Abstract
Emergency and difficult tracheal intubations are hazardous undertakings where successive laryngoscopy–hypoxaemia–re-oxygenation cycles can escalate to airway loss and the ‘can't intubate, can't ventilate’ scenario. Between 2013 and 2014, we extended the apnoea times of 25 patients with difficult airways who were undergoing general anaesthesia for hypopharyngeal or laryngotracheal surgery. This was achieved through continuous delivery of transnasal high-flow humidified oxygen, initially to provide pre-oxygenation, and continuing as post-oxygenation during intravenous induction of anaesthesia and neuromuscular blockade until a definitive airway was secured. Apnoea time commenced at administration of neuromuscular blockade and ended with commencement of jet ventilation, positive-pressure ventilation or recommencement of spontaneous ventilation. During this time, upper airway patency was maintained with jaw-thrust. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) was used in 15 males and 10 females. Mean (SD [range]) age at treatment was 49 (15 [25–81]) years. The median (IQR [range]) Mallampati grade was 3 (2–3 [2–4]) and direct laryngoscopy grade was 3 (3–3 [2–4]). There were 12 obese patients and nine patients were stridulous. The median (IQR [range]) apnoea time was 14 (9–19 [5–65]) min. No patient experienced arterial desaturation < 90%. Mean (SD [range]) post-apnoea end-tidal (and in four patients, arterial) carbon dioxide level was 7.8 (2.4 [4.9–15.3]) kPa. The rate of increase in end-tidal carbon dioxide was 0.15 kPa.min−1. We conclude that THRIVE combines the benefits of ‘classical’ apnoeic oxygenation with continuous positive airway pressure and gaseous exchange through flow-dependent deadspace flushing. It has the potential to transform the practice of anaesthesia by changing the nature of securing a definitive airway in emergency and difficult intubations from a pressured stop–start process to a smooth and unhurried undertaking.
Introduction
The principal objective of airway management during anaesthesia is maintenance of oxygenation. As the patient transitions from wakefulness to anaesthesia and receives neuromuscular blockade, the anaesthetist is afforded a finite time (‘apnoeic window’) during which to secure a definitive airway. Failure to do so normally results in recommencement of facemask ventilation, re-oxygenation and a further attempt at securing a definitive airway. In some patients, the combination of unfavourable pharyngolaryngeal anatomy and reduced apnoea time due to cardiorespiratory decompensation makes this stop-start approach hazardous. Multiple attempts at difficult laryngoscopy increase the risk of airway trauma, which in turn makes subsequent attempts at laryngoscopy and facemask ventilation more difficult [1]. This can deleteriously impact on human factors that are intrinsic to a highly pressured clinical scenario [2], and can readily cascade into a ‘cannot intubate, cannot ventilate’ scenario with significant attending morbidity and mortality [3,4].
The mainstay method of increasing the apnoeic window is through pre-oxygenation, which entails spontaneous facemask ventilation with 100% oxygen [5]. Pre-oxygenation denitrogenises the lungs and creates an alveolar oxygen reservoir [6]. The size of this reservoir can be increased by reducing dependent atelectasis through head-up patient positioning [7] and raising mean airway pressure [8] but ultimately, the size of the oxygen reservoir is fixed at the end of pre-oxygenation and once apnoea begins, it does not get replenished.
Aventilatory mass flow (AVMF) [9] is a physiological phenomenon in which, provided that a patent air passageway exists between the lungs and the exterior, the difference between the alveolar rates of oxygen removal and carbon dioxide excretion generates a negative pressure gradient of up to 20 cmH2O [10] that drives oxygen into the lungs [9,11–16]. The clinical application of this phenomenon is known in modern anaesthetic practice as apnoeic oxygenation (i.e. AVFM and apnoeic oxygenation are synonymous). Apnoeic oxygenation has been used both experimentally and clinically as a strategy to extend the apnoeic window by providing a pharyngeal oxygen reservoir [9,11–14,16,17]. We report our early experience with OptiFlow™, a commercial transnasal humidified oxygen delivery system (Fisher and Paykel Healthcare Limited, Panmure, Auckland, New Zealand) to increase apnoea time in difficult airway patients undergoing general anaesthesia.
Methods
Between 2013 and 2014, 25 adult patients presenting for surgery, in whom the presence of a difficult airway was known based on previous anaesthetics or strongly anticipated based on unfavourable pharyngolaryngeal anatomy, and whose BMI or underlying cardiorespiratory disease made rapid arterial oxygen desaturation at induction of anaesthesia likely, were clinically judged as likely to benefit from using the Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) technique. Patients were undergoing surgery for laryngotracheal stenosis, vocal fold pathology and obstructive sleep apnoea, and benign and malignant hypopharyngeal obstruction.
All patients were pre-oxygenated at 40 degrees of head-up inclination with the OptiFlow nasal cannula (Fig.1) at a rate of 70 l.min−1 for 10 min. Intravenous induction of anaesthesia then commenced with boluses of 2–3 mg.kg−1 propofol, 1–2 μg.kg−1 fentanyl, and 0.5 mg.kg−1 rocuronium, followed by a peripheral infusion of propofol at a rate of 0.2–0.3 mg.kg−1.min−1. Jaw-thrust was performed immediately the patient became unconscious and was maintained throughout the apnoeic period, to ensure upper airway patency. Facemask ventilation was confirmed and discontinued. The patient's angle of inclination was reduced to 20 degrees for laryngoscopy. The first attempt at laryngoscopy was with a standard metal Macintosh laryngoscope and if this was unsuccessful, an A.P. Advance™ videolaryngoscope (Venner Medical Deutschland GmbH, Dänischenhagen, Germany) with a Macintosh blade was used. If this also proved unsuccessful, an A.P. Advance difficult airway blade was used. Meticulous care was taken in all laryngoscopies not to traumatise the airway. Nasal oxygenation was maintained at the same rate of 70 l.min−1 until the definitive airway was secured. Apnoea time referred to the time between administration of neuromuscular blockade and commencement of jet ventilation, positive-pressure ventilation or recommencement of spontaneous ventilation.
Figure 1.
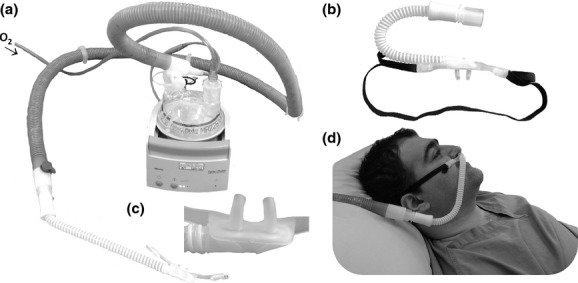
The OptiFlow high-flow humidified oxygen delivery system. The oxygen humidification unit (a) receives oxygen from a standard oxygen regulator and delivers humidified oxygen to a custom-built transnasal oxygen cannula (b and c) like a standard nasal oxygen cannula (d).
Information about patients' age, sex, ASA grade, burden of general morbidities (which was quantified using the Charlson co-morbidity score [18]), BMI, indication for and the nature of the procedure undertaken, and Mallampati [19] and Cormack-Lehane direct laryngoscopy grades [20] were recorded. Duration of apnoea was determined by clinical need in all cases and the nature of the airway placed at the end of the apnoeic period and procedure were recorded. Maximum heart rate and minimum oxygen saturations during apnoea, and end-tidal carbon dioxide levels once a definitive airway had been placed, were recorded from the anaesthetic record. In four patients with longer apnoea times and in whom arterial cannulation had been required, arterial blood gases were also measured. The correlation between carbon dioxide levels at the end of apnoea and apnoea time was assessed with simple correlation. Data were analysed using MedCalc (MedCalc Software bvba, Ostend, Belgium). As data were collected as part of delivering standard care following introduction of a new technique into clinical practice, formal ethical committee approval was not sought. However, we consulted the Caldicott guardian for approval to analyse the data and present the study.
Results
Twenty-five patients underwent induction of anaesthesia using the THRIVE technique between 2013 and 2014. There were 15 males and 10 females and mean age (SD [range]) at treatment was 49 (15 [25–81]) years. The median (IQR [range]) ASA grade was 3 (2–3 [1–4]). The median (IQR [range]) BMI was 30 (23–36 [18–52]) kg.m−2. The median (IQR [range]) age-adjusted Charlson co-morbidity index was 2 (0–4 [0–5]). Ten patients underwent treatment for benign laryngeal conditions, two patients had surgery for obstructive sleep apnoea and four patients had treatment for benign or malignant head and neck conditions. Nine patients had acute airway compromise with stridor on presentation. The median (IQR [range]) Mallampati grade was 3 (2–3 [2–4]) and direct laryngoscopy grade was 3 (3–3 [2–4]). The median (IQR [range]) apnoea time was 14 (9–19 [5–65]) min. No patient experienced arterial desaturation < 90% (Fig.2).
Figure 2.
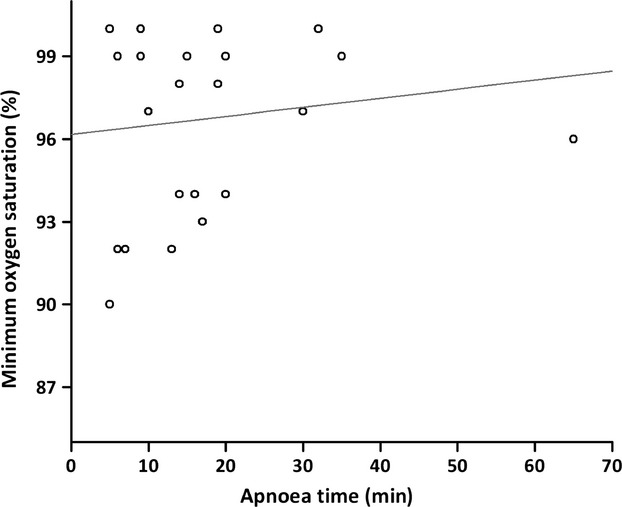
The relationship between apnoea time and oxygen saturation levels (n = 25). The line represents linear regression with r = 0.136 and p = 0.51.
The surgical procedures required different forms of definitive airway management: in 14 patients the definitive airway was suspension laryngoscopy and jet ventilation; and four patients were tracheally intubated. Furthermore, four patients had a laryngeal mask airway placed after THRIVE, one patient had a tracheostomy, and for two patients, THRIVE was the sole mode of ventilation throughout the procedure. Figure3 illustrates the relationship between duration of apnoea and carbon dioxide levels at the end of the apnoeic period.
Figure 3.
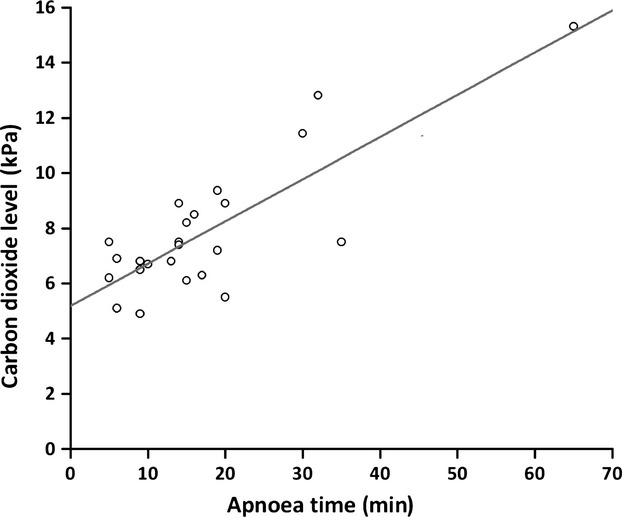
The relationship between apnoea time and end-tidal (and in four patients, arterial) carbon dioxide levels (n = 24). The line represents linear regression with r = 0.82 and p < 0.0001. The regression equation was CO2 = (5.2 ± 0.5) + (0.15 ± 0.02) × apnoea time.
Discussion
We found deployment of THRIVE beneficial in extending apnoea time in our patients with difficult airways undergoing general anaesthesia [1,21]. There were no desaturations below 90%, despite an average apnoea time of 17 min and none of the patients developed cardiac arrhythmias or other complications suggestive of carbon dioxide toxicity [13,22].
High-flow nasal oxygenation has successfully been used, predominantly in intensive care [23] but also in emergency department [24] settings, to treat acute respiratory failure [25–29], to prevent postoperative atelectasis [30], and to alleviate dyspnoea in acute heart failure [31]. Interest is also emerging in the use of this technique to increase the apnoeic window in the context of tracheal intubation in the intensive care unit [32]. Our findings extend the application of this technique to managing patients with difficult airways undergoing general anaesthesia.
In 23 of 25 of our patients, termination of apnoea was planned and THRIVE made securing the definitive airway a smooth and unpressured undertaking. One stridulous patient with acute airway compromise due to severe tracheobronchomalacia and a BMI of 34 kg.m−2 desaturated to 92% at 7 min after induction and is likely to have done so sooner without THRIVE, and a second patient with spasmodic dysphonia and a BMI of 52 kg.m−2 desaturated to 90% after 5 min of apnoea. In both of these cases, the modestly extended apnoeic window allowed suspension laryngoscopy and jet ventilation to be atraumatically established and saturations returned to 99% once jetting commenced. In two other patients, THRIVE was used throughout the procedure; these patients had apnoea times of 32 and 65 min, and this allowed pharyngolaryngeal surgery to be performed.
The physiological nomenclature for describing ‘apnoeic oxygenation’ has changed several times since the phenomenon was described by Volhard in 1908 [10]. It has been described as ‘diffusion respiration’ by Draper and Whitehead [33], as ‘AVMF’ by Bartlett et al. [9] and as ‘apnoeic oxygenation’ by Frumin et al. [13]. What all of these studies describe is oxygenation using only the difference in the rates of excretion of carbon dioxide and absorption of oxygen as the driver of gaseous flow. It was rapidly recognised that while apnoeic oxygenation alone could largely match the oxygen demands of the subject, it did not prevent a potentially rapid and dangerous rise in carbon dioxide concentration. In Frumin et al.'s experiments, two of eight human trials were prematurely terminated owing to development of ventricular arrhythmias [13], and in Draper et al.'s experiments, one of the 12 dogs died, most likely from carbon dioxide toxicity [11]. There were also early suggestions of patients' death and altered cerebral function following ‘classical’ apnoeic oxygenation [34,35]. Joels and Samueloff demonstrated that classical apnoeic oxygenation causes a progressive respiratory acidosis that rapidly overwhelms the blood's buffering mechanisms and progresses into a mixed acidosis that proves fatal [36]. Death is principally due to limited tolerance of the myocardial contractile [37] and conductive [22] mechanisms to acidosis. Joels and Samueloff's experiments placed the upper limit of the 95% CI for occurrence of death due to acidosis at a pH of 6.9 [36].
We provide further evidence that classical apnoeic oxygenation provides little clearance of carbon dioxide. We have plotted the rate of rise of carbon dioxide in three studies of classical apnoeic oxygenation [13,15,16], along with one study by Stock et al., who measured the rate of increase in carbon dioxide during airway obstruction (Fig.4) [38]. In all of these studies, the rate of rise of carbon dioxide levels was between 0.35 and 0.45 kPa.min−1, suggesting that classical apnoeic oxygenation provides a similarly low level of carbon dioxide clearance to that if the airway was obstructed.
Figure 4.
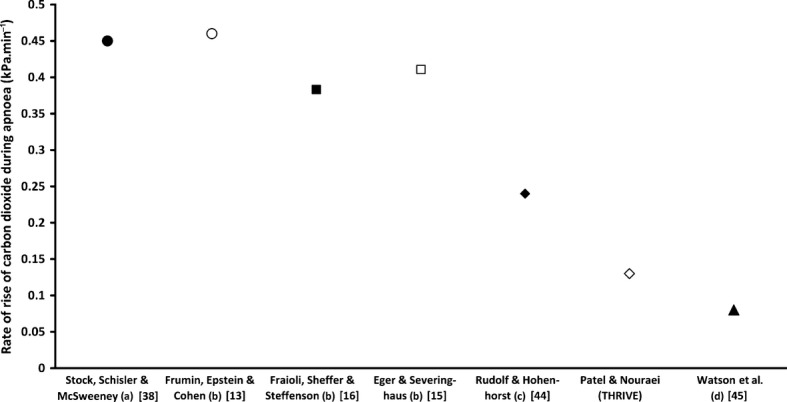
Rate of rise of carbon dioxide levels under different apnoea conditions undertaken within the study referred to: (a) airway obstruction; (b) classical apnoeic oxygenation; (c) low-flow intra-tracheal cannula and (d) high-flow intratracheal cannula.
It seems, therefore, that ‘apnoeic oxygenation’, while almost certainly a contributor to oxygenation during THRIVE, is an incomplete description for the technique. Perhaps, there is a contribution from a high-flow pre-oxygenation element, since very high flows of oxygen before or at induction may reduce rebreathing and maximise oxygen stores [39]. Moreover, we believe that a better explanation for the physiology that is being clinically observed can be derived from Meltzer and Auer's ‘respiration without respiratory movements’ experiments [40]. They maintained prolonged ventilation, apparently without carbon dioxide toxicity, through continuous insufflation of oxygen into the trachea [40]. They chose the calibre of the cannula to be smaller than tracheal diameter to allow gases to be exchanged with the exterior. We believe that continuous insufflation is the critical component of THRIVE, which achieves a continuous positive airway pressure of approximately 7 cmH2O [41] that splints the upper airways and reduces shunting [42,43]. Continuous insufflation facilitates oxygenation [17,44] and carbon dioxide clearance through gaseous mixing and flushing of the deadspace. Evidence for the existence of flow-dependent, non-rhythmic ventilatory exchange can be provided by comparing the increase in rise of carbon dioxide under different continuous insufflation apnoeic conditions. Rudolf and Hohenhorst [45] performed a study in which ventilation was achieved through an intratracheal catheter delivering oxygen at a rate of 0.5 l.min−1. This achieved a rate of carbon dioxide increase of 0.24 kPa.min−1. Watson et al. used a high-flow tracheal cannula at 45 l.min−1 and achieved a steady-state carbon dioxide level within 5 min of the start of apnoea [46]. With THRIVE, the rate of carbon dioxide increase was 0.15 kPa.min−1 (Fig.3) and a steady-state carbon dioxide level was not reached.
Our study was limited by the fact that it was observational and cross-sectional, and we only maintained THRIVE until a definitive airway had been secured. Furthermore, we only recorded those measurements of oxygenation and carbon dioxide excretion that were necessary as part of delivering routine clinical care. Furthermore, experimental studies are needed to characterise safe upper limits of THRIVE in different patient groups. We encountered two instances of desaturation, although not hypoxaemia. One case occurred in the presence of severe obesity and a second in the presence of severe tracheobronchomalacia and obesity. We are mindful of the fact that the apnoeic window, while extended through post-oxygenation compared with pre-oxygenation alone, is unlikely to be the same in obese as in non-obese patients. Based on our preliminary findings, the safe upper limit of apnoea in the presence of morbid obesity can be as low as 5 min, but this needs to be confirmed through an experimental human physiology study. It is also unlikely that THRIVE can readily rescue those patients who have total airway obstruction and its use in the presence of a known or suspected cranial base fracture is also not advised.
In conclusion, we have shown that THRIVE, as currently administered through a standard commercially available nasal high-flow oxygen delivery system, could maintain oxygen saturations after commencement of apnoea to levels that could change the nature of difficult intubations from a hurried stop-start, potentially traumatic undertaking, to a smooth event undertaken within an extended safe apnoeic window.
Competing interests
No external funding or competing interests declared. AP has contributed to the design of the A.P. Advance videolaryngoscopy system.
References
- 1.Griesdale DEG, Bosma TL, Kurth T, Isac G, Chittock DR. Complications of endotracheal intubation in the critically ill. Intensive Care Medicine. 2008;34:1835–42. doi: 10.1007/s00134-008-1205-6. [DOI] [PubMed] [Google Scholar]
- 2.Schulz CM, Endsley MR, Kochs EF, Gelb AW, Wagner KJ. Situation awareness in anesthesia: concept and research. Anesthesiology. 2013;118:729–42. doi: 10.1097/ALN.0b013e318280a40f. [DOI] [PubMed] [Google Scholar]
- 3.Cook TM, Woodall N, Frerk C. Major Complications of Airway Management in the United Kingdom: Report and Findings 4th National Audit of the Royal College of Anaesthetists and the Difficult Airway Society. London: National Patient Safety Agency; 2011. [DOI] [PubMed] [Google Scholar]
- 4.Mort TC. The incidence and risk factors for cardiac arrest during emergency tracheal intubation: a justification for incorporating the ASA Guidelines in the remote location. Journal of Clinical Anesthesia. 2004;16:508–16. doi: 10.1016/j.jclinane.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Baraka AS, Taha SK, Aouad MT, El-Khatib MF, Kawkabani NI. Preoxygenation: comparison of maximal breathing and tidal volume breathing techniques. Anesthesiology. 1999;91:612–6. doi: 10.1097/00000542-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Annals of Emergency Medicine. 2012;59:165–75. doi: 10.1016/j.annemergmed.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Dixon BJ, Dixon JB, Carden JR, et al. Preoxygenation is more effective in the 25 degrees head-up position than in the supine position in severely obese patients: a randomized controlled study. Anesthesiology. 2005;102:1110–5. doi: 10.1097/00000542-200506000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. American Journal of Respiratory and Critical Care Medicine. 2006;174:171–7. doi: 10.1164/rccm.200509-1507OC. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett RG, Jr, Brubach HF, Specht H. Demonstration of aventilatory mass flow during ventilation and apnea in man. Journal of Applied Physiolology. 1959;14:97–101. doi: 10.1152/jappl.1959.14.1.97. [DOI] [PubMed] [Google Scholar]
- 10.Volhard F. Uber kunstliche Atmung durch Ventilation der Trachea und eine einfache Vorrichtung zur hytmischen kunstlichen Atmung. München Medizinische Wochenschrift. 1908;55:209–11. [Google Scholar]
- 11.Draper WB, Whitehead RW, Spencer JN. Studies on diffusion respiration. III. Alveolar gases and venous blood pH of dogs during diffusion respiration. Anesthesiology. 1947;8:524–33. doi: 10.1097/00000542-194709000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Holmdahl MH. Pulmonary uptake of oxygen, acid-base metabolism, and circulation during prolonged apnoea. Acta Chirurgica Scandinavica Supplementum. 1956;212:1–128. [PubMed] [Google Scholar]
- 13.Frumin MJ, Epstein RM, Cohen G. Apneic oxygenation in man. Anesthesiology. 1959;20:789–98. doi: 10.1097/00000542-195911000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Weitzner SW, King BD, Ikezono E. The rate of arterial oxygen desaturation during apnea in humans. Anesthesiology. 1959;20:624–7. doi: 10.1097/00000542-195909000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Eger EI, Severinghaus JW. The rate of rise of PaCO2 in the apneic anesthetized patient. Anesthesiology. 1961;22:419–25. doi: 10.1097/00000542-196105000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Fraioli RL, Sheffer LA, Steffenson JL. Pulmonary and cardiovascular effects of apneic oxygenation in man. Anesthesiology. 1973;39:588–96. doi: 10.1097/00000542-197312000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Teller LE, Alexander CM, Frumin MJ, Gross JB. Pharyngeal insufflation of oxygen prevents arterial desaturation during apnea. Anesthesiology. 1988;69:980–2. doi: 10.1097/00000542-198812000-00035. [DOI] [PubMed] [Google Scholar]
- 18.Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4:94. doi: 10.1186/1471-2407-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42:487–90. doi: 10.1111/j.1365-2044.1987.tb04039.x. [DOI] [PubMed] [Google Scholar]
- 20.Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105–11. [PubMed] [Google Scholar]
- 21.Jaber S, Amraoui J, Lefrant J-Y, et al. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: A prospective, multiple-center study. Critical Care Medicine. 2006;34:2355–61. doi: 10.1097/01.CCM.0000233879.58720.87. [DOI] [PubMed] [Google Scholar]
- 22.Gertler MM, Hoff HE, Humm DG. Acid tolerance of the dog heart. American Journal of Physiology. 1946;146:478–86. doi: 10.1152/ajplegacy.1946.146.3.478. [DOI] [PubMed] [Google Scholar]
- 23.Parke RL, McGuinness SP, Eccleston M. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respiratory Care. 2011;56:265–70. doi: 10.4187/respcare.00801. [DOI] [PubMed] [Google Scholar]
- 24.Lenglet H, Sztrymf B, Leroy C, Brun P, Dreyfuss D, Ricard J-D. Humidified high flow nasal oxygen during respiratory failure in the emergency department: a feasibility and efficacy study. Respiratory Care. 2012;57:1873–8. doi: 10.4187/respcare.01575. [DOI] [PubMed] [Google Scholar]
- 25.Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respiratory Care. 2010;55:408–13. [PubMed] [Google Scholar]
- 26.Millar J, Lutton S, O'Connor P. The use of high-flow nasal oxygen therapy in the management of hypercarbic respiratory failure. Therapeutic Advances in Respiratory Disease. 2014;8:63–4. doi: 10.1177/1753465814521890. [DOI] [PubMed] [Google Scholar]
- 27.Rello J, Perez M, Roca O, et al. High-flow nasal therapy in adults with severe acute respiratory infection: a cohort study in patients with 2009 influenza A/H1N1v. Journal of Critical Care. 2012;27:434–9. doi: 10.1016/j.jcrc.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Kugelman A, Riskin A, Said W, Shoris I, Mor F, Bader D. A randomized pilot study comparing heated humidified high-flow nasal cannulae with NIPPV for RDS. Pediatric Pulmonology. 2014 doi: 10.1002/ppul.23022. Mar 12; doi: 10.1002/ppul.23022. [DOI] [PubMed] [Google Scholar]
- 29.Beggs S, Wong ZH, Kaul S, Ogden KJ, Walters JA. High-flow nasal cannula therapy for infants with bronchiolitis. Cochrane Database of Systematic Reviews. 2014;1:CD009609. doi: 10.1002/14651858.CD009609.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Futier E, Paugam-Burtz C, Constantin JM, Pereira B, Jaber S. The OPERA trial – comparison of early nasal high flow oxygen therapy with standard care for prevention of postoperative hypoxemia after abdominal surgery: study protocol for a multicenter randomized controlled trial. Trials. 2013;14:341. doi: 10.1186/1745-6215-14-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roca O, Perez-Teran P, Masclans JR, et al. Patients with New York Heart Association class III heart failure may benefit with high flow nasal cannula supportive therapy: high flow nasal cannula in heart failure. Journal of Critical Care. 2013;28:741–6. doi: 10.1016/j.jcrc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Montanes RG, Messika J, Bertrand F, et al. Interest in High Flow Oxygen Therapy for Improving Oxygenation Pre- and Per- Intubation in the ICU 3rd Paris International Conference on Intensive Care (SRLF & ISICEM) Paris: The French Society of Intensive Care; 2013. [Google Scholar]
- 33.Draper WB, Whitehead RW. Diffusion respiration in the dog anesthetized with pentothal sodium. Anesthesiology. 1944;5:262–73. [Google Scholar]
- 34.Busse EW, Parry TM, Goldensohn ES, Whitehead RW, Draper WB. Alteration of cerebral function in man produced by diffusion respiration and prolonged inhalation of carbon dioxide. Diseases of the Nervous System. 1952;13:35–41. [PubMed] [Google Scholar]
- 35.Sims JL, Morris LE, Orth OS, Waters RM. The influence of oxygen and carbon dioxide levels during anesthesia upon postsurgical hepatic damage. Journal of Laboratory and Clinical Medicine. 1951;38:388–96. [PubMed] [Google Scholar]
- 36.Joels N, Samueloff M. Metabolic acidosis in diffusion respiration. Journal of Physiology. 1956;133:347–59. doi: 10.1113/jphysiol.1956.sp005591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell JH, Wildenthal K, Johnson RL. The effects of acid-base disturbances on cardiovascular and respiratory function. Kidney International. 1972;1:375–89. doi: 10.1038/ki.1972.48. [DOI] [PubMed] [Google Scholar]
- 38.Stock MC, Schisler JQ, McSweeney TD. The PaCO2 rate of rise in anesthetized patients with airway obstruction. Journal of Clinical Anesthesia. 1989;1:328–32. doi: 10.1016/0952-8180(89)90070-6. [DOI] [PubMed] [Google Scholar]
- 39.Pandit JJ, Duncan T, Robbins PA. Total oxygen uptake with two maximal breathing techniques and the tidal volume breathing technique. Anesthesiology. 2003;99:841–6. doi: 10.1097/00000542-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Meltzer SJ, Auer J. Continuous respiration without respiratory movements. Journal of Experimental Medicine. 1909;11:622–5. doi: 10.1084/jem.11.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritchie JE, Williams AB, Gerard C, Hockey H. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesthesia and Intensive Care. 2011;39:1103–10. doi: 10.1177/0310057X1103900620. [DOI] [PubMed] [Google Scholar]
- 42.Tokics L, Hedenstierna G, Strandberg A, Brismar B, Lundquist H. Lung collapse and gas exchange during general anesthesia: effects of spontaneous breathing, muscle paralysis, and positive end-expiratory pressure. Anesthesiology. 1987;66:157–67. doi: 10.1097/00000542-198702000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Duncan SR, Mihm FG, Guilleminault C, Raffin TA. Nasal continuous positive airway pressure in atelectasis. Chest. 1987;92:621–4. doi: 10.1378/chest.92.4.621. [DOI] [PubMed] [Google Scholar]
- 44.Engström J, Hedenstierna G, Larsson A. Pharyngeal oxygen administration increases the time to serious desaturation at intubation in acute lung injury: an experimental study. Critical Care. 2010;14:R93. doi: 10.1186/cc9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudolf B, Hohenhorst W. Use of Apneic Oxygenation for the Performance of Pan-endoscopy. Otolaryngolology – Head and Neck Surgergy. 2013;149:235–9. doi: 10.1177/0194599813486248. [DOI] [PubMed] [Google Scholar]
- 46.Watson RJ, Szarko R, Mackenzie CF, Sequeira AJ, Barnas GM. Continuous endobronchial insufflation during internal mammary artery harvest. Anesthesia and Analgesia. 1992;75:219–25. doi: 10.1213/00000539-199208000-00012. [DOI] [PubMed] [Google Scholar]


