Abstract
Objective
To investigate whether inter-professional simulation training influenced the rate of red blood cell (RBC) transfusions after birth.
Design
Two cohorts were compared retrospectively using a pre–post design.
Setting
Norwegian university hospital with 4800 deliveries annually.
Population
Women with estimated blood loss >500 mL within 24 h after birth in 2009 and 2011.
Methods
In 2010, all maternity staff attended a 6-h, scenario-based training on emergency obstetrics including postpartum hemorrhage, using a birthing simulator. The simulation focused on prevention, identification, and treatment of postpartum hemorrhage and on communication and leadership. Debrief immediately after the scenarios involved reflection and self-assessment.
Main outcome measures
The frequency of women receiving RBC transfusions as a marker for blood loss. Secondary outcome was the frequency of surgical procedures in the management of postpartum hemorrhage.
Results
In 2009, 111/534 (20.8%) women with estimated blood loss >500 mL after birth received RBC transfusions vs. 67/546 (12.3%) in 2011 (p < 0.01). The adjusted odds ratio for women receiving RBC transfusions in 2011 vs. 2009 was 0.53 (95% CI 0.38–0.74). Parity, oxytocin augmentation, duration of second stage, episiotomy, operative vaginal delivery, and sphincter injury were included in the final model. The odds ratio was stable in all combinations of possible confounders. We observed a significant reduction in the frequencies of curettage (p < 0.01) and uterine artery embolizations (p = 0.01).
Conclusion
We found a significant reduction in RBC transfusions after birth, which might be associated with mandatory simulation training. A causal link cannot be documented because of complex interactions of several variables.
Keywords: Post-partum hemorrhage, blood transfusion, simulation training, morbidity
Key Message
We examined whether inter-professional simulation training could influence the rate of red blood cell transfusions after birth and observed a significant reduction in red blood cell transfusions, curettages, and uterine artery embolization after introduction of mandatory simulation training on management of postpartum hemorrhage.
Introduction
Postpartum hemorrhage (PPH) is a major cause of maternal mortality worldwide (1–3), and it is the most preventable cause (4). PPH is diagnosed as estimated blood loss >500 mL after vaginal birth and >1000 mL after cesarean section (CS) (3). Additionally, PPH can be defined as excessive bleeding causing symptoms or signs of hypovolemia. The reported rate of PPH varies from 1 to 20% (5–7), and the incidence seems to be increasing (8). Uterine atony is responsible for at least 80% of the cases of PPH (5,9). Recognizing the correct amount of lost blood is challenging, and it is most commonly based on a visual estimation where underestimation seems to be proportional with excessive blood loss (7,10). Estimation skills can be improved by simple education (11). Unnecessary transfusion of blood products should be avoided because of potential risks associated with the procedure, side effects, and the limited availability of blood units (12). Inter-professional training is potentially effective in the prevention of errors and thereby patient safety can be improved (13–16). The notion of self-efficacy is known as the extent or strength of one's belief in one's own ability to complete tasks and reach goals (17). Medical simulation training might strengthen the level of self-efficacy, group efficacy, and competence pertaining to the management of PPH (17). Evaluation of a training program should take place on four levels: reaction, learning, behavior, and results. Measuring results as patient outcome is considered the most challenging part (18). Studies analyzing the effects of inter-professional simulation training have indicated improved patient outcomes based on Apgar scores and hypoxic–ischemic encephalopathy (19), but we did not find any studies showing a significant reduction of PPH related to scenario-based training (20,21).
In 2009, 11.2% of the mothers who gave birth at Stavanger University Hospital had an estimated blood loss >500 mL. In this group, 20.8% received red blood cell (RBC) transfusions. In 2010 an inter-professional scenario-based training started with a systemic approach to prevent human error (22). Facilitators helped fellow staff members to reflect on the practice carried out during the scenarios and to stimulate the participants to carefully examine their understanding of PPH (23). We hypothesized that training might reduce bleeding and that RBC transfusion is a better marker for bleeding than estimated blood loss. The aim of this study was to investigate whether the simulation training might have influenced the rate of RBC transfusions.
Material and methods
This study was carried out at Stavanger University Hospital, Norway, which serves an unselected population of 320 000 citizens including approximately 4800 deliveries annually. There was no change in birth cohort visitations during the periods. The study population comprised all women who delivered newborns with gestational age ≥23 weeks and with an estimated blood loss exceeding 500 mL within 24 h after birth in 2009 and 2011. The time line for receiving RBC transfusions was the period of admission. The attending midwife, or obstetrician in the case of CS, visually estimated the blood loss. Data were extracted from the registry of the Department of Immunology and Transfusion Medicine and from the hospital's electronic birth registration system. To ensure reliable data quality, we individually checked the hospital's electronic patient record system for relevant information on all the women with estimated blood loss >500 mL in 2009 and 2011.
Two cohorts were compared retrospectively using a pre–post design. The frequency of women receiving RBC transfusions was the primary outcome measure regarded as a surrogate marker for the actual blood loss after birth, and secondary outcomes were the frequencies of curettage, B-Lynch sutures, uterine embolization, and hysterectomy.
Vacuum extraction was the preferred operative delivery method in the department and episiotomies were medio-lateral or lateral. The hemoglobin (Hgb) levels before admission, the lowest Hgb level during admission, and the Hgb level at discharge were recorded as g/dL. Hypertension at admission was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, and the start of the active phase of labor was defined as when cervical dilatation was ≥4 cm combined with regular contractions. Epidural analgesia was given as a combination of low-dose fentanyl/ropivacaine and perineal tears grade 3–4 were defined as a sphincter injury.
In 2010, the hospital implemented a yearly, mandatory, 6-h inter-professional training for 180 midwives, doctors, and nurse assistants in the maternity wards. The simulation training comprised lectures and discussions on local guidelines for obstetric emergencies, such as PPH with a focus on atony being the major cause of PPH, pre-defined learning objectives, scenarios with role play, and reflections in inter-professional teams.
The scenario-based training on PPH involved small teams being exposed to scenarios on excessive blood loss after birth. A fellow midwife using the strap-on birthing simulator “MamaNatalie” (Laerdal Global Health, Stavanger, Norway) acted as a laboring woman (Figure1) featuring a possible blood loss ≤1200 mL and bleeding in a manner corresponding with atony after birth. Uterine massage was an important part of the protocol on PPH and was emphasized in all PPH scenarios as contributing to the prevention of excessive blood loss. According to the protocol, the midwife consults the obstetrician on duty when estimated blood loss exceeds 1000 mL. Simulation training focused on the algorithm regarding PPH, where the midwife was supposed to perform active management of the third stage of labor, including thorough uterine massage and routine administration of oxytocin. After running PPH scenarios, the teams were challenged on visual estimation of the blood loss. In cases of ongoing hemorrhage, the midwife is supposed to call for help, administer a high-dose oxytocin infusion, give misoprostol rectally, empty the urinary bladder, and repair bleeding perineal tears. In case of excessive blood loss, the protocol recommends bimanual compression of the uterus and transfer of the mother to the operating theater. Scenario-based training was limited to the emergency situation and the treatment given before transfer to the operating theatre. According to protocol, curettage and, if needed, uterine artery embolization (available round-the-clock) were to be carried out in the operating theater if excessive bleeding remained. For women with severe PPH after cesarean section, B-Lynch sutures were recommended. Hysterectomy would be the last option, performed only as a life-saving procedure.
Figure 1.
Simulation training using MamaNatalie.
The facilitators, being fellow midwives trained to facilitate multi-professional teams, challenged the groups on their experiences, understanding, and actions, with the aim of strengthening self-efficacy and adherence to local procedures to prevent human errors. Simulation training at Stavanger University Hospital was carried out at the Stavanger Acute Medicine Foundation for Education and Research, a simulation center outside the hospital premises.
Inter-professional teamwork was maintained as a focus area in the clinic after the training sessions. The maternity staff at Stavanger University Hospital did not record the performance of uterine massage and non-technical skills like proper communication and cooperation in the patient files. Hence, the clinical effect of simulation training in inter-professional teams had to be measured indirectly by patient outcome.
Statistical analysis
Categorical variables were compared with the chi-squared test or Fisher exact test and continuous variables with the t-test or Mann–Whitney U-test. We performed logistic regression analyses using RBC transfusions as a dependent variable and the time period before and after the first training as the main variable (2009 vs. 2011). We tested parity, maternal age, body mass index, gestational age, previous PPH, previous CS, hypertension at admission, induction of labor, oxytocin augmentation, epidural analgesia, duration of the active phase of labor, duration of the second stage of labor, CS, operative vaginal delivery, episiotomy, sphincter injury, placental weight, and birthweight as possible confounders. The duration of the active phase was recorded as zero and not as a missing value in women where a CS was performed before the start of the active phase. Variables with unadjusted p-values <0.25 were included in the multivariable analysis. The confounding effect was set to at least a 10% change in the estimate of the main dependent variable. The analyses were conducted with the IBM SPSS Statistics, Version 21.0 (IBM Corp., Armonk, NY, USA).
The Regional Committee for Medical and Health Research Ethics assessed the study to be quality work not needing ethical approval (2012/308/REK West). Approvals for access to and analyses of patient data were granted from the Research Department and the Data Protection Officer at Stavanger University Hospital.
Results
In 2009, 4777 women delivered their infants at Stavanger University Hospital and 534 (11.2%) had an estimated blood loss >500 mL, compared with 546/4872 (11.2%) in 2011. The mean estimated blood loss did not change significantly and was 1109 mL (median 800 mL) in 2009 vs. 1051 mL (median 800 mL) in 2011 (p = 0.22). The mean estimated blood loss for women receiving RBC transfusions was 1898 mL (median 1500 mL) vs. 1911 mL (median 1600 mL) accordingly (p = 0.95). However, we observed a significant reduction (41%) of women who received RBC transfusions in the study population. In 2009, 111/534 (20.8%) women with estimated blood loss >500 mL after birth received RBC transfusions vs. 67/546 (12.3%) in 2011 (p < 0.01). For women with vaginal deliveries, 80/389 (20.6%) of the study population received RBC transfusions in 2009 vs. 53/410 (12.9%) in 2011 (p < 0.01).For women with CS the RBC frequencies were 31/145 (21.4%) in 2009 vs. 14/136 (10.3%) in 2011 (p = 0.01). The overall numbers of RBC units given to the study population were 393 in 2009 vs. 205 in 2011. The frequencies of plasma transfusions and platelet transfusions were not significantly changed. The frequency of RBC transfusions to women with estimated blood loss <500 mL within the first 24 h (not a part of the study population) was 1.2% in 2009 vs. 0.7% in 2011; p = 0.02. The overall frequency of blood transfusions in the maternity wards was 3.4% in 2009 vs. 2.0% in 2011; p < 0.01.
We observed no significant differences in maternal characteristics between the time periods. However, the frequency of oxytocin augmentation was 29.2% in 2009 and 21.2% in 2011 (p < 0.01). The median duration of the active phase of labor was 300 min in 2009 and 316 min in 2011 (p = 0.05). Details of maternal and labor characteristics are presented in Table 1. Uterine artery embolization was performed on 10 women in 2009 compared with one woman in 2011 (p = 0.01), and the frequency of women undergoing curettage was 11.0% vs. 6.0% (p < 0.01). We observed no change in the number of women needing B-Lynch sutures or hysterectomies. The Hgb level before birth, lowest Hgb level during admission, and Hgb level at discharge (mean values) were not significantly different between time periods. Characteristics associated with PPH are presented in Table 2.
Table 1.
Maternal and labor characteristics
| Before training, 2009 (n = 534) | After training, 2011 (n = 546) | p-value | |||
|---|---|---|---|---|---|
| Maternal characteristics | |||||
| Nulliparous women | 285 | 53.4% | 283 | 51.8% | 0.61 |
| Maternal age (years) | 30.8 | SD = 5.2 | 30.2 | SD = 5.1 | 0.05 |
| BMI (kg/m2) (n = 482/518) | 25.1 | SD = 5.2 | 24.6 | SD = 5.0 | 0.09 |
| Gestational age (days) | 283 | IQR = 15 | 283 | IQR = 14 | 0.61 |
| Previous PPH | 21 | 3.9% | 29 | 5.3% | 0.28 |
| Previous cesarean section | 69 | 12.9% | 86 | 15.8% | 0.19 |
| Hypertension at admission | 120 | 26.7% | 119 | 27.3% | 0.83 |
| Labor characteristics | |||||
| Induction of labor | 133 | 24.9% | 155 | 28.4% | 0.20 |
| Oxytocin augmentation | 156 | 29.2% | 116 | 21.2% | < 0.01 |
| Epidural analgesia | 230 | 43.1% | 239 | 43.8% | 0.82 |
| Duration of active phase (min) (n = 440/459) | 300 | IQR = 326 | 316 | IQR = 371 | 0.05 |
| Duration of second stage (min) (n = 357/364) | 32 | IQR = 42 | 31 | IQR = 47 | 0.95 |
| Caesarean section | 145 | 27.2% | 136 | 24.9% | 0.40 |
| Operative vaginal delivery | 115 | 21.5% | 100 | 18.3% | 0.19 |
| Episiotomy | 153 | 28.7% | 139 | 25.5% | 0.24 |
| Sphincter injury | 24 | 4.5% | 20 | 3.7% | 0.49 |
| Birthweight (g) | 3612 | SD = 647 | 3599 | SD = 654 | 0.74 |
| Placental weight (g) | 695 | SD = 168 | 709 | SD = 170 | 0.16 |
Standard deviation is given in combination with the mean, interquartile range (IQR) is given with the median, and the percentage (%) is given with the number of participants. The p-values refer to t-test for differences in mean scores, Mann–Whitney U-test for differences in medians, or chi-squared test for differences in proportions.
BMI, body mass index; IQR, interquartile range; PPH, postpartum hemorrhage; SD, standard deviation.
Table 2.
Variables associated with postpartum hemorrhage
| Before training, 2009 (n = 534) | After training, 2011 (n = 546) | p-value | |||
|---|---|---|---|---|---|
| Estimated blood loss (mL) | 1109 | SD = 872 | 1051 | SD = 653 | 0.22 |
| RBC transfusions ≥1 unit | 111 | 20.8% | 67 | 12.3% | < 0.01 |
| 1 unit | 2 | 0.4% | 3 | 0.5% | 1.0* |
| 2 units | 67 | 12.5% | 41 | 7.5% | <0.01 |
| 3 units | 15 | 2.8% | 4 | 0.7% | <0.01 |
| 4 units | 12 | 2.2% | 11 | 2.0% | 0.79 |
| ≥5 units | 15 | 2.8% | 8 | 1.5% | 0.13 |
| Platelet transfusions ≥1 unit | 14 | 2.8% | 8 | 1.5% | 0.18 |
| Plasma transfusions ≥1 unit | 27 | 5.1% | 18 | 3.3% | 0.15 |
| Iron sucrose injections ≥1 unit | 43 | 8.1% | 52 | 9.5% | 0.39 |
| Hgb level before birth (n = 585) | 11.4 | SD = 1.2 | 11.6 | SD = 1.2 | 0.11 |
| Lowest Hgb level (n = 1070) | 9.1 | SD = 1.5 | 9.3 | SD = 1.4 | 0.18 |
| Lowest hematocrit (n = 658) | 27.4 | SD = 4.3 | 28.0 | SD = 4.3 | 0.09 |
| Hgb level at discharge (n = 1066) | 9.6 | SD = 1.1 | 9.6 | SD = 1.2 | 0.750 |
| Manual removal of placenta | 47 | 8.8% | 52 | 9.5% | 0.68 |
| Curettage | 59 | 11.0% | 33 | 6.0% | <0.01 |
| B-Lynch sutures | 8 | 1.5% | 8 | 1.5% | 0.96 |
| Hysterectomy | 3 | 0.6% | 2 | 0.4% | 0.68* |
| Uterine artery embolization | 10 | 1.9% | 1 | 0.2% | 0.01 |
Standard deviation is given in combination with the mean; percentage (%) is given with the number of participants. The p-values refer to t-test for differences in means and chi-squared test or Fisher's exact test *for differences in proportions.
Hgb, hemoglobin; RBC, red blood cell; SD, standard deviation.
The unadjusted odds ratio for women receiving RBC transfusions in 2011 was 0.53 (95% CI 0.38–0.74) compared with 2009, and the odds ratio was stable in all combinations of possible confounders. The odds ratio was 0.53 (95% CI 0.38–0.75) in the final multivariable model, which is presented in Table 3. Body mass index showed a significant unadjusted association, odds ratio 0.94 (95% CI 0.91–0.98); however, this variable was excluded from the final model because of missing information in 7% of the cases. The reduction in oxytocin augmentation had no significant influence on the frequency of women receiving RBC transfusions in the multivariable logistic regression analysis (Table 3). None of the possible confounders changed the estimate of time periods by more than 4%.
Table 3.
Multivariate logistic regression analyses using red blood cell transfusion (yes/no) as a dependent variable (n = 178)
| Unadjusted OR | (95% CI) | p-value | Adjusted OR | (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Time periods (2009/2011) | 0.53 | 0.38–0.74 | <0.01 | 0.53 | 0.38–0.75 | <0.01 |
| Sphincter injury (yes/no) | 3.08 | 1.63–5.83 | <0.01 | 2.68 | 1.38–5.21 | <0.01 |
| Episiotomy (yes/no) | 1.71 | 1.22–2.40 | <0.01 | 1.67 | 1.07–2.58 | 0.02 |
| Parity (0/1) | 0.71 | 0.52–0.99 | 0.04 | 0.83 | 0.57–1.20 | 0.32 |
| Operative vaginal delivery (yes/no) | 1.35 | 0.92–1.98 | 0.12 | 0.77 | 0.47–1.28 | 0.32 |
| Duration of active second stage (min) | 1.00 | 1.00–1.00 | 0.21 | 1.00 | 1.00–1.00 | 0.69 |
| Oxytocin augmentation (yes/no) | 1.24 | 0.87–1.77 | 0.24 | 0.97 | 0.63–1.48 | 0.88 |
OR, odds ratio.
Discussion
Our main finding was a significant reduction in RBC transfusions, and the frequencies of curettage and uterine artery embolization were significantly reduced. We observed no significant changes in Hgb levels at discharge. The frequency of women with estimated blood loss >500 mL remained approximately the same in 2011 and 2009.
The results suggest that there was a reduction in blood loss based on a significant reduction in mothers receiving RBC transfusions, no observed changes in Hgb levels at discharge, and the reduced need for surgical procedures; although, the estimation of blood loss remained the same. The validity of assessing changes in blood loss based on visual estimation could be questioned. Practical training on the estimation of blood loss has in other studies been associated with increased estimates (11). The birth simulator MamaNatalie has a tank containing a maximum of 1200 mL artificial blood. Immediately after running PPH scenarios, the staff members were challenged on estimation of the actual blood loss and this training might explain why the estimates remained high in 2011. Focus on estimation procedure is likely to heighten the attention to blood loss, as well as improve the accuracy and increase the estimation of blood loss. Therefore, it is reasonable to have confidence in the other quality indicators: the number of RBC transfusions, Hgb level at discharge, and rate of surgical procedures, and to conclude that blood loss was probably reduced after the simulation training (2011) compared with the period before training (2009). Using blood transfusions as a surrogate marker for bleeding can be seen as relevant because of the unreliability of visual estimation of blood loss. Reducing the transfusion rates while maintaining the same level of Hgb concentration supports the hypothesis that bleeding has actually diminished. Otherwise it could be expected that women in the second period would be more anemic at discharge.
At Stavanger University Hospital, RBC transfusions as treatment for excessive bleeding after birth are given based on clinical indications, circulatory symptoms like hypovolemia, pre-shock, ongoing excessive bleeding not under control, or an Hgb level <7 g/dL (24). These indications remained the same throughout the study period. The intention to practice stricter indications for RBC transfusions was verbally implemented in 2011 because of increasing knowledge of the side effects of blood transfusions (12). Still, mothers were discharged with the same Hgb levels as in 2009; so we do not think this factor can explain the reduction of blood transfusions. A lower mean Hgb could be expected if the blood loss was equal in both periods.
There are no changes in the groups concerning maternal and labor characteristics besides oxytocin augmentation and sphincter ruptures. Both factors had no confounding effect in the multivariable logistic regression analysis.
The main strength of the study was the attendance by all maternity staff at the 6-h, mandatory simulation training with scenarios on obstetric emergencies including PPH. Another main strength was the obstetric data being consecutively recorded in electronic patient journals and that all journals for the study population were individually checked for quality control. The biggest limitation is the pre–post study design because no causality can be achieved, and only possible associations discussed. Unfortunately the use of balloon tamponade was not recorded in the electronic journal and we lack this information. The use of balloon tamponade was introduced in our hospital before 2009 and the hospital procedures on the use of balloon tamponade did not change within the study period. Another limitation is that the study population was based on visual estimation of blood loss. Studies confirm that estimation of blood loss generally is inaccurate and proportional with excessive bleeding (7,10). We could have tried cluster randomization; although, from an ethical perspective, it is difficult to randomize maternity wards so as not to carry out practical training to improve patient safety. Only the medical records of women with estimated blood loss >500 mL were investigated. Blood transfusions were also given to some mothers with estimated blood loss <500 mL. The frequency of RBC transfusions in this group was significantly lower in 2011 compared with 2009.
Simulation in medical education represents a major paradigm shift that leads to increased competence among health professionals. This educational method allows health workers to practice relevant procedures without the patient being present and subject to potential harm. Multiple studies have shown the effectiveness of simulation training related to technical and nontechnical skills, but few previous studies have demonstrated improved clinical outcomes (25). A study from Copenhagen did not find a significant effect of multi-professional obstetric skills training on the rate of RBC transfusion in vaginal births – 1.5% (2003), 1.6% (2005), and 1.2% (2007) – but a reduction after CS – 2.4%, 2.1%, and 0.7% (p < 0.01) (20,21). The overall RBC transfusion rate in our hospital was 3.4% before simulation training in 2009 and 2.0% after training. A possible explanation of the significant change in transfusion rate at Stavanger University Hospital could be the relatively high frequency of RBC transfusions before training and thereby more chance to find a significant effect of simulation training compared with the Danish study. In the Danish study, 26% of women receiving RBC transfusions were excluded, whereas in the study at Stavanger University Hospital all RBC transfusions related to PPH during admission are reported in our overall frequencies (3.4% vs. 2.0%).
The inter-professional training at Stavanger University Hospital differed from similar simulation training in many other hospitals. There was a continuous focus on local implementation to obtain improvements as illustrated by the Utstein Formula of Survival (Figure2), emphasizing the importance of medical science, educational efficiency and local implementation (26). One essential difference between the training at Stavanger University Hospital and similar exercises in other hospitals was the yearly, mandatory 6-h training for the entire maternity staff using the birthing simulator MamaNatalie, which was newly developed and offered a highly realistic way to simulate excessive bleeding (21). Actual competences may have increased because of relevant and realistic PPH scenarios. Another important factor was the review sessions involving reflection and self-assessment immediately after the scenario (27). Self-assessment has the highest impact on learning (28). The learning objectives in the simulation training were actions according to PPH protocol, including appropriate communication between staff members. Debriefing may have stimulated self-evaluation and group reflection on how to prevent, identify, and treat PPH most efficiently (28,29), resulting in more effective handling of PPH situations. By enabling maternity staff to more accurately identify critical blood loss and respond more promptly and efficiently according to the PPH protocol, clinical outcomes seem to have improved. A causal link cannot be documented because of complex interactions of several variables, and our tentative conclusion needs to be supported by studies implementing stronger research designs with better controls for alternative explanations.
Figure 2.
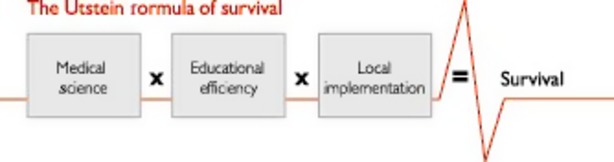
The Utstein formula of survival.
Acknowledgments
We thank Solveig Schouw, Elsa Lindtjørn, and Johanne E Huurnink for data sampling and Hege Ersdal for study design input.
Glossary
- CS
cesarean section
- Hgb
hemoglobin
- PPH
postpartum hemorrhage
- RBC
red blood cell
Funding
Signe Egenberg is PhD candidate funded by the Laerdal Foundation for Acute Medicine.
References
- 1.Winter C, Macfarlane A, Deneux-Tharaux C, Zhang WH, Alexander S, Brocklehurst P, et al. Variations in policies for management of the third stage of labour and the immediate management of postpartum haemorrhage in Europe. BJOG. 2007;1471:845–54. doi: 10.1111/j.1471-0528.2007.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skjeldestad FE, Oian P. Blood loss after cesarean delivery: a registry-based study in Norway, 1999-2008. Am J Obstet Gynecol. 2012;206:76. doi: 10.1016/j.ajog.2011.07.036. e1–7. [DOI] [PubMed] [Google Scholar]
- 3.Mousa HA, Blum J, Abou El Senoun G, Shakur H, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database Syst Rev. 2014;13:134. doi: 10.1002/14651858.CD003249.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. MDG 5: improve maternal health. WHO; 2013. Available online at: http://www.who.int/topics/millennium_development_goals/maternal_health/en/ (accessed June 24, 2014) [Google Scholar]
- 5.Sheldon WR, Blum J, Vogel JP, Souza JP, Gülmezoglu AM, Winikoff B, et al. Postpartum haemorrhage management, risks, and maternal outcomes: findings from the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121:5–13. doi: 10.1111/1471-0528.12636. [DOI] [PubMed] [Google Scholar]
- 6.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265–72. doi: 10.1111/j.1471-0528.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 7.Sloan NL, Durocher J, Aldrich T, Blum J, Winikoff B. What measured blood loss tells us about postpartum bleeding: a systematic review. BJOG. 2010;117:788–800. doi: 10.1111/j.1471-0528.2010.02567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossen J, Okland I, Nilsen OB, Eggebo TM. Is there an increase of postpartum hemorrhage, and is severe hemorrhage associated with more frequent use of obstetric interventions? Acta Obstet Gynecol Scand. 2010;89:1248–55. doi: 10.3109/00016349.2010.514324. [DOI] [PubMed] [Google Scholar]
- 9.Lutomski JE, Byrne BM, Devane D, Greene RA. Increasing trends in atonic postpartum haemorrhage in Ireland: an 11-year population-based cohort study. BJOG. 2012;119:306–14. doi: 10.1111/j.1471-0528.2011.03198.x. [DOI] [PubMed] [Google Scholar]
- 10.Bose P, Regan F, Paterson-Brown S. Improving the accuracy of estimated blood loss at obstetric haemorrhage using clinical reconstructions. BJOG. 2006;113:919–24. doi: 10.1111/j.1471-0528.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- 11.Al-Kadri HM, Dahlawi H, Airan MA, Elsherif E, Tawfeeq N, Mokhele Y, et al. Effect of education and clinical assessment on the accuracy of post partum blood loss estimation. BMC Pregnancy Childbirth. 2014;14:110. doi: 10.1186/1471-2393-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alter HJ, Klein HG. The hazards of blood transfusion in historical perspective. Blood. 2008;112:2617–26. doi: 10.1182/blood-2008-07-077370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGaghie WC, Issenberg SB, Petrusa ER, Scalese RJ. A critical review of simulation-based medical education research: 2003-2009. Med Educ. 2010;44:50–63. doi: 10.1111/j.1365-2923.2009.03547.x. [DOI] [PubMed] [Google Scholar]
- 14.Gundry R, Siassakos D, Crofts JF, Draycott TJ. Simulation training for obstetric procedures and emergencies. Fetal Maternal Med Rev. 2010;21:323–45C. [Google Scholar]
- 15.Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27:10–28. doi: 10.1080/01421590500046924. [DOI] [PubMed] [Google Scholar]
- 16.Rall M, Dieckmann P. General principles of managing critical situations and preventing errors in anesthesia and intensive care medicine. Eur Soc Anesthesiol. 2005 Available online at: https://www.guysandstthomas.nhs.uk/resources/education-training/sail/reading/crisis-mgt-pt-safety.pdf (accessed June 24, 2014) [Google Scholar]
- 17.Bandura A. Self-efficacy: the exercise of control. New York: W.H. Freeman and Company; 1997. [Google Scholar]
- 18.Kirkpatrick DL, Kirkpatrick JD. Evaluating training programs. The four levels. 3rd edn. San Francisco, CA: Berrett-Koehler Publisher; 2006. [Google Scholar]
- 19.Draycott T, Sibanda T, Owen L, Akande V, Winter C, Reading S, et al. Does training in obstetric emergencies improve neonatal outcome? BJOG. 2006;113:177–82. doi: 10.1111/j.1471-0528.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 20.Markova V, Sorensen JL, Holm C, Norgaard A, Langhoff-Roos J. Evaluation of multi-professional obstetric skills training for postpartum hemorrhage. Acta Obstet Gynecol Scand. 2012;91:346–52. doi: 10.1111/j.1600-0412.2011.01344.x. [DOI] [PubMed] [Google Scholar]
- 21.Sorensen JL, Lokkegaard E, Johansen M, Ringsted C, Kreiner S, McAleer S. The implementation and evaluation of a mandatory multi-professional obstetric skills training program. Acta Obstet Gynecol Scand. 2009;88:1107–17. doi: 10.1080/00016340903176834. [DOI] [PubMed] [Google Scholar]
- 22.Reason J. Human error: models and management. BMJ. 2000;2000:768–70. doi: 10.1136/bmj.320.7237.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudolph JW, Simon R, Rivard P, Dufresne RL, Raemer DB. Debriefing with good judgment: combining rigorous feedback with genuine inquiry. Anesthesiol Clin. 2007;25:361–76. doi: 10.1016/j.anclin.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Eggebø TM, Lichtenberg SM, Torkildsen EA. Hgb/anemi. Extend Quality System; Kvalitetshåndbok Kvinneklinikken SUS. Available online at: http://eqs-kk.sus.no/exportkk/docs/doc_12593/index.html (accessed October 6, 2014)
- 25.Siassakos D, Bristowe K, Draycott TJ, Angouri J, Hambly H, Winter C, et al. Clinical efficiency in a simulated emergency and relationship to team behaviours: a multisite cross-sectional study. BJOG. 2011;118:596–607. doi: 10.1111/j.1471-0528.2010.02843.x. [DOI] [PubMed] [Google Scholar]
- 26.Soreide E, Morrison L, Hillman K, Monsieurs K, Sunde K, Zideman D, et al. The formula for survival in resuscitation. Resuscitation. 2013;84:1487–93. doi: 10.1016/j.resuscitation.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Steinwachs B. How to facilitate a debriefing. Simul Gaming. 1992;23:186–95. [Google Scholar]
- 28.Hattie J. Visible learning: a synthesis of over 800 meta-analyses relating to achievement. New York: Routledge; 2009. [Google Scholar]
- 29.Prick BW, Vos AA, Hop WC, Bremer HA, Steegers EA, Duvekot JJ. The current state of active third stage management to prevent postpartum hemorrhage: a cross-sectional study. Acta Obstet Gynecol Scand. 2013;92:1277–83. doi: 10.1111/aogs.12238. [DOI] [PubMed] [Google Scholar]


