Abstract
Introduction
Patients with rheumatoid arthritis (RA) treated with abatacept (ABT) are at increased risk for vaccine-preventable infections. The aim of the present study is to evaluate the humoral response to 23-valent pneumococcal polysaccharide (PPSV23) vaccination in RA patients receiving ABT.
Methods
The immunogenicity study was nested within a randomized, double-blind placebo-controlled study, designed to evaluate the efficacy of the PPSV23. PPSV23 was given to 111 RA patients, who were classified into three groups: RA control (n = 35), methotrexate (MTX) alone (n = 55), and ABT (n = 21). Before and 4–6 weeks after vaccination, we measured the patients’ concentrations of antibodies against pneumococcal serotypes 6B and 23F using an enzyme-linked immunosorbent assay and determined their antibody functionality using a multiplexed opsonophagocytic killing assay, reported as the opsonization index (OI).
Results
The pneumococcal serotype-specific IgG concentrations and OIs were both significantly increased in all treatment groups in response to PPSV23 vaccination. In the ABT group, the IgG responses for the 6B serotype were lower compared with those in the MTX alone or control groups, whereas the OI responses were similar to those in the other two groups. In a subgroup analysis, the pneumococcal serotype-specific IgG responses were significantly lower in both serotypes (6B and 23F) in the ABT/MTX group; however, the OI responses in the ABT group were not different from the control group. There was no association between the pneumococcal serotype-specific IgG and OI responses for the 6B serotype in patients receiving ABT in contrast to the control or MTX alone patients. No severe adverse effects were observed in any of the treatment groups.
Conclusions
OI responses indicate antibody functionality rather than simply their amount, so the similarity of these measurements between all three groups suggests that RA patients receiving ABT still benefit from receiving the PPSV23 vaccination, even though they produce less IgG in response to it. The results suggest an influence of ABT on the humoral response to PPSV23 vaccination under MTX treatment; however, preserved opsonin responses are expected in RA patients treated with ABT plus MTX.
Trial registration
University Hospital Medical Information Network Clinical Trials Registry: UMIN000009566. Registered 12 December 2012.
Keywords: Abatacept, Methotrexate, Opsonization index, Rheumatoid arthritis, 23-valent pneumococcal polysaccharide
Introduction
Patients with autoimmune rheumatic diseases are more susceptible to infectious complications during the course of their diseases. Rheumatoid arthritis (RA) treatment can induce immunosuppression and increase the risk of infection [1]. The introduction of biologics has been a major achievement in treating these diseases, but an increased risk of infection associated with these therapies has become evident [2]. Some infections can be prevented by vaccination, which if used appropriately will decrease the burden of infection [3]. It is important to determine if RA patients receiving biologics have normal responses to vaccines.
Abatacept (ABT) selectively modulates the CD80/CD86:CD28 co-stimulatory signal required for full T-cell activation [4]. The efficacy of ABT has previously been demonstrated both in RA patients with an inadequate response to methotrexate (MTX) and in RA patients with an inadequate response to anti-tumor necrosis factor (TNF) therapy [5], and ABT has been approved for the treatment of RA in a number of countries, including Japan. The impact of ABT on humoral responses to T-cell-dependent antigens, such as bacteriophage X174 and keyhole limpet hemocyanin, was previously evaluated in patients treated with ABT, and the responses to these antigens were reduced [6].
Polysaccharides are able to elicit immune responses in the absence of T-cell help, although the magnitude of the response can be marginally affected by immunosuppressive treatments [7]. Recent studies with pneumococcal polysaccharide vaccines with a limited number of RA patients were performed, without control groups, suggesting an adequate response [8]. Regarding the pneumococcal vaccine, the polysaccharide and less T cell-dependent nature of the antigen [9], may account for the preserved immune response during costimulatory modulation with ABT. The results from the earlier report would be strengthened by an inclusion of the relevant control groups because it is crucial to have a group of age-matched RA patients under treatment with MTX alone for comparison [10]. The objective of this study was to investigate the 23-valent pneumococcal polysaccharide vaccine (PPSV23) in patients with established RA who were being treated with ABT alone or in combination with MTX.
Methods
Study design and patient population
This immunogenicity study was nested within a randomized, double-blind, controlled trial designed to evaluate the effectiveness of the PPSV23 in reducing the incidence of pneumonia as a primary endpoint. Patients with clinically diagnosed RA were recruited in Japanese National Hospital Organization (NHO) hospitals across Japan (n = 32) from September 2010 to December 2012 [11]. A total of 932 RA patients were enrolled and randomized 1:1 to receive either the PPSV23 or placebo. Of these, paired serum samples were obtained before and after vaccination from 703 patients, 353 of which received PPSV23. Among these 353 patients,121 patients receiving disease-modifying anti-rheumatic drugs (DMARDs), MTX, or ABT with/without MTX were subjected to the nested study for vaccine immunogenicity (Fig. 1).
Fig. 1.
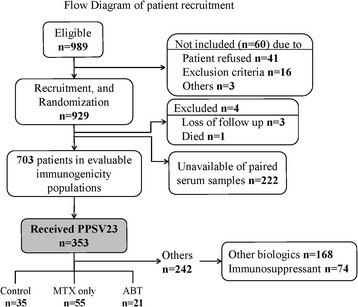
Flow diagram of patient recruitment
Eligible patients were also found to be at risk for developing respiratory infections. RA patients were divided into the following groups: (1) patients with rheumatoid lung disease, (2) patients with RA treated with biological agents, and (3) patients treated with immunosuppressive agents. Patients who had received PPSV23 previously were excluded from this study. This study complies with the principles of the Declaration of Helsinki and was approved by the appropriate institutional review boards at each participating center. All patients provided written informed consent. This study was approved by the ethical committees of NHO central IRB (No. 0512014, 2012) for all participating NHO hospitals and was registered with UMIN-CTR (UMIN000009566).
Intervention
Patients were randomly assigned to receive either 0.5 ml (25 μg) of PPSV23 (Pneumovax NP, Merck Sharp & Dohme Corp., Tokyo, Japan) or 0.5 ml of a placebo (sodium chloride) subcutaneously in the upper arm. The vaccines were prepared in a masked fashion for those who administered them, blinding both the administrator of the vaccine and the patient to the type of vaccine given. Vaccine and placebo were presented in identical single dose syringes and needle combinations that were labelled with sequential study numbers only. A statistician who was not on the study team carried out the randomization using a random number table and numbered the containers accordingly.
Enzyme-linked immunosorbent assays for serotype-specific IgG
Blood samples were drawn at vaccination and four to six weeks thereafter, and stocked at −30 °C. Enzyme-linked immunosorbent assays (ELISAs) for serotype-specific IgG were performed to measure the concentration of each type of antibody as previously described [12]. For the measurement of IgG specificity for the 6B and 23F serotypes, we specifically performed our ELISAs according to the World Health Organization (WHO) standard procedure that used the international reference serum, 89SF-3 (graciously supplied by Dr. Carl E. Frasch). To improve the specificity of the assay, a pneumococcal cell wall polysaccharide (C-PS) and pneumococcal 22F polysaccharide pre-absorption step was performed on the samples. The reference serum was pre-absorbed with only C-PS [13, 14]. Detailed protocols are available at www.vaccine.uab.edu/ELISAProtocol(89SF).pdf.
Multiplexed opsonophagocytic assays
To measure antibody functionality against pneumococcus, we performed multiplexed opsonophagocytic assays (OPAs) for pneumococcal serotypes 6B and 23F, using differentiated HL-60 cells and an antibiotic-resistant target bacteria strain, at the Research Institute for Microbial Disease, Osaka University, as previously described [15]. The quality control serum included in each assay was prepared from pooled sera of adults immunized with PPV23. Opsonization indices (OIs) were defined as the serum dilution that led to 50 % death of target bacteria. Opsotiter 3, an excel-based data processing program, was used to convert colony counts to OIs, according to the WHO protocol available at www.vaccine.uab.edu/UAB-MOPA.pdf.
Antibody response
Fold increases relative to pre-vaccination values (post-vaccination value to pre-vaccination value ratios) were determined. Positive antibody response was defined as a two-fold or more increase in IgG concentrations or as a ten-fold or more increase in OIs as described previously [12].
Statistical analysis
The study population was classified into three groups based on the RA treatment at the time of vaccination. Clinical and demographic characteristics of each group were expressed as mean ± standard deviation or as a percentage. Changes in IgG geometric mean concentrations (GMCs) and OPA titers before and after the vaccination were compared using the paired-sample t test. To compare categorical variables in response rates between groups, the Pearson chi-square test was used. Continuous variables were compared using Mann–Whitney tests. For all tests, probability values (p values) less than 0.05 were considered statistically significant. All the statistical analyses were performed using the Statistical Analysis System (SAS) and SPSS version 18 software (SPSS, Chicago, IL, USA).
Results
Clinical and demographic characteristics
A total of 989 RA patients were assessed for eligibility, and 929 patients were recruited and randomized. Of these, 121 patients receiving disease-modifying anti-rheumatic drugs (DMARDs), MTX, or ABT with/without MTX were subjected to the nested study for vaccine immunogenicity (Fig. 1). The clinical and demographic characteristics of these 121 subjected patients are summarized in Table 1. The study population was classified into three groups: DMARD treatment only (RA control group; n = 35), MTX monotherapy (MTX alone group, n = 55), and ABT treatment (n = 24, mean dose; 547 + 127.9 mg/4 weeks). The mean ages of patients in the ABT group were significantly lower compared to those in the control group. The three groups were otherwise similar. All patients fulfilled the criteria of safety required for vaccine injection, and no serious side effects were observed after vaccination.
Table 1.
Clinical and demographic characteristics of RA patients prior to pneumococcal vaccination
| RA control | MTX group | ABT group | p Values between treatment groups | |
|---|---|---|---|---|
| n = 35 | n = 55 | n = 21 | ||
| Male/female | 12/23 | 11/44 | 4/17 | 0.251 |
| Age, mean ± SD (years) | 70.5 ± 10.8 | 63.8 ± 11.5 | 59.8 ± 12.0 | 0.002 |
| Weight, mean ± SD (Kg) | 53.3 ± 9.5 | 52.9 ± 11.8 | 55.2 ± 10.1 | 0.501 |
| BMI, mean ± SD | 21.8 ± 3.5 | 21.7 ± 3.7 | 23.2 ± 4.9 | 0.344 |
| RA duration, mean ± SD (years) | 11.7 ± 12.5 | 14.1 ± 10.9 | 13.5 ± 11.2 | 0.144 |
| MTX dose, mean ± SD (mg/week) | - | 7.8 ± 2.4 | 7.2 ± 3.4(15/21) | - |
| ABT dose, mean ± SD (mg/4 week) | - | - | 547.6 ± 127.9 | - |
| Use of prednisolone, number of patients (%) | 21 (60.0) | 30 (54.5) | 13(61.9) | 0.798 |
| Prednisolone dose, mean ± SD (mg/day) | 6.06 ± 4.23 | 4.98 ± 2.97 | 5.04 ± 2.85 | 0.769 |
| DAS28(CRP), mean ± SD | 2.79 ± 1.17 | 2.61 ± 0.98 | 2.48 ± 1.31 | 0.565 |
| SDAI, mean ± SD | 9.03 ± 6.32 | 8.15 ± 7.33 | 8.32 ± 7.29 | 0.593 |
| CDAI, mean ± SD | 7.83 ± 5.40 | 7.46 ± 6.73 | 7.87 ± 6.54 | 0.721 |
| IP (%) | 6 (17.1) | 7 (12.7) | 3(14.3) | 0.844 |
| COPD (%) | 3 (8.6) | 1 (1.8) | 2(9.5) | 0.251 |
| Smoking history (%) | 10(28.6) | 13(23.6) | 6(28.6) | 0.839 |
Data were obtained immediately before pneumococcal vaccination. p values between treatment groups were determined using the Kruskal-Wallis test. p values were calculated with the chi-square test for qualitative data. RA rheumatoid arthritis, MTX methotrexate, ABT abatacept, BMI body mass index, DAS28 Disease Activity Score 28, SDAI simplified disease activity index, CDAI clinical disease activity index, IP interstitial pneumonia, COPD chronic obstructive pulmonary disease
Pneumococcal serotype-specific IgG concentrations
To evaluate the effect of ABT treatment on the level of pneumococcal serotype-specific IgG produced following PPS23V vaccination in RA patients, enzyme-linked immunosorbent assays were performed to measure the serotype 6B- and 23F-specific IgG levels in patients from each of the three groups before and after vaccination. The ratios between post- and pre-vaccination antibody concentrations are summarized in Table 2. After vaccination with PPSV23, the geometric mean concentrations (GMCs) of both serotype 6B- and 23F-specific IgG were increased in all groups. However, there were large differences in the fold induction of GMC responses among the groups with regard to treatments; for 6B serotypes, a higher post-GMC was obtained in the control (2.38 times) and MTX alone (1.75 times) groups compared with that in the ABT (1.23 times, no significant increase) group.
Table 2.
Concentrations of pneumococcal polysaccharide antigen serotype-specific IgG antibodies and opsonization indices in the RA treatment groups before and after 23-valent pneumococcal polysaccharide vaccination
| RA Control | MTX group | ABT group | ||
|---|---|---|---|---|
| n = 35 | n = 55 | n = 21 | ||
| IgG GMCs (μg/ml) | ||||
| 6B | Before | 0.84(0.58 to 1.11) | 1.42(0.86 to 1.97) | 1.12(0.80 to 1.45) |
| After | 4.05(2.13 to 5.97)* | 4.36(2.17 to 6.55)* | 2.29(0.76 to 3.83) | |
| Fold increase | 2.38(1.41 to 5.62) | 1.75(1.15 to 3.11) | 1.41(0.87 to 3.09) | |
| 23F | Before | 1.17(0.85 to 1.48) | 1.79(1.33 to 2.25) | 1.22(0.79 to 1.65) |
| After | 11.61(4.16 to 19.07)* | 7.41(4.48 to 10.33)* | 4.61(2.95 to 6.27)* | |
| Fold increase | 3.36(1.85 to 9.42) | 2.00(1.27 to 5.48) | 2.45(1.23 to 7.44) | |
| GM-OIs | ||||
| 6B | Before | 17.24(10.96 to 23.53) | 150.79(14.85 to 286.74) | 61.55(13.78 to 109.32) |
| After | 981.15(407.24 to 1555.05)* | 584.29(270.29 to 898.28)* | 1345.19(383.97 to 2306.41)* | |
| Fold increase | 10.22(1.92 to 79.48) | 2.57(1.22 to 22.40) | 14.83(2.93 to 163.03) | |
| 23F | Before | 63.21(−6.79 to 133.20) | 52.11(14.04 to 90.18) | 138.40(−87.83 to 364.63) |
| After | 713.49(307.97 to 1119.01)* | 724.56(336.93 to 1112.19)* | 887.76(172.11 to 1603.42)* | |
| Fold increase | 6.86(2.50 to 27.14) | 3.75(1.47 to 38.32) | 2.97(1.37 to 76.09) | |
IgG GMCs and GM-OIs are expressed as the mean (95 % CI). Fold increases are expressed as the median (IQR). Differences between pre- and post-vaccination GMCs of serotype-specific IgG were assessed using a paired-sample t test
RA rheumatoid arthritis, MTX methotrexate, ABT abatacept, GMC geometric mean concentration, GM-OI geometric mean opsonization index, IQR interquartile range
*p < 0.05 compared with pre-vaccination IgG GMCs or GM-OIs
Opsonophagocytic killing assays
To determine the antibody functionality in these groups, we performed multiplexed opsonophagocytic killing assays and reported the results as the opsonization index (OI). The post-vaccination OIs increased significantly in all treatment groups. The ratios between pre- and post-vaccination are provided in Table 2. In contrast to the GMC (6B) results, there were no differences in the fold induction of OIs for either serotype (6B or 23F) among the ABT and control or MTX alone groups. In this assay, the ABT group showed an antibody response rate that was equivalent to those in the control and MTX groups.
Antibody response rates
The GMC response rates, given as the percentage of patients with a positive antibody response, for patients in the ABT group were significantly decreased compared with those for patients in the control or MTX alone groups for serotype 6B (Fig. 2a). For OIs specific to serotype 6B and 23F, the ABT group showed an equivalent antibody response rate, similarly defined as the percentage of patients with a positive OI response, compared with the control or MTX groups (Fig. 2b).
Fig. 2.
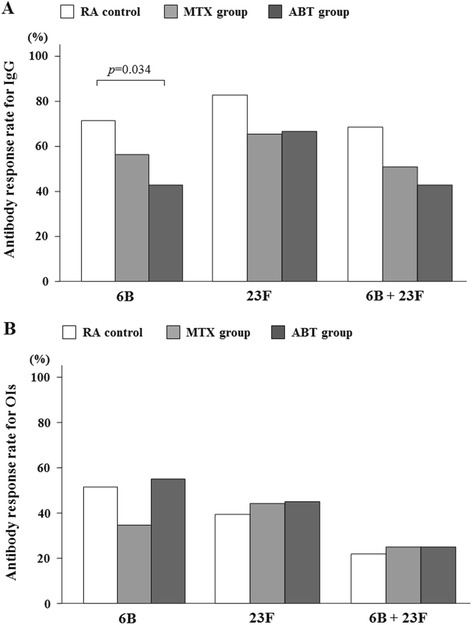
a Comparison of post-vaccination GMC responses in patients receiving DMARDs (control), MTX and ABT. Percentages of patients with an increase in 6B or 23F serotype-specific IgG concentration greater than two-fold are shown. There was a significant difference in the 6B serotype-specific IgG response rates between control and ABT groups (p = 0.034). Data were compared using the Pearson chi-square test. b Comparison of post-vaccination OIs responses in patients receiving DMARDs (control), MTX and ABT. Percentage of patients with an increase in OIs for serotypes 6B or 23F greater than ten-fold are shown. There was no significant difference in the response rates among control, MTX and ABT groups. GMC geometric mean concentration, DMARDS disease modifying anti-rheumatic drugs, MTX methotrexate, ABT abatacept, OIs opsonization index
Subgroup analysis for patients receiving ABT/MTX combination treatment
Notably, ABT is primarily used in combination with MTX, and ABT monotherapy is limited in Japan. Therefore, we compared the patients receiving both ABT and MTX (ABT/MTX) to control patients (receiving DMARDs alone or MTX alone). It appears that ABT/MTX groups had an insignificant increase in the GMC for the 6B serotype and an even lower increase in the GMC for the 23F serotype after PPSV23 vaccination compared with control groups (Table 3). However, the post-vaccination OI responses for both serotypes in the patients receiving ABT/MTX combination therapy were equivalent to those in the control group patients. Similarly, a lower proportion of RA patients receiving ABT/MTX had a geometric mean concentration (GMC) increase greater than two-fold for both serotypes; however, the rates of patients with OIs greater than ten-fold for both serotypes were not different between the ABT/MTX and control groups (Fig. 3). Because the opsonophagocytic activity (OPA) is a measurement of antibody function, these results suggest that while the GMC response rate is lower in patients receiving ABT/MTX combination therapy, the antibodies that are produced in response to PPSV23 vaccination by this group are similarly functional.
Table 3.
Concentrations of pneumococcal polysaccharide antigen serotype-specific IgG antibodies and opsonization indices in the RA treatment groups before and after 23-valent pneumococcal polysaccharide vaccination
| RA Control | MTX group | ABT/MTX group | ||
|---|---|---|---|---|
| n = 35 | n = 55 | n = 15 | ||
| IgG GMCs (μg/ml) | ||||
| 6B | Before | 0.84(0.58 to 1.11) | 1.42(0.86 to 1.97) | 1.19(0.73 to 1.65) |
| After | 4.05(2.13 to 5.97)* | 4.36(2.17 to 6.55)* | 2.49(0.29 to 4.70) | |
| Fold increase | 2.38(1.41 to 5.62) | 1.75(1.15 to 3.11) | 1.19(0.77 to 2.44) | |
| 23F | Before | 1.17(0.85 to 1.48) | 1.79(1.33 to 2.25) | 1.17(0.63 to 1.71) |
| After | 11.61(4.16 to 19.07)* | 7.41(4.48 to 10.33)* | 3.78(1.87 to 5.70)* | |
| Fold increase | 3.36(1.85 to 9.42) | 2.00(1.27 to 5.48) | 1.96(1.15 to 5.99) | |
| GM-OIs | ||||
| 6B | Before | 17.24(10.96 to 23.53) | 150.79(14.85 to 286.74) | 43.33(0.15 to 86.51) |
| After | 981.15(407.24 to 1555.05)* | 584.29(270.29 to 898.28)* | 1454.80(109.29 to 2800.31)* | |
| Fold increase | 10.22(1.92 to 79.48) | 2.57(1.22 to 22.40) | 14.65(2.96 to 193.79) | |
| 23F | Before | 63.21(−6.79 to 133.20) | 52.11(14.04 to 90.18) | 178.13(−130.27 to 486.54) |
| After | 713.49(307.97 to 1119.01)* | 724.56(336.93 to 1112.19)* | 880.73(−81.54 to 1843.01)* | |
| Fold increase | 6.86(2.50 to 27.14) | 3.75(1.47 to 38.32) | 2.50(1.33 to 35.74) | |
IgG GMCs and GM-OIs are expressed as the mean (95 % CI). Fold increases are expressed as the median (IQR). Differences between pre- and post-vaccination GMCs of serotype-specific IgG were assessed using a paired-sample t test
RA rheumatoid arthritis, MTX methotrexate, ABT abatacept, GMC geometric mean concentration, GM-OI geometric mean opsonization index, CI, confidence interval, IQR interquartile index
*p < 0.05 compared with pre-vaccination IgG GMCs or GM-OIs
Fig. 3.
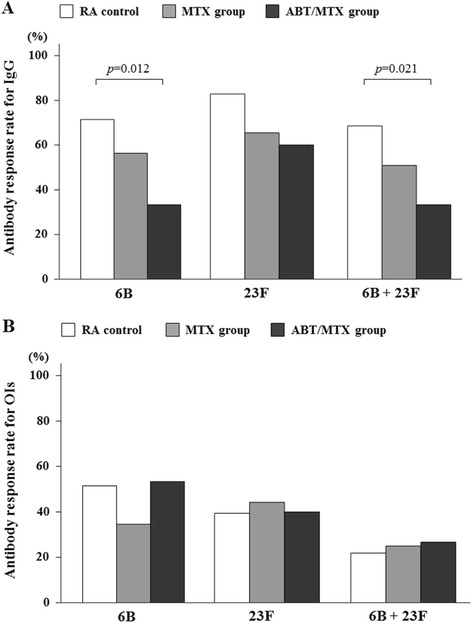
a Comparison of post-vaccination GMC responses in patients receiving DMARDs (control), MTX and ABT/MTX. Percentages of patients with an increase in 6B and 23F serotype-specific IgG concentration greater than two-fold are shown. There were significant differences in the 6B and 6B/23F serotype-specific IgG response rates between control and ABT/MTX groups (6B; p = 0.012, 6B + 23F; p = 0.021). Data were compared using the Pearson chi-square test. b Comparison of post-vaccination OI responses in patients receiving DMARDs (control), MTX and ABT/MTX. Percentage of patients with an increase in OIs for serotypes 6B and 23F greater than ten-fold are shown. There was no significant difference in the response rates among control, MTX and ABT/MTX groups. Data were compared using the Pearson chi-square test. GMC geometric mean concentration, DMARDS disease modifying anti-rheumatic drugs, MTX methotrexate, ABT abatacept, OIs opsonization index
Associations between pneumococcal serotype-specific IgG and OI responses
We assessed the associations between the pneumococcal serotype-specific IgG and OI responses for serotype 6B, in which lower 6B-specific IgG responses were demonstrated in the ABT group. In the control or MTX groups, the functional OI responses were almost exclusively observed in patients who had an optimum pneumococcal serotype-specific IgG response (Fig. 4a). In contrast, in the MTX/ABT group, a positive OI response was also observed in patients who lacked an optimum pneumococcal serotype-specific IgG response (Fig. 4a). The OIs in patients with negative IgG responses were higher in the MTX/ABT group than those in the control or MTX groups (Fig. 4b). Therefore, an association between the pneumococcal serotype-specific IgG response and the OI response was not demonstrated in patients receiving MTX/ABT combination therapy.
Fig. 4.
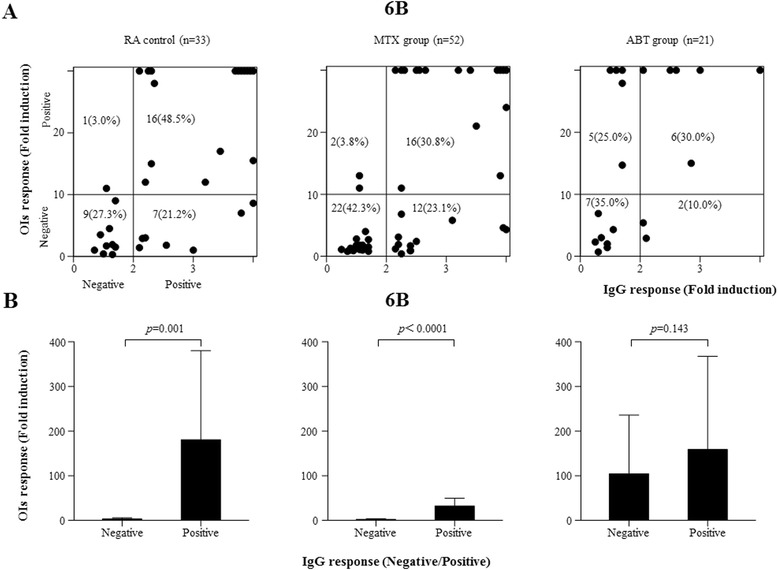
a Relationship between IgG and OI responses after PPSV23 vaccination. The 6B serotype-specific IgG (X axis) and OIs (Y axis) responses were plotted in the comparison of three groups (control, MTX, ABT). Positive OI responses were demonstrated in patients receiving ABT with negative IgG responses. b Comparisons of means of OIs between patients with negative or positive serotype 6B-specific IgG responses among three treatment groups (control, MTX, ABT). Error bars represent SD of mean OIs. There was no significant difference in mean OIs between patients with or without positive serotype-6B-specific IgG response in ABT group. IgG immunoglobulin G, OIs opsonization indices, MTX methotrexate, ABT abatacept, SD standard deviation
Safety
There were no reported adverse events associated with PPSV23 vaccination in the patients from this study.
Discussion
This study was nested within a randomized, double-blind, controlled trial designed to evaluate the effectiveness of the PPSV23, and immunogenicity of PPSV23 vaccination in patients receiving ABT was investigated. ABT, a CTLA-4-immunoglobulin-fusion protein, inhibits T cell activation by binding to CD80 and CD86, thus blocking the interaction with CD28 [4]. ABT acts by inhibiting the costimulatory pathway, which is essential for the generation of an immune response to protein and peptide antigens [16]. The requirement of T-cell co-stimulation for B-cell affinity maturation and for the production of high affinity IgG antibodies has potential implications for pneumococcal vaccination [17]. In an open-label, controlled study in healthy subjects, tetanus toxoid vaccine and PPSV23 were each used to assess the impact of ABT on the memory response to a T-cell-dependent protein antigen and to a less T-cell-dependent polysaccharide antigen, respectively [18]. While ABT blunted the immune response (geometric mean titers) to both vaccinations, it did not significantly inhibit the ability of healthy subjects to develop a two-fold response to either vaccine [18]. The reduced antibody response to the T-cell dependent protein tetanus toxoid antigen is consistent with the known ABT modulation of memory T-cell activation, whereas the inhibition of B–T cell help is likely responsible for the reduced antibody response to T cell-independent polysaccharide antigens, such as the pneumococcal vaccine [19]. Kapetanovic et al. studied the effect of ABT on the antibody response to PPSV23 in patients with RA (compared with TCZ) and reported marginally diminished antibody responses in the ABT group [8]. Thus, although there is a paucity of data, it appears that ABT is able to blunt the effectiveness of the immune response, but does not significantly inhibit the ability of healthy subjects and patients with RA to develop a clinically significant positive immune response to PPSV23. RA patients frequently use ABT in combination with traditional DMARDs, including MTX [20]. However, MTX was shown to be associated with reduced immune responses to PPSV23 [10]. It is, therefore, crucial to evaluate the immunogenicity of PPSV23 by comparing RA patients treated with ABT with those treated with MTX. Blockage of the CD80/CD86 co-stimulatory molecules by a variety of activated immune cells can regulate the immune responses [21]. This may result in T cell activation and diminished B-cell immunological response as the consequence of inadequate differentiations into plasma cells.
In the present study, the magnitude of the IgG response to PPSV23 was decreased in RA patients treated with ABT. However, the decreased IgG responses to PPSV23 did not affect the OI responses in these patients. This may have resulted from a diminished B cell immune response as a consequence of inadequate stimulation, such as a lack of the T cell help needed for B cell differentiation. However, we also demonstrated that ABT, even with MTX, did not affect the OI responses to PPSV23 vaccination in RA patients. The mechanism by which ABT with or without MTX affects the IgG responses, but not the OI responses against PPSV23, has not been examined in this study. Unlike ELISA results, OPA results are ideal surrogate markers for vaccine efficacy as they mimic the host defense responses [9]. Recent evidence has shown that older adults have a lower capacity to opsonize pneumococci despite normal IgG levels, owing to a lack of anti-pneumococcal IgM antibodies [22]. These poor correlations between opsonic activity and IgG levels have also been shown in patients who are in immunosuppressive states [23]. RA patients receiving ABT/MTX showed a similar dissociation between opsonic activity and levels of IgG against the polysaccharide capsule of pneumococci.
Pneumococcal polysaccharides are T cell-independent antigens. ABT/MTX treatment may partly impair the T cell-independent antibody response that is normally triggered by PPSV23 vaccination because B cells also express CD80 and CD86 [24], and ABT may have some effects on B cell activation in the presence of MTX, as described previously [21]. In contrast, ABT/MTX treatment did not inhibit PPSV23 vaccination-induced OPA. The relative activities of IgG and IgM antibodies may contribute to the preserved OPA in RA patients treated with ABT/MTX as described previously [25]. Strong correlations between ELISA results and OPA results have been observed in many studies [26]; however, no such correlation was found in RA patients receiving ABT. In this setting, OPA has become a useful measure of pneumococcal vaccine immunogenicity. The introduction of pneumococcal conjugate vaccines may expand the options available for protecting RA patients against pneumococcal infections. Abatacept-treated RA patients had decreased antibody response against pneumococcal conjugate vaccines 7 (PCV7) compared to controls and tocilizumab-treated RA patients [8]. Abatacept attenuates activation of T cells by blocking the interaction between CD80/86 and CD28, a co-stimulation signal for T cell activation, which may contribute to the impaired antibody responses after PCV7 vaccination. Whereas another immunogenicity studies comparing PPSV23 and PCV7 revealed that the OPA and geometric antibody titers PCV7 were comparable to those of PPSV23in RA patients [27]. Further immunogenicity studies comparing PPSV23 and PCV13 are needed.
The primary limitation of this study is the relatively small number of RA patients in each group, particularly the group for ABT/MTX combination treatment. Other limitations include the fact that the antibody response is only a surrogate marker of vaccine-induced protection and the inclusion of a number of patients who were treated with ABT in combination with remedies other than MTX. Furthermore, we choose to investigate serotypes 6B and 23F because they are the main causative serotypes of penicillin-resistant pneumococcal pneumonia in Japan [28]. Lastly, the antibody concentrations necessary for protection against invasive pneumococcal disease in adults have not been clearly defined [29]. Strengths of the present study are the standardized blood sampling and the way that the analyses were blinded for demographic and treatment data.
Conclusions
In conclusion, our results suggest that the T cell co-stimulation modulator, ABT, with or without MTX, has an influence on the humoral responses to PPSV23 vaccination; however, even in patients concomitantly treated with MTX, the opsonization responses against PPSV23 were preserved in RA patients treated with ABT. These data suggest immunization with PPSV23 resulted in a preserved immune response in RA patients treated with ABT.
Acknowledgments
The study was supported by research grants research funds from the National Hospital Organization (NHO)-EBM study, Japan.
Financial support
The study was supported by research funds from the National Hospital Organization (NHO)-EBM study, Japan.
Abbreviations
- ABT
Abatacept
- ELISA
Enzyme-linked immunosorbent assay
- GMCs
Geometric mean concentrations
- MTX
Methotrexate
- OI
Opsonization index
- OPA
Opsonophagocytic assay
- PPSV23
23-valent pneumococcal polysaccharide vaccination
- RA
Rheumatoid arthritis
- TCZ
Tocilizumab
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KM, YA, MA, HFu, and KO participated in the design of the study. HK and YJ analyzed the data. ST, FHi, HI, RM, ES, TMi, SM, TF, YI, NI, HT, KS, TY, SO, NM, AM, KT, SY, YKa, YS, TO, NH, YKo and TMa collected the clinical data. All authors wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
Kiyoshi Migita, Phone: +81-957-52-3121, Email: migita@nagasaki-mc.com.
Yukihiro Akeda, Email: akeda@biken.osaka-u.ac.jp.
Manabu Akazawa, Email: makazawa@my-pharm.ac.jp.
Shigeto Tohma, Email: s-touma@sagamihara-hosp.gr.jp.
Fuminori Hirano, Email: hirano@asahikawa.hosp.go.jp.
Haruko Ideguchi, Email: ideguchi@cronos.ocn.ne.jp.
Hideko Kozuru, Email: hkozuru@nagasaki-mc.com.
Yuka Jiuchi, Email: jiuchi@nagasaki-mc.com.
Ryutaro Matsumura, Email: ryu-ma@cehpnet.com.
Eiichi Suematsu, Email: suematsu@qmed.hosp.go.jp.
Tomoya Miyamura, Email: miyamura@kyumed.jp.
Shunsuke Mori, Email: moris@saisyunsou1.hosp.go.jp.
Takahiro Fukui, Email: opmeisterf@yahoo.co.jp.
Yasumori Izumi, Email: yizumi@nagasaki-mc.com.
Nozomi Iwanaga, Email: n.iwanaga@nagasaki-mc.com.
Hiroshi Tsutani, Email: htsutani@kitagata.hosp.go.jp.
Kouichirou Saisyo, Email: saisyo@miyakonojo.hosp.go.jp.
Takao Yamanaka, Email: yamanaka.takao@momc.jp.
Shiro Ohshima, Email: ohshimas@ommc-hp.jp.
Naoya Mori, Email: ueda.chiken@gmail.com.
Akinori Matsumori, Email: matsumoria@kochi2.hosp.go.jp.
Koichiro Takahi, Email: ktakahi@toneyama.go.jp.
Shigeru Yoshizawa, Email: syoshi@mfukuoka2.hosp.go.jp.
Yojiro Kawabe, Email: ykawabe@uresino.go.jp.
Yasuo Suenaga, Email: rheuma@beppu.hosp.go.jp.
Tetsuo Ozawa, Email: ozawa@niigata-nh.go.jp.
Norikazu Hamada, Email: hamadan@kure-nh.go.jp.
Yasuhiro Komiya, Email: y-komiya@subsigaraki1.hosp.go.jp.
Toshihiro Matsui, Email: t-matsui@sagamihara-hosp.gr.jp.
Hiroshi Furukawa, Email: h-furukawa@sagamihara-hosp.gr.jp.
Kazunori Oishi, Email: oishik@nih.go.jp.
References
- 1.Falagas ME, Manta KG, Betsi GI, Pappas G. Infection-related morbidity and mortality in patients with connective tissue diseases: a systematic review. Clin Rheumatol. 2007;26:663–70. doi: 10.1007/s10067-006-0441-9. [DOI] [PubMed] [Google Scholar]
- 2.Winthrop KL. Infections and biologic therapy in rheumatoid arthritis: our changing understanding of risk and prevention. Rheum Dis Clin North Am. 2012;38:727–45. doi: 10.1016/j.rdc.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira I, Isenberg D. Vaccines and biologics. Ann Rheum Dis. 2014;73:1446–54. doi: 10.1136/annrheumdis-2014-205246. [DOI] [PubMed] [Google Scholar]
- 4.Romo-Tena J, Gómez-Martín D, Alcocer-Varela J. CTLA-4 and autoimmunity: new insights into the dual regulator of tolerance. Autoimmun Rev. 2013;12:1171–6. doi: 10.1016/j.autrev.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 6.Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, et al. CTLA4 Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–52. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melmed GY, Agarwal N, Frenck RW, Ippoliti AF, Ibanez P, Papadakis KA, et al. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:148–54. doi: 10.1038/ajg.2009.523. [DOI] [PubMed] [Google Scholar]
- 8.Crnkic Kapetanovic M, Saxne T, Jönsson G, Truedsson L, Geborek P. Rituximab and abatacept but not tocilizumab impair antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis. Arthritis Res Ther. 2013;15:R171. doi: 10.1186/ar4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CJ, Lee LH, Frasch CE. Protective immunity of pneumococcal glycoconjugates. Crit Rev Microbiol. 2003;29:333–49. doi: 10.1080/713608018. [DOI] [PubMed] [Google Scholar]
- 10.Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2014;66:1016–26. doi: 10.1002/acr.22246. [DOI] [PubMed] [Google Scholar]
- 11.Migita K, Akeda Y, Akazawa M, Tohma S, Hirano F, Ideguchi H, et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tacrolimus. Arthritis Res Ther. 2015;17:149. doi: 10.1186/s13075-015-0662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori S, Ueki Y, Akeda Y, Hirakata N, Oribe M, Shiohira Y, et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tocilizumab therapy. Ann Rheum Dis. 2013;72:1362–6. doi: 10.1136/annrheumdis-2012-202658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2001;8:266–72. doi: 10.1128/CDLI.8.2.266-272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wernette CM, Frasch CE, Madore D, Carlone G, Goldblatt D, Plikaytis B, et al. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol. 2003;10:514–9. doi: 10.1128/CDLI.10.4.514-519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton RL, Nahm MH. Development and validation of a four fold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006;13:1004–9. doi: 10.1128/CVI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oosterwegel MA, Greenwald RJ, Mandelbrot DA, Lorsbach RB, Sharpe AH. CTLA-4 and T cell activation. Curr Opin Immunol. 1999;11:294–300. doi: 10.1016/S0952-7915(99)80047-8. [DOI] [PubMed] [Google Scholar]
- 17.Wu ZQ, Khan AQ, Shen Y, Schartman J, Peach R, Lees A, et al. B7 requirements for primary and secondary protein- and polysaccharide-specific Ig isotype responses to Streptococcus pneumoniae. J Immunol. 2000;165:6840–8. doi: 10.4049/jimmunol.165.12.6840. [DOI] [PubMed] [Google Scholar]
- 18.Tay L, Leon F, Vratsanos G, Raymond R, Corbo M. Vaccination response to tetanus toxoid and 23-valent pneumococcal vaccines following administration of a single dose of abatacept: a randomized, open-label, parallel group study in healthy subjects. Arthritis Res Ther. 2007;9:R38. doi: 10.1186/ar2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Defrance T, Taillardet M, Genestier L. T cell-independent B cell memory. Curr Opin Immunol. 2011;23:330–6. doi: 10.1016/j.coi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Guyot P, Taylor P, Christensen R, Pericleous L, Poncet C, Lebmeier M, et al. Abatacept with methotrexate versus other biologic agents in treatment of patients with active rheumatoid arthritis despite methotrexate: a network meta-analysis. Arthritis Res Ther. 2011;13:R204. doi: 10.1186/ar3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cutolo M, Nadler SG. Advances in CTLA-4-Ig-mediated modulation of inflammatory cell and immune response activation in rheumatoid arthritis. Autoimmun Rev. 2013;12:758–67. doi: 10.1016/j.autrev.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Park S, Nahm MH. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect Immun. 2011;79:314–20. doi: 10.1128/IAI.00768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parkkali T, Väkeväinen M, Käyhty H, Ruutu T, Ruutu P. Opsonophagocytic activity against Streptococcus pneumoniae type 19F in allogeneic BMT recipients before and after vaccination with pneumococcal polysaccharide vaccine. Bone Marrow Transplant. 2001;27:207–11. doi: 10.1038/sj.bmt.1702779. [DOI] [PubMed] [Google Scholar]
- 24.Sahoo NC, Rao KV, Natarajan K. CD80 expression is induced on activated B cells following stimulation by CD86. Scand J Immunol. 2002;55:577–84. doi: 10.1046/j.1365-3083.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 25.Simell B, Nurkka A, Ekstrom N, Givon-Lavi N, Kayhty H, Dagan R. Serum IgM antibodies contribute to high levels of opsonophagocytic activities in toddlers immunized with a single dose of the 9-valent pneumococcal conjugate vaccine. Clin Vaccine Immunol. 2012;19:1618–23. doi: 10.1128/CVI.00248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song JY, Moseley MA, Burton RL, Nahm MH. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother. 2013;19:412–25. doi: 10.1007/s10156-013-0601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crnkic Kapetanovic M, Saxne T, Truedsson L, Geborek P. Persistence of antibody response 1.5 years after vaccination using 7-valent pneumococcal conjugate vaccine in patients with arthritis treated with different antirheumatic drugs. Arthritis Res Ther. 2013;15:R1. doi: 10.1186/ar4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oishi K, Yoshimine H, Watanabe H, Watanabe K, Tanimura S, Kawakami K, et al. Drug-resistant genes and serotypes of pneumococcal strains of community-acquired pneumonia among adults in Japan. Respirology. 2006;11:429–36. doi: 10.1111/j.1440-1843.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 29.Romero-Steiner S, Frasch C, Concepcion N, Goldblatt D, Käyhty H, Väkeväinen M, et al. Multilaboratory evaluation of a viability assay for measurement of opsonophagocytic antibodies specific to the capsular polysaccharides of Streptococcus pneumoniae. Clin Diagn Lab Immunol. 2003;10:1019–24. doi: 10.1128/CDLI.10.6.1019-1024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


