Abstract
Sleep disturbance is a common feature of posttraumatic stress disorder (PTSD), but is not a focus of standard PTSD treatments. Psychological trauma exposure is associated with considerable physical and mental health morbidity, possibly due to the alterations in neuroendocrine function and inflammation observed in trauma exposed individuals. Although PTSD treatments are efficacious, they are associated with high drop-out rates in clinical trials and clinical practice. Finally, individuals with PTSD stemming from exposure to interpersonal violence represent an especially under-treated population with significant sleep disturbance. Community-based participatory research was utilized to design and prepare a clinical trial that randomizes recent survivors of interpersonal violence who have PTSD, depression, and insomnia to receive either: (1) Cognitive Behavioral Therapy for Insomnia (CBTi) followed by Cognitive Processing Therapy (CPT) for trauma, or (2) attention control followed by CPT. Outcome measures include subjective and objective measures of sleep, clinician-administered PTSD and depression scales, inflammatory cytokines, and salivary cortisol. Assessments are conducted at baseline, following the sleep or control intervention, and again following CPT. The design allows for: (1) the first test of a sleep intervention in this population; (2) the comparison of sequenced CBTi and CPT to attention control followed by CPT, and (3) assessing the roles of neuroendocrine function, inflammatory processes, and objective sleep markers in mediating treatment outcomes. The study’s overarching hypothesis is that treating insomnia will produce reduction in insomnia, PTSD, and depression severity, allowing patients to more fully engage in, and derive optimal benefits from, cognitive processing therapy.
Keywords: sleep, insomnia, posttraumatic stress disorder, depression, interpersonal violence, cognitive-behavioral therapy, community-based participatory research
1. Introduction
Up to 30% of individuals who experience a traumatic event will eventually be diagnosed with posttraumatic stress disorder (PTSD) [1–3]. Of those individuals with PTSD, 50% will also have a comorbid diagnosis of Major depressive disorder (MDD) [4–6]. A striking feature of both PTSD and MDD is the high prevalence of sleep disturbance. Indeed, sleep disturbances are considered the hallmark of PTSD [7]. Insomnia (difficulty initiating or maintaining sleep) occurs in 60–90% of PTSD patients and is the most commonly endorsed PTSD symptom [8]. Further, these high rates of insomnia persist following targeted behavioral and psychopharmacological treatments for both depression [9–10] and PTSD [11–13].
The demonstrated links between these psychiatric disorders and insomnia support the need for interventions aimed explicitly at targeting both domains. Although there is evidence for successful treatment of sleep disturbance in PTSD patients with insomnia, this has only been accomplished in pilot studies in which both PTSD and depressive symptoms appear to remain high [14–16]. This suggests that sleep intervention in isolation is not likely to be an effective treatment for PTSD with comorbid depression, but may serve as an important adjunct to evidence-based PTSD and MDD psychotherapies as has been suggested by one case study [17]. Despite recommendations for such adjunctive or combined approaches [14–17], and further, despite their potential to alter mechanistic pathways of mental and physical morbidity, integrated approaches have not been tested. Our study combines Cognitive Behavioral Therapy for Insomnia (CBTi) with Cognitive Processing Therapy (CPT).
CBTi is a well-supported and highly effective insomnia treatment to improve insomnia symptoms [18]. Historically, the standard number of sessions for CBTi has ranged from 6–8 sessions. We [19] and others [20] have shown that the intervention can be delivered in fewer sessions without compromising effect. In this study, all standard components are delivered over 4 individual sessions (sleep education, stimulus control, sleep restriction, sleep hygiene, cognitive therapy, and relapse prevention).
Participants arrive at session 1 having completed daily sleep diaries for the preceding week recording time to bed, minutes to fall asleep, number and length of awakenings, time of final awakening and the time out of bed for the day. Sleep diaries are maintained throughout the treatment period. At the first and subsequent sessions, weekly averages for the variables recorded are calculated by therapist entry into an excel sheet which also calculates average total sleep time for the week. Sleep efficiency, an important variable used to guide treatment, is calculated by dividing average nightly total sleep time for the week by the average time in bed and presenting this as a percentage (e.g., 6 hours of total sleep time in 8 hours of time in bed equates to a 75% sleep efficiency). Session 1 includes the delivery of sleep psychoeducation including an introduction to normal sleep, a diathesis-stress model of insomnia, and the concept of how insomnia may become conditioned. Stimulus control is introduced, which limits the amount of time patients spend awake in bed or in the bedroom and also begins to develop a more consistent sleep schedule. The stimulus control instructions include: (1) use an alarm to keep a fixed wake time every day, regardless of how much sleep you get during the night; (2) do not nap during the day; (3) avoid any behavior in the bed or bedroom other than sleep or sexual activity; (4) lie down to go to sleep only when you are sleepy; (5) leave the bedroom when awake for approximately 15–20 minutes or when you begin to feel frustrated about not sleeping; (6) return to bed only when sleepy. At the end of the first session a fixed rise time is established as well as a target bed time such that the total time in bed is no less than the average total sleep time and no more than 8 hours.
Session 2 includes discussion and establishment of a consistent pre-bedtime routine and a review of a sleep hygiene list with instructions geared toward helping the maintain good sleep habits, such as keeping an environment and routine conducive to sleep, and avoiding tobacco, alcohol, large meals and vigorous exercise for several hours prior to bed. Items relevant to the participant are identified with goals (and action steps to achieve them) set to change each identified sleep hygiene factor. This session also includes sleep education related to sleep-wake regulation, which serves as a rationale for sleep restriction therapy. Sleep restriction therapy limits the amount of time patients spend in bed to an amount that matches their ability fill this with mostly sleep. Here the prescribed nightly total time in bed for the coming week is set equal to the average total sleep time for the week calculated from the current daily sleep diary. Once the fixed wake/rise time from the prior week is confirmed or adjusted, the prescribed bed time is established (e.g., if total sleep time has averaged 6 hours and the preferred rise time is 7:00 a.m., the bedtime is set at 1:00 a.m. Participants are instructed to maintain this schedule while continuing to keep their daily sleep diaries. In subsequent week the length of time in bed is adjusted upwards by 15 minutes if sleep efficiency for the week has met or exceeded 90%.
In session 3, the prescribed time in bed is adjusted based on sleep diaries, progress is reviewed and suggestions made for any adjustments to stimulus control and/or sleep hygiene goals, and cognitive therapy is introduced. Here the overall focus is on the negative thoughts and/or maladaptive beliefs about sleep, insomnia, and its consequences and utilizing cognitive restructuring to challenge the veracity and usefulness of these unhelpful thoughts and beliefs and then to change or modify them. After being introduced to the steps of cognitive restructuring, participants are provided with a thought log to record problematic or sleep-interfering thoughts, challenges to those thoughts and the resulting alternative thought.
In session 4, progress and discussion of all prior CBTi components continues, the total sleep time is again adjusted, the participant is instructed on how to continue to make changes to total sleep time on their own, and there is discussion of how to maintain gains and prevent relapse.
CPT is a trauma treatment with demonstrated efficacy in reducing PTSD and MDD symptoms [21]. CPT consists of 12 one-hour, weekly sessions. In the first session, participants are presented with psychoeducation on PTSD symptoms and a rationale for CPT. CPT is based on social-cognitive theory, which states that the way an individual cognitively processes a traumatic event directly impacts emotions. According to this theory, recovery from PTSD relies on the activation and subsequent correction of maladaptive beliefs related to the traumatic event. The therapy focuses on five major dimensions of one’s life that are often dramatically altered by a traumatic event: safety, trust, power and control, esteem, and intimacy. In session 2, participants read a statement about the impact of the traumatic event on their beliefs regarding each dimension.
In sessions 3–5, participants use worksheets to help them identify the relationship between thoughts, feelings, and behaviors. Specifically, the therapist helps participants identify thoughts and behaviors that interfere with their recovery and process the natural emotions associated with the event. Participants are taught to examine how avoidance behaviors reinforce distorted beliefs about the trauma, which become generalized to current life situations. In session 4, participants read their trauma account and the therapist and participant identify maladaptive beliefs, such as self-blame, associated with the traumatic event that perpetuate the symptoms of PTSD. Session 5 begins with participants reading a rewritten version of the trauma account. Using worksheets, the participants are taught to challenge beliefs that interfere with recovery, which is continued in sessions 6 and 7.
Sessions 8–12 explore how beliefs regarding safety, trust, power and control, esteem, and intimacy were altered by the traumatic event and challenge maladaptive beliefs associated with each of the dimensions. In the last session, the participants read a final account of the impact of the event on their life. The goal of therapy is to actively challenge patterns of problematic thinking and teach participants to develop more accurate thoughts regarding the trauma, accept that the event occurred, and successfully integrate the experience into their life.
An overarching aim of this study is to identify the effect of targeted sleep treatment (CBTi) on psychiatric symptoms both before and following an evidence-based PTSD and MDD treatment (CPT). Further, there are likely neurobiological mechanisms through which insomnia treatment may bolster psychological well-being and PTSD and MDD treatment outcomes. First, sleep disturbance is associated with alterations in inflammation, including increased levels of inflammatory markers such as interleukin (IL)-6, and neuroendocrine function, including less reliable changes in diurnal cortisol [22–24]. These same alterations are observed in patients suffering with PTSD and MDD [25], and mechanistic models of depression and PTSD underscore neuroendocrine and inflammatory contributions to behavioral and emotional symptoms [26–28]. Second, insomnia, PTSD, and depression are each characterized by similar alterations in sleep architecture, including decrements in slow wave sleep (SWS) [29–32]. Further, patients with PTSD have more arousals in rapid eye movement (REM) sleep compared to healthy controls, which may exacerbate PTSD symptoms [32–33].
Taken together, insomnia may play a key role in neurobiological pathways linking trauma exposure to PTSD and depression symptomatology. If so, addressing insomnia directly and prior to PTSD and MDD treatments may promote regulation of neuroendocrine and inflammatory pathways, sleep architecture, and psychological well-being in traumatized individuals. Such effects should, in turn, strengthen individuals’ capacity to manage the emotional stress, and thereby increase effectiveness, of trauma-targeted treatments.
To identify the role of insomnia treatment in PTSD and MDD treatment outcomes, and mechanisms of effects, we implemented a novel randomized controlled trial (RCT) to test the following hypotheses: (1) Patients with comorbid PTSD and MDD receiving CBTi, compared to patients in a phone contact only (no sleep treatment) control condition, will show greater improvements immediately following CBTi, but prior to CPT, in three domains: (a) declines in insomnia, PTSD, and MDD symptoms; (b) lower levels of cortisol and IL-6; and (c) fewer arousals in REM sleep and more SWS; (2) CBTi treatment, compared to the control condition, will lead to (a) greater reductions in PTSD and MDD symptom severity; (b) lower PTSD and MDD remission rates; and (c) lower overall cortisol output and IL-6 levels following CPT; (3) CBTi effects on PTSD and MDD symptom severity will be mediated in part by lower cortisol and IL-6 levels, as well as decreased REM arousals and improvements in SWS. As our prior work identified a critical need for insomnia, PTSD, and MDD treatment among interpersonal violence (IPV) survivors [34], we are testing the efficacy of CBTi in improving CPT outcomes in this trauma-exposed population.
2. Methods and Materials
2.1 Preparatory Phase: Community-Based Participatory Research
In community based participatory research (CBPR), researchers consult with potential intervention participants prior to the study design and implementation [35,36]. Important information needed prior to intervention development includes understanding the target populations’ needs and wants relative to care, the manner in which the care might be delivered including where and when, and how the intervention might be labeled so as to not limit participation. Using this information to develop trials greatly enhances the likelihood of success in trial implementation. To prepare for design of the current trial, investigators [CC; WP] conducted two assessments: a) a survey of 121 court-based trauma survivors about their sleep issues, comorbidities and treatment concerns and b) a survey with 47 individuals with a IPV-related trauma to understand their treatment desires with respect to addressing sleep disturbances. The proposed study design was subsequently presented to a IPV survivor group for their feedback.
The first survey utilized the Modified PTSD Symptom Scale [37], the Conflict Tactics Scale-2 Short [38], The Center for Epidemiological Studies Depression Scale [39], and the Insomnia Severity Index [40]. The findings revealed that 46% of participants suffered from clinically meaningful insomnia and that insomnia was associated with more severe depression and PTSD [34]). In additional qualitative data collected, both service providers and survivors were concerned that a traditional approach of prescribing sleep medications would limit survivors’ abilities to execute safety plans in the night should their perpetrators attempt to break-in, and may limit effective parenting as many of them have children they are responsible for during the night. To determine next steps to meet this treatment need, while balancing the concerns of the target population, we conducted a follow-up survey.
For the second survey (unpublished), participants were recruited from two community-based locations: a local family court waiting room and a domestic violence shelter. Participants completed a brief two-page survey created for intervention development and to follow-up on the qualitative data above. We queried participants about sociodemographics, sleep issues and concerns, and treatment preferences. Forty-seven individuals participated including 44 women (94%) and 3 men (6%). The mean age was 36 years old (SD = 13). The sample was racially and ethnically diverse, with approximately 34% endorsing being black, 55% white, 10% non-white Hispanic/Latino and 6% other or multi-racial. As a proxy of socioeconomic status, 43% of the sample received Medicaid with an additional 10% under the age of 55 who received Medicare. Importantly, we learned that 96% of the sample had some form of health insurance. Sixty percent of participants reported an insomnia complaint, in response to one item (“Do you have insomnia – difficulty falling asleep or staying asleep?”) and the mean duration of the problem was 7.6 months (SD = 2.0). More than half the participants (62%) indicated they would participate in a study to treat their sleep needs. Individuals were asked to rate their interests in different methods for addressing sleep issues on a Likert scale, ranging from Not at all (1) to Extremely (5). Of those that responded, 66% were not interested in medication to address their sleep issues in isolation of other options. The majority, (57%), selected an interest in “therapy or counseling for insomnia” (somewhat, very and extremely interested); the second most popular choice was a combination of counseling and medication, while very few chose self-help. Participants also preferred individual therapy (70%) over group counseling (58%) and several provider types were rated highly especially psychologist (70%), social worker (66%), and nurse (63%). While the type of counseling was not specified by name (i.e., CBTi), as most individuals would be unfamiliar with the titles or acronyms, it was specified as “sleep counseling”. This alleviated potential concerns about survivors feeling hesitant to seek help from a medical or mental health provider. To determine the location for this study, we asked participants where they would like to receive services: at the court, a local domestic violence shelter, a downtown location, a local hospital or a community-based medical office park. The majority of participants, 28 (60%), selected the hospital as their first choice. When asked for more information regarding the choice for the hospital, participants indicated a hospital-based setting would allow for the use of Medical Motors, a free transportation option for many individuals meeting circumstances of poverty.
Based on the information gathered, we designed the current clinical trial to meet the participants’ needs, their providers’ concerns, and address scientific questions about addressing sleep concerns in addition to addressing PTSD. Because of sleep issues, survivors expressed being unable to meet basic needs, including safety concerns. We determined that sequencing CBTi with CPT would be a way to meet all of these needs. The proposed study design was presented to a IPV survivor group prior to grant submission; participants of that group supported the proposed design and affirmed the potential utility of the sequenced treatment proposed. The lead researcher created an interdisciplinary team that would be able to address sleep (WP), trauma and safety (CC), neuroendocrine and inflammatory processes (KH) and the randomization needs to ensure that the experimental and control groups were evenly matched in terms of gender, recruitment site, and antidepressant medication class (HC).
2.2 Design and Trial Procedures
The university-based Research Subjects Review Board approved all aspects of the study, and the investigators obtained a Federal Certificate of Confidentiality to further protect participant privacy. Participants provide verbal informed consent prior to an initial screening and written informed consent prior to a subsequent, full, eligibility and baseline assessment interview. Participants receive up to $430 (in cash) for their participation prorated by study procedures completed.
2.3. Study sample
Study staff recruit participants from community sites: a County Domestic Violence Integrated Family Court (Family Court), the outpatient services of a local 38-bed battered women’s shelter, and community referrals. Alternatives for Battered Women (ABW, Inc.), the shelter, is over four decades old and was the third emergency shelter in the state of New York. ABW is the only licensed New York State provider of domestic violence services in Monroe County, New York, the study site. The programs are divided into five key areas: Hotline, Emergency Shelter, Prevention Education, Counseling, and Court Advocacy. The staff answer over 5,000 calls per year and act as triage for safety planning, crisis counseling, and referrals to other community-based agencies. The program most integral to this study is the Court Advocacy Program. Each year, they help over 1,700 people obtaining resources and protection orders. The Family Court was established February 26, 1998, and has partnered with our university since 1999 for research and evaluation. Family Court has county-wide jurisdiction which includes IPV cases meeting certain requirements that occur in the City of Rochester, as well as 29 towns and villages. Throughout New York State, our court created the first dedicated, full-service domestic violence court, which issues protection orders. Based on power analyses, planned enrollment was set at 150 participants with the goal of retaining 120 participants, earlier work in this setting indicated this sample could be recruited in our anticipated time table.
While therapeutic studies conducted with IPV survivors have had widely divergent attrition rates (13%–83%) [41], we based our 20% attrition rate on our past and current history of research with this population that began in 1999. Our retention efforts include: reach out via phone; the opportunity for participants to watch a video describing the study, the study site and study staff; assistance with transportation and childcare; reminder calls; and the ability to reschedule appointments within a window of time depending on the protocol.
Inclusion criteria include: between 18–64 years of age; report of exposure to trauma from interpersonal violence in the past year that serves as one of the index events for symptoms of PTSD; and meet diagnostic criteria for full or subthreshold PTSD (DSM-IV diagnostic criteria [42], full diagnostic criteria for MDD or clinical cutoff for minor depression (> 10) on the Patient Health Questionnaire (PHQ-9) [43], and insomnia severity and research diagnostic criteria for insomnia disorder [44]. The decision to include subthreshold PTSD is based on its association with multiple impairments, including poor physical and social functioning, and recommendations for its inclusion in research and clinical practice [45].
Study exclusion criteria include: currently cohabitating with their perpetrators; evidence of some untreated sleep disorders (other than insomnia or nightmares), such as sleep apnea, circadian rhythm disorders, restless legs syndrome, and narcolepsy as assessed by the validated, but currently unpublished, Sleep Disorders Symptoms Checklist; dementia or cognitive impairment; history of schizophrenia or bipolar I disorder; current or recent suicidality; health conditions with immunological components or undergoing or taking immunosuppressive therapies; active alcohol dependence (or in remission less than 3 months); pregnancy; medication use including antipsychotics, opiate analgesics, and sleep medications including prescription sleep medications, off-label use of sedating medications used at night for sleep such as trazodone and quetiapine, and other-the-counter sleep aids such as melatonin; unstable use (i.e., new or change of dose within 3 months of study participation) of anti-depressant, anti-hypertensive, cholesterol-reducing medication, non-narcotic, prescription pain medication, and daytime use of anti-anxiety medication. Participants with sleep apnea and self-reported use of continuous positive airway pressure are deemed eligible though adherence to this treatment is not objectively verified. Prazosin for the treatment of nightmares is not exclusionary.
2.4. Recruitment and Initial Screening
Recruitment methods include study flyers provided to recruitment locations, periodic presentations by study staff to community groups with agencies providing services to violence survivors, in person contact by study recruiters at Family Court, referrals to the study from providers at each site and in response to inquiries from individuals or community providers meeting the needs of violence survivors.
The most intense recruitment method occurs at the Family Court described above, which has private, locked waiting rooms where survivors who are seeking safety via a protection order wait for their cases to be called. The area is staffed by ABW advocates, who also provide outpatient services to men and women who have experienced IPV. Study recruiters are on site in this waiting room and approach potential participants according to an IRB approved screening script that includes a stepped process beginning with whether the person would be interested in hearing about the study. Potential participants are given several opportunities to decline before being asked if they would like to complete a brief screening survey to determine eligibility. Verbal consent is obtained from potential participant and the survey is then presented on a tablet computer.
The community-based study sites lend themselves to having potential participants who have experienced IPV within the year: a community-based Family Court is addressing current issues with an intimate partner, family member, or household member. The community-based domestic violence shelter, ABW, provides myriad services as described above including residential and non-residential services such as a hotline, day counseling, and transitional services. While some of these individuals may be experiencing acute stress from very recent traumas, many have concurrent PTSD (an inclusion criteria for the current study) given their current and lifetime traumas.
For potential participants who are not recruited in-person and/or who choose to call the study recruiter, rather than complete the screening in-person, the brief screen is conducted via telephone following verbal consent. For all potential participants, the initial screening measures include: (i) a one page self-report survey including questions about age, height, weight, if interpersonal violence occurred in the past year, current living arrangements, sleep complaints, substance use, and use of sleep medications and (ii) instruments outlined in the measures section below. Screening data is collected and managed using a secure, web-based application (REDCap) [46] designed to support data capture. Per above, data is captured through the use of a tablet computer and downloaded instantly to a secure web portal hosted on a University server. Initial eligibility is determined instantly and potential participants are informed of study eligibility. Persons who are ineligible are provided with a variety of referral and other community resources. If the individual is eligible, research staff describe the study further and provide a study brochure detailing the study. If the individual remains interested in participating, they are scheduled for an informed consent and baseline assessment, which takes place at hospital offices. They are also provided with a copy of the written informed consent document.
The full informed consent process takes place with the study coordinator immediately prior to the baseline interview. Participants have the opportunity to read the consent document, ask questions and discuss any aspects of the study.
2.5. Baseline assessment - Time 1 (T1)
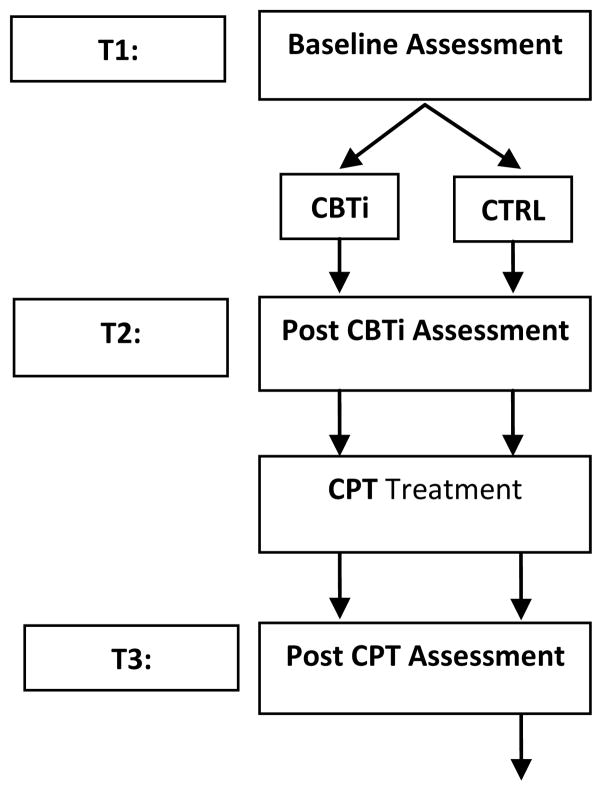
A participant study schedule is outlined in Figure 1. The Time 1 (T1) Baseline Assessment occurs following the informed consent process and consists of two parts: (i) clinician-administered and self-report instruments and (ii) laboratory assessments, including evening and morning blood draws for IL-6, saliva samples for cortisol, and overnight polsomnography (PSG) for SWS and REM. Participants complete the baseline instruments approximately 1–2 weeks prior to their laboratory assessment.
Figure 1. Participant Schedule.
CBTi: Cognitive Behavioral Therapy for Insomnia; CTRL: Control group; CPT: Cognitive Processing Therapy
For the laboratory assessment, participants arrive at the Sleep & Neurophysiology Research Laboratory (Sleep Lab) at 7:00 p.m. to complete self-report instruments, blood draws, salivary cortisol collection, and overnight polysomnography (PSG). For PSG, the sleep period matches their preferred sleep phase (but not later than midnight) and all participants are recorded for approximately 8 hours. The PSG recording montage follows the recommended montage established by the American Academy of Sleep Medicine AASM [47]. PSGs are visually scored in 30-second epochs according to AASM scoring guidelines [47] by technicians meeting laboratory inter-rater reliability and blinded to the participant’s randomized assignment.
For blood draws, two blood samples of approximately 10 ccs each are drawn by venipuncture from the preferred arm of participants at an evening draw within two hours prior to preferred bedtime and a morning draw one hour following rise time. Each sample is centrifuged and placed into four 1 mL cryovials that are frozen and stored at −80º C in an onsite psychoneuroimmunology laboratory. For salivary cortisol collection, saliva for cortisol is collected using the passive drool method. Samples are refrigerated at 4º C immediately after sampling (two evening samples: [7 p.m. and 9 p.m.] and five morning samples [at waking, and then 15, 30, 60 and 90 minutes later]). Saliva is aliquoted into sterile microcentrifuge tubes and stored at −80º C.
2.6. Blinding and Randomization to CBTi or Control Condition
This is a single blind study in which study recruiters, assessors, sleep technicians, CPT therapists, research assistants entering data, and the statistician are blinded to study condition. The CBTi therapists and staff conducting control condition phone calls are necessarily not blinded to condition.
Randomization occurs through Wei’s urn model randomization, which provides overall balance at the end of accrual but also gives good, often near-perfect, balance within many strata [48]. Randomization strata include gender, recruitment site, and antidepressant medication class. Assignment to condition is not predetermined, but generated by a computer program that takes into account study balance to date across randomization strata. After all baseline assessments are completed (including baseline PSG), participants are randomized to condition by study staff who remain blinded as the randomization program provides an assignment to condition “A” or Condition “B”. Based on this designation, a study investigator (WP) who is not blinded, then assigns the participant to either a CBTi therapist, who informs the participant of their assignment and schedules the first CBTi therapy appointment, or to a the CTRL condition staff, who informs the participant of their assignment to the CTRL condition and schedules their first phone check-in.
Participants in the CBTi condition attend 4 individual therapy sessions with the same therapist. This consists of a structured, multi-component CBT intervention described in the introduction. The study CBTi therapists are licensed mental health providers or advanced clinical psychology doctoral students trained in CBTi. Participant progress and adherence is monitored by daily sleep diaries and weekly homework logs.
Participants in the CTRL condition receive phone calls from the clinical coordinator equal in number and timing as the treatment contacts received in the CBTi condition. Content of the calls include brief check-ins and reminders about the protocol, when to start their next one week sleep diary, and their next appointment.
2.7. Post-CBTi assessment - Time 2 (T2)
The Time 2 (T2) post-CBTi assessment takes place at the Sleep Lab approximately 1–3 weeks following the end of the CBTi treatment or CTRL period to allow for some flexibility in scheduling and follows the same procedures as those conducted at the baseline assessment with the exception that PSG has a reduced montage (no breathing, effort, or limb movement channels) and participants in the CBTi condition are asked to complete an intervention feedback survey.
2.8. Cognitive Processing Therapy (CPT)
All participants receive CPT following the T2 assessment. CPT consists of a standard, structured 12-session protocol described in the introduction. It is delivered in individual weekly sessions by experienced CPT therapists, certified in CPT. Progress and adherence is monitored by weekly homework logs.
2.9. Post-CPT assessment - Time 3 (T3)
The Time 3 (T3) post-CPT assessment takes place at the Sleep Lab approximately 1–3 weeks following the CPT treatment period and follows the same procedures as those conducted at T2. In addition, all participants complete surveys to provide feedback on their intervention and research experience. Once all individual data collection activities are completed at the T3 assessment, CTRL participants are offered the CBTi intervention adding approximately 5 weeks to their study involvement, if accepted.
2.10 Therapist fidelity
Therapist fidelity is assessed following established procedures [49] with IRB-approved audio recordings of sessions rated by expert clinicans, one with expertise in CBTi and the other with extensive clinical experience in CPT. Fidelity raters use rating scale instruments developed by two Veterans Health Administration work groups: the ‘Work Group on CBTi Dissemination’ and the ‘Work Group on CPT Dissemination.’ Raters rate approximately 10% of treatment sessions.
2.11. Measures
Preliminary and baseline screening instruments
See Table 1 for an overview of the assessments completed at each time point. Preliminary screening instruments include the PTSD Checklist-civilian version (PCL-C) [50], the PHQ-9 [43], and the Insomnia Severity Index (ISI) [40]. Responses on the PCL-C are used to assess our PTSD inclusion criterion. The PCL-C has 17 items asking respondents to rate how much they have been bothered by particular PTSD symptoms in the past month on a 1–5 scale. The PHQ-9 is used to assess the MDD inclusion criterion. The PHQ-9 is a 9-item self-report questionnaire that assesses the frequency of depressive symptoms over the past 2 weeks with a specific focus on the symptoms necessary to meet criteria for DSM-IV diagnosis of MDD. Individuals scoring 10 or above are considered eligible, with a score of 10–14 identifying moderate depressive symptoms, and 15 or above indicating severe depressive symptoms. To determine if individuals meet the insomnia criterion, the ISI is completed. The ISI is a validated 7-item scale with individual items rated 0–4 and total scores ranging from 0–28. The recently validated cutoff of ≥ 10 for clinically meaningful insomnia is used as an inclusionary threshold [40].
Table 1.
Data collection schedule
| Preliminary | Time 1 | Time 2 | Time 3 | |
|---|---|---|---|---|
| PCL-C | X | |||
| PHQ-9 | X | |||
| ISI | X | X | X | X |
| Demographics | X | |||
| Medical history | X | |||
| MMSE | X | |||
| SDS-CL | X | |||
| MINI | X | X* | X* | |
| CAPS | X | X | X | |
| HRSD-17 | X | X | X | |
| PSG | X | X | X | |
| Serum | * X** | * X** | * X** | |
| Saliva | ** X*** | ** X*** | ** X*** |
PCL-C: PTSD Checklist – Civilian Version; PHQ-9: Physician Health Questionnaire – 9; ISI: Insomnia Severity Index; Medical history includes medication usage and sleep symptoms; SDS-CL: Sleep Disorders Symptoms Checklist; MMSE: Mini Mental Status Exam; MINI: MINI International Neuropsychiatric Exam; CAPS: Clinician-Administered PTSD Scale; HRSD-17: Hamilton Rating Scale for Depression – 17; PSG: Polysomnography.
At Time 2 and Time 3 only the Depression module of the MINI is administered.
One blood draw is conducted within two hours prior to going to bed; another within one hour of rising.
Two evening samples are collected at 7:00 PM and 9:00 PM. Five morning samples are collected at waking, then waking + 15 minutes, +30 minutes, +60 minutes and +90 minutes.
Baseline assessment instruments include instruments to record demographics, medications, medical history and sleep symptoms. The Sleep Disorders Symptoms Checklist (SDS-CL) was developed in our lab to screen for 7 major sleep disorders (insomnia, nightmares, sleep apnea, circadian rhythm disorders, restless legs syndrome, and narcolepsy); the instrument has been validated, but is not yet published. In addition, the Mini Mental State Examination (MMSE) [51] is used to assess cognitive mental status and the DSM-IV version of the MINI International Neuropsychiatric Exam (MINI) [52] is used to further screen for exclusionary disorders and confirm minor depression or MDD diagnosis.
Primary subjective and objective measures
Primary subjective measures are well-validated instruments administered at T1-T3 assessments. The ISI measures insomnia severity, which is also administered at pre-baseline screening, is used at all three time points along with other primary subjective outcome measures. The Clinician Administered PTSD Scale (CAPS) [53] is the gold standard measure of PTSD with both intensity and frequency for each of 17 symptoms rated on a 0–4 scale. The CAPS is used to determine PTSD diagnosis (defined as ≥ 1 re-experiencing symptom(s), ≥ 3 avoidance and numbing symptoms, and ≥ 2 hyperarousal symptoms all meeting the “1–2 rule” [ ≥ 1 in frequency; ≥ 2 in intensity]) [54]. The total CAPS score (minus insomnia items) is used as the primary PTSD outcome measure. The Hamilton Rating Scale for Depression-17 (HRSD) [55] is used as the primary measure of depressive symptoms and is also a clinician-administered depression instrument. Some items are scored on a 0–2 scale and others on a 0–4 scale. The total score (minus insomnia items) is used as the primary depression outcome measure. To identify PTSD remission status at T3, the CAPS is used to determine diagnosis per above. The depression module of the MINI is used to identify depression status based on DSM-IV-TR criteria. All baseline and subsequent assessments are administered by a research coordinator who is blind to study condition.
Objective Sleep Measures are derived from visual scoring of overnight PSG include Sleep Continuity Variables (sleep latency, number of awakenings, wake after sleep onset time, total sleep time and sleep efficiency), and Sleep Architecture Variables (minutes and percent of total sleep time spent in each sleep stage), which are all secondary sleep outcomes. The primary objective sleep outcomes are REM arousals and Slow Wave Activity. Arousals of 3–10 seconds in duration are scored from PSG recordings according to established guidelines [47]. Arousals occurring in REM sleep that are not associated with respiratory events constitute the raw number of REM arousals, which are divided by hours of REM sleep to calculate a REM arousal index. Slow wave activity is obtained by applying Power Spectral Analysis (PSA) to the digital EEG data from the PSG recordings using time series analysis software (Prana Software Suite; PhiTools, Inc., Strasbourg, France).
Salivary cortisol and inflammatory cytokine levels are also assessed at T1-T3. Saliva for cortisol assessment and serum for measuring circulating interleukin-6 (IL-6) level, the primary inflammatory cytokine outcome, are stored at −80° C until assay. Salivary cortisol is assayed in a University laboratory using a cortisol high sensitivity (HS) enzyme immunoassay kit (Salimetrics, PA). The test has a lower limit of sensitivity of .007 μl/dl, range of sensitivity from .007 to 1.2 μg/dl, and average intra-and inter-assay coefficients of variation 4.13% and 8.89%, respectively. IL-6 is assayed in the same laboratory using Quantikine HS ELISA kits (R&D Systems, Minneapolis, MN), which have mean minimum detectable limits of 0.039 pg/mL.
The primary outcome for salivary cortisol measurements is the cortisol awakening response (CAR), indexed by cortisol area under the response curve (AUC) calculated from 5 morning saliva samples; evening cortisol levels are calculated as the mean cortisol level across the two evening samples and will serve as a secondary measure of HPA function (for instance, evening levels would be expected to be lower than morning levels) and evaluated for use in analyses (for instance, if evening HPA activity is related to morning HPA activity, evening cortisol level may be used as a covariate in analyses to determine independent effects of CBTi on CAR). IL-6 levels in the evening and morning will be examined separately.
2.12. Data analysis plan
Power analyses considerations
For analyses involving mean differences (hypotheses 1 and 2), the Optimal Design software program was used to assess statistical power [56]. Given the robust effect sizes (Cohen’s d ≥ .5 [57]) noted in the literature for CBTi effects on PTSD and depression [14,58], we expect to be adequately powered with a sample size of 120 (given 20% attrition over the life of the study) to detect moderate effects. Here, one notes that even under the most conservative estimates (no variance explained by covariates), there is .8 power to detect a moderate effect size of .51. However, with a more reasonable assumption of baseline covariates explaining 30% of the variance in the outcome, .8 power is able to detect an effect size of .43.
For the structural equation model to be tested, the logic of MacCallum, Browne, and Sugawara was followed [59]. Here, adequate power (> .95) to reject an hypothesis of close fit (RMSEA > .08) with 200 degrees of freedom (a degrees of freedom of 200 is a somewhat conservative estimate given that the proposed model, if left unmodified, contains 251 degrees of freedom), given a population RMSEA of .05 was obtained. That is, if the true value of RMSEA = .08, and we test the hypothesis that RMSEA ≤ .05, power is > .98 for rejecting the hypothesis of close fit in the population. Additionally, adequate power (.92) to reject a hypothesis of not-close fit (RMSEA ≥ .05) was obtained. Here, if model fit is actually extremely good (RMSEA < .01), and we test the hypothesis that fit is not close, we have greater than .90 power to reject the null hypothesis that RMSEA ≥ .05.
Hypotheses testing
Study outcomes will be examined using intent-to-treat principles. A baseline check of the effects of random assignment will be conducted for each demographic, mediational, and outcome variable collected prior to assessing for intervention effects. Categorical variables will be analyzed using chi-square tests; continuous variables will use simple t-tests. Because we are more interested in establishing baseline equivalence as opposed to differences, a more conservative alpha level (p < .20) will be used.
To assess the effects of CBTi on PTSD and MDD symptoms, IL-6 and cortisol levels, and REM and SWS, prior to CPT (hypothesis 1), Analysis of Covariance (ANCOVA) will be used to examine mean differences between the CBTi versus control conditions in, respectively, (a) the ISI, CAPS, HRSD scores; (b) IL-6 levels and cortisol area under the curve (AUC); and (c) REM arousals and SWS activity. In all instances, alpha = .05 and covariates will include gender, ethnicity (dummy coded), age, number of trauma exposures, and the outcome baseline score as well as any other variables found to differ at baseline.
To assess outcomes effects of combining CBTi and CPT (hypothesis 2), similar to above, ANCOVAs will be assessed, examining mean differences between the CBTi versus control conditions, following CPT, in CAPS, HRSD, IL-6 levels and cortisol AUC. In addition, to test remission rates, logistic regression will be used to predict a PTSD and MDD remission (CAPS and HRSD-based).
Finally, to test the mediating role of IL-6, cortisol, REM and SWS in CBTi effects on PTSD and MDD symptoms post-CPT (hypothesis 3), structural equation modeling (SEM) will be used with mental health status at T3 (PTSD and MDD) as the key outcome of interest.
While we will make every effort to collect all data at each data collection point, missing data will likely remain an issue. For analyses examining mean differences, pairwise deletion of missing data will be used, thus retaining all available information. The structural equation model to be tested will make use of the full-information maximum likelihood (FIML) estimation method. FIML uses estimates throughout the model to provide more accurate estimates where data is missing. Full information estimation has been shown to provide more realistic parameter estimates than other missing data techniques (e.g., listwise, pairwise, mean imputation; [60]), providing data is missing at random or missing completely at random.
Attrition analyses
To further understand the effects of any biased attrition, two additional forms of sensitivity analyses will be conducted. First, multiple imputation of missing data will be conducted. In short, multiple imputation uses a regression based approach to impute values for data that are missing. So that variance is not artificially constrained, multiple imputation incorporates random error into the imputation process [61,62]. Each intervention condition is imputed separately (i.e., CBTi participants imputed; wait-list control participants imputed) and then the resulting datasets are merged. Ten imputations will be performed and each of the 10 resulting datasets will be analyzed as above. The results obtained from each will be combined following the techniques employed by Rubin [63]. Multiple imputation has consistently demonstrated less biased parameter estimates than most other traditional approaches to the handling of missing data (i.e., listwise, pairwise, mean imputation, single regression imputation; [60,63–64]). The second approach will adopt Hedecker and Gibbons’ pattern-mixture modeling approach to missing data in longitudinal studies [66]. This approach essentially groups participants based on their patterns of missing data and incorporates group membership into the outcome analyses with particular emphasis on group membership by condition interaction terms.
3. Discussion
We have designed and are executing a CBPR-informed clinical trial to determine the effects of delivering CBTi, a behavioral insomnia treatment, prior to delivering CPT, an evidence-based trauma treatment. Our sample consists of survivors of recent interpersonal violence with PTSD, depression, and insomnia. Outcomes for this study include both clinically relevant symptom severity scores and biologic markers associated with chronic disease amongst survivors of recent interpersonal violence.
The novelty of this clinical trial lies in the integrative approach to both testing targeted treatment in PTSD, and to understanding significant augmenters of PTSD and MDD symptomatology. First, we see considerable merit in prefacing PTSD and MDD treatment with a sleep intervention. Others identify sleep as a residual problem following PTSD treatment and have noted the potential value in targeting sleep in the context of PTSD treatment [67], yet to our knowledge this is the first trial to explicitly preface an evidenced-based PTSD treatment with CBTi, a highly efficacious behavioral sleep intervention. Having sleep problems and being in sleep treatment carry far less stigma than being diagnosed and treated for PTSD or depression. Thus, sleep treatment may be a gateway to engagement with evidenced-based PTSD and MDD treatment, especially in populations for whom mental health treatment seeking is more stigmatizing. Moreover, improved sleep may decrease the severity of both PTSD and MDD symptoms, as we found in our own pilot work [68], while increasing a person’s capacity to cope with persistent symptoms. Beyond this, however, improving sleep prior to a PTSD intervention may enhance the effect of PTSD interventions by improving adherence and retention rates and/or by allowing patients to engage more fully in treatment because of improved sleep.
Second, the inflammatory and neuroendocrine alterations observed in insomnia have not yet been applied to understanding comorbid PTSD and MDD symptomatology. Yet, there are considerable commonalities in neurobiological dysregulation between these disorders. The proposed work leverages the longitudinal nature of an intervention study to also focus on mechanistic questions and aims. In this way, CBTi becomes a probe to test an innovative, integrative model of sleep, endocrine and inflammatory pathways that we suggest play a significant role in PTSD with MDD. The capture of both symptom severity and biomarkers in a reasonably large clinical sample over an observation period that includes three time points that will allow for modeling is itself unique. Further, chronic PTSD and depression increase risk for chronic diseases [69]. Cortisol and inflammatory processes are linked to the most common and costly of chronic diseases for which PTSD may be a risk factor (e.g., type II diabetes, hypertension, coronary heart disease, and stroke) [69–71]. Thus, clarifying the role of neuroendocrine and inflammation in links between PTSD and sleep can help with novel development of comprehensive and efficacious approaches to reducing PTSD-associated health risk.
Potential limitations of the current trial include the heterogeneity that may be introduced by variability in PTSD chronicity and severity, the greater amount of attention provided to participants assigned to CBTI versus the phone call control condition, and the generalizability of findings given the use of DSM-IV assessments. With regard to PTSD chronicity and severity, it should be noted that the population of interest is survivors of recent interpersonal violence (IPV) with PTSD and MDD. In the current study, this is operationalized as IPV within the past year, and currently meeting diagnostic criteria for full or subthreshold PTSD and MDD or minor depression. Based on our earlier work, we anticipate a range in reported IPV (e.g., from pushing and shoving to more violent acts that lead to greater physical harm, such as broken bones and lost teeth. Although participants will meet criteria for full or subthreshold PTSD, it is possible that some participants will have experienced very recent traumas (or experience them during the course of their participation) and, thus, be experiencing acute stress symptoms in addition to post-traumatic symptoms. Such participants are not excluded, but each of the assessments capture the time of occurrence of any recent traumas, making them available for analysis. The attention control condition was designed to equate the number and frequency of contact with the participants in the CBTi condition, as it was discerned that equating the duration of each contact between groups was not feasible (phone calls would be required to last 40–60 minutes). Nevertheless, contact duration could be a potential confound. Finally, the American Psychiatric Association introduced the DSM-5 approximately 12 months after data collection commenced for this protocol. Given the number of enrolled participants at the time the revised PTSD and MDD measures were available, it would not have been prudent to adopt these measures. Thus, interpreting findings from the current study will require consideration of distinctions between DSM-IV and DSM-5 diagnostic requirements.
A court-based IPV population was selected as the main recruitment site. Already a vulnerable population, survivors of IPV have to deal with the aftermath of violence in their lives, but also comorbid issues such as depression and PTSD. Much work remains to address their sleep issues in ways that continue to promote their safety (such as exploring the impact of psychotherapy when compared to medications). This study is a first step in our research program, which also includes understanding how sleep interventions might enhance survivor participation in the court processes as well as safety planning. Because sleep is crucial for overall physical and mental health, our sleep intervention is a preliminary step to improving survivors’ depression and trauma, which in turn, should improve overall quality of life. Confirmation of the hypotheses would support immediate translation of this research approach to clinical practice. Additionally, by conducting these studies outside the clinical walls, with survivor input, we can more readily translate this intervention to community partners where individuals are more likely to seek help for violence issues.
Acknowledgments
The work presented here was funded by the National Institute of Nursing Research at the National Institute of Health (R01NR013909). The trial is registered with clinical trilas.gov (NCT01743339).
Footnotes
Disclosures
Author WP is an employee of the United States (U.S.) Department of Veterans Affairs (VA); the views or opinions expressed herein do not necessarily represent those of the VA or the U.S. Government.
Author WP is on the speaker’s bureau for Merck, Sharpe & Dohme; the authors declare no other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Jong JTVM, Komproe IH, Van Ommeren M, El Masri M, Araya M, Khaled N, van de Put W, Somasundaram D. Lifetime events and posttraumatic stress disorder in 4 postconflict settings. JAMA. 2001;286:555–62. doi: 10.1001/jama.286.5.555. [DOI] [PubMed] [Google Scholar]
- 2.Campbell R, Greeson MR, Bybee D, Raja S. The co-occurrence of childhood sexual abuse, adult sexual assault, intimate partner violence, and sexual harassment: a mediational model of posttraumatic stress disorder and physical health outcomes. J Consulting Clin Psych. 2008;76:194–207. doi: 10.1037/0022-006X.76.2.194. [DOI] [PubMed] [Google Scholar]
- 3.Pimlott-Kubiak S, Cortina LM. Gender, victimization, and outcomes: reconceptualizing risk. J Consulting Clin Psych. 2003;71:528–39. doi: 10.1037/0022-006x.71.3.528. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 5.Cascardi M, O’Leary KD, Schlee KA. Co-occurrence and correlates of posttraumatic stress disorder and major depression in physically abused women. J Family Violence. 1999;14:227–49. [Google Scholar]
- 6.Stein MB, Kennedy C. Major depressive and post-traumatic stress disorder comorbidity in female victims of intimate partner violence. J Affective Disorders. 2001;66:133–38. doi: 10.1016/s0165-0327(00)00301-3. [DOI] [PubMed] [Google Scholar]
- 7.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbances as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146:697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 8.Green BL. Disasters and posttraumatic stress disorder. In: Davidson JR, Foa EB, editors. Posttraumatic stress disorder: DSM-IV and beyond. Washington D.C: American Psychiatric Press; 1993. [Google Scholar]
- 9.Manber R, Rush J, Thase ME, Arnow B, Klein D, Trivedi MH, Korenstein SG, Markowitz JC, Dunner DL, munsaka M, Borian FE, Keller MB. The effects of psychotherapy, nefazodone, and their combination on subjective assessment of disturbed sleep in chronic depression. Sleep. 2003;26:130–36. doi: 10.1093/sleep/26.2.130. [DOI] [PubMed] [Google Scholar]
- 10.Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry. 2007;68:254–60. doi: 10.4088/jcp.v68n0211. [DOI] [PubMed] [Google Scholar]
- 11.Belleville G, Guay S, Marchand A. Persistence of sleep disturbances following cognitive-behavior therapy for posttraumatic stress disorder. J Psychosom Res. 2011;70:318–27. doi: 10.1016/j.jpsychores.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Stein DJ, Davidson J, Seedat S, Beebe K. Paroxetine in the treatment of post-traumatic stress disorder: pooled analysis of placebo-controlled studies. Expert Opinion on Pharmacotherapy. 2003;4:1829–38. doi: 10.1517/14656566.4.10.1829. [DOI] [PubMed] [Google Scholar]
- 13.Davidson JRT, Rothbaum BO, van der Kolk BA, Sikes CR, Farfel GM. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry. 2001;58:485–92. doi: 10.1001/archpsyc.58.5.485. [DOI] [PubMed] [Google Scholar]
- 14.Ulmer C, Bosworth H, Edinger J, Calhoun P, Almirall D. A Brief Intervention for Sleep Disturbance in Ptsd: Pilot Study Findings. J Gen Intern Med. 2010;25:202–06. [Google Scholar]
- 15.Germain A, Shear MK, Hall M, Buysse DJ. Effects of a brief behavioral treatment for PTSD-related sleep disturbances: A pilot study. Behaviour Research and Therapy. 2007;45:627–32. doi: 10.1016/j.brat.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Swanson LM, Favorite TK, Horin E, Arnedt JT. A combined group treatment for nightmares and insomnia in combat veterans: A pilot study. Journal of Traumatic Stress. 2009;22:639–42. doi: 10.1002/jts.20468. [DOI] [PubMed] [Google Scholar]
- 17.Baddeley JL, Gros DF. Cognitive behavioral therapy for insomnia as a preparatory treatment for exposure therapy for posttraumatic stress disorder. Am J Psychotherapy. 2013;67:199–210. doi: 10.1176/appi.psychotherapy.2013.67.2.203. [DOI] [PubMed] [Google Scholar]
- 18.Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive Behavioral Treatment of Insomnia. New York: Springer Science Business Media Inc; 2005. [Google Scholar]
- 19.Pigeon WR, Funderburk J. Delivering a brief insomnia intervention to depressed VA primary care patients. Cognitive Behavioral Practice. 2014;21:252–60. [Google Scholar]
- 20.Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;2:177–82. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- 21.Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. Journal of consulting and clinical psychology. 1992;60:748–56. doi: 10.1037//0022-006x.60.5.748. [DOI] [PubMed] [Google Scholar]
- 22.Vgontzas AN, Tsigos C, Bixler EO, Stratakis CA, Zachman K, Kales A, Vela-Bueno A, Chrousos GP. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 23.Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin HM, Vela-Bueno A, Kales A, Chrousos GP. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51:887–92. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- 24.Burgos I, Richter L, Klein T, Feibich B, Feige B, Lieb K, Voderholzer U, Riemman D. Increased nocturnal Interleukin-6 excetion in patients with primary insomnia: a pilot study. Brain, Behavior, and Immunity. 2006;20:246–53. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Gill J, Luckenbaugh D, Charney D, Vythilingam M. Sustained elevation of serum interleukin-6 and relative insensitivity to hydrocortisone differentiates posttraumatic stress disorder with and without depression. Biol Psychiatry. 2010;68:999–1006. doi: 10.1016/j.biopsych.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Mehta D, Binder EB. Gene x environment vulnerability factors in PTSD: The HPA axis. Neuropharmacology. 2011;62:654–62. doi: 10.1016/j.neuropharm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Pace TWW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: From risk factors to medical comorbidities. Brain Behavior and Immunity. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 30.Perlis ML, Buysse DJ, Thase ME, Tu XL, Giles DE, Kupfer DJ. Which depressive symptoms relate to which sleep EEG variables. Biol Psychiatry. 1997;42:904–13. doi: 10.1016/S0006-3223(96)00439-8. [DOI] [PubMed] [Google Scholar]
- 31.Pigeon WR, Perlis ML. Sleep homeostasis in primary insomnia. Sleep Med Reviews. 2006;10:247–54. doi: 10.1016/j.smrv.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: Integrative review and neurobiological hypotheses. Sleep Med Reviews. 2008;12:185–95. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: A meta-analytic review. Psychophysiology. 2007;44:660–9. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 34.Pigeon WR, Cerulli C, Richards H, He H, Perlis ML, Caine ED. Sleep disturbances and their association with mental health among women exposed to intimate partner violence. J Womens Health. 2011;20:1923–9. doi: 10.1089/jwh.2011.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annual Review of Public Health. 1998;19:173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- 36.Wallerstein NB, Duran B. Using community-based participatory research to address health disparities. Health Promotion Practice. 2006;7:312–23. doi: 10.1177/1524839906289376. [DOI] [PubMed] [Google Scholar]
- 37.Falsetti SA, Resnick HS, Resick PA, Kilpatrick DG. The Modified PTSD Symptom Scale: A brief self-report measure of posttraumatic stress disorder. The Behavior Therapist. 1993;16:161–62. [Google Scholar]
- 38.Straus MA, Hamby SL, BoneyMcCoy S, Sugarman DB. The revised Conflict Tactics Scales (CTS2) - Development and preliminary psychometric data. J Family Issues. 1996;17:283–316. [Google Scholar]
- 39.The CES-D scale: a self-report depressive scale for research in the general population. J Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 40.Morin CM, Belleville G, Belanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warshaw, Sullivan, Rivera 2013 http://www.nationalcenterdvtraumamh.org/wp-content/uploads/2013/03/NCDVTMH_EBPLitReview2013.pdf.
- 42.Grubaugh AL, Magruder KM, Waldrop AE, Elhai JD, Knapp RG, Frueh BC. Subthreshold PTSD in primary care - Prevalence, psychiatric disorders, healthcare use, and functional status. J Nervous Mental Disease. 2005;193:658–664. doi: 10.1097/01.nmd.0000180740.02644.ab. [DOI] [PubMed] [Google Scholar]
- 43.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9 - Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Jamieson AO, McCall WV, Morin CM, Stepanski EJ. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 45.Schnurr PP. A guide to the literature on partial PTSD. PTSD Research Quarterly. 2014;25:1–8. [Google Scholar]
- 46.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iber C, Ancoli-Israel S, Chesson A, Jr, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 48.Wei L. An application of an urn model to the design of sequential controlled trials. Journal of the American Statistical Association. 1978;73:559–63. [Google Scholar]
- 49.Waltz J, Addis ME, Koerner K, Jacobson NS. Testing the integrity of a psychotherapy protocol - assessment of adherence and competence. J Consulting and Clin Psych. 1993;61:620–30. doi: 10.1037//0022-006x.61.4.620. [DOI] [PubMed] [Google Scholar]
- 50.Weathers FW, Ford J. Psychometric review of PTSD Checklist (PCL-C, PCL-S, PCL-M, PCL-PR) In: Stamm BH, editor. Measurement of stress, trauma, and adaptation. Lutherville, MD: Sidram; 1996. pp. 250–2. [Google Scholar]
- 51.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 52.Sheehan DV, Lecrubier Y, Sheehan KH, Janavs J, Weiller E, Keskiner A, Schinka J, Knapp E, Sheehan MF, Dunbar GC. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. European Psychiatry. 1997;12:232–41. [Google Scholar]
- 53.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The Development of A Clinician-Administered Ptsd Scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 54.Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the clinician administered posttraumatic stress disorder scale. Psychological Assessment. 1999;11:124–33. [Google Scholar]
- 55.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spybrook J, Raudenbush SW, Congdon R, Martinez A. Optimal design for longitudinal and multilevel research: Documentation for the “Optimal Design” software. 2011 Available: http://www.wtgrantfdn.org/resources/overview/research_tools/research_tools.
- 57.Cohen J. The earth is round (p <. 05) Am Psychol. 1994;49:997–1003. [Google Scholar]
- 58.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo, Melanie G, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacCallum RC, Browne MW, Sugawara HM. Power analysis and determination of sample size for covariance structure modeling. Psychological Methods. 1996;1:130–49. [Google Scholar]
- 60.Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling: Issues and techniques. Mahwah, NJ: Erlbaum; 1996. pp. 243–277. [Google Scholar]
- 61.Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–77. [PubMed] [Google Scholar]
- 62.Allison PD. Missing data. Newbury Park: Sage; 2002. [Google Scholar]
- 63.Rubin DB. Multiple imputation for nonresponse in surveys. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 64.Graham JW. Missing data analysis: Making it work in the real world. Annual Rev Psych. 2009;60:549–76. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 65.Roth PL. Missing data: A conceptual review for applied psychologists. Personnel Psych. 1994;47:537–60. [Google Scholar]
- 66.Hedeker D, Gibbons RD. Application of random-effects pattern-mixture modes for missing data in longitudinal studies. Psychological Methods. 1997;2:64–78. [Google Scholar]
- 67.Galovski TE, Monson C, Bruce SE, Resick PA. Does Cognitive-Behavioral Therapy for PTSD Improve Perceived Health and Sleep Impairment? Journal of Traumatic Stress. 2009;22:197–204. doi: 10.1002/jts.20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pigeon WR, Matteson-Rusby S, Knox KL. CBT for sleep disturbances in combat veterans: preliminary findings. Sleep. 2010:33S. [Google Scholar]
- 69.Schnurr PP, Green BL. Understanding relationships among trauma, posttraumatic stress disorder, and health outcomes. In: Schnurr PP, Green BL, editors. Trauma and health: Physical health consequences to exposure to extreme stress. Washington D.C: American Psychological Association; 2003. pp. 247–75. [Google Scholar]
- 70.Schnurr PP, Spiro A. Combat exposure, posttraumatic stress disorder symptoms, and health behaviors as predictors of self-reported physical health in older veterans. Journal of Nervous and Mental Disease. 1999;187:353–9. doi: 10.1097/00005053-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Boscarino JA. Diseases among men 20 years after exposure to severe stress: Implications for clinical research and medical care. Psychosom Med. 1997;59:605–14. doi: 10.1097/00006842-199711000-00008. [DOI] [PubMed] [Google Scholar]