104:895–9. [DOI] [PubMed] [Google Scholar]
- 20. Trengove NJ, Stacey MC, McGechie DF, Mata S. Qualitative bacteriology and leg ulcer healing. J Wound Care 1996;5:277–80. [DOI] [PubMed] [Google Scholar]
- 21. Hansson C, Hoborn J, Möller A, Swanbeck G. The microbial flora in venous leg ulcers without clinical signs of infection. Repeated culture using a validated standardised microbiological technique. Acta Derm Venereol 1995;75:24–30. [DOI] [PubMed] [Google Scholar]
- 22. Moore K, Hall V, Paull A, Morris T, Brown S, McCulloch D, Richardson M, Harding K. Surface bacteriology of venous leg ulcers and healing outcome. J Clin Pathol 2010;63:830–4. [DOI] [PubMed] [Google Scholar]
- 23. Lansdown AB. Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol 2006;33:17–34. [DOI] [PubMed] [Google Scholar]
- 24. Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. J Hosp Infect 2005;60:1–7. [DOI] [PubMed] [Google Scholar]
- 25. Hareendran A, Bradbury A, Budd J, Geroulakos G, Hobbs R, Kenkre J, Symonds T. Measuring the impact of venous ulcers on quality of life. J Wound Care 2005;14:53–7. [DOI] [PubMed] [Google Scholar]
- 26. Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition‐specific questionnaire to assess health‐related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004;1:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iglesias CP, Birks Y, Nelson EA, Scanlon E, Cullum NA. Quality of life of people with venous leg ulcers: a comparison of the discriminative and responsive characteristics of two generic and a disease specific instruments. Qual Life Res 2005;14:1705–18. [DOI] [PubMed] [Google Scholar]
- 28. Drew P, Posnett J, Rusling L. The cost of wound care for a local population in England. Int Wound J 2007;4:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vowden K, Vowden P, Posnett J. The resource costs of wound care in Bradford and Airedale primary care trust in the UK. J Wound Care 2009;18:93–102. [DOI] [PubMed] [Google Scholar]


