Abstract
Hypothesis
Electrode-to-modiolus distance is correlated with clinically-programmed stimulation levels.
Background
Conventional wisdom has long supposed a significant relationship between cochlear implant (CI) stimulation levels and electrode-to-modiolus distance; however, to date, no such formal investigation has been completed. Thus the purpose of this project was to investigate the relationship between stimulation levels and electrode-to-modiolus distance. A strong correlation between the two would suggest that stimulation levels might be used to estimate electrode-to-modiolus geometry.
Methods
Electrode-to-modiolus distance was determined via CT imaging using validated CI position analysis software in 137 implanted ears from the three manufacturers holding FDA approval in the United States. Analysis included 2,365 total electrodes, with 1,472 from pre-curved arrays. Distances were compared to clinically-programmed C/M levels which were converted to charge units.
Results
Mean modiolar distance with perimodiolar and lateral wall electrodes was 0.47 mm and 1.15 mm, respectively. Mean suprathreshold charge values were significantly different between each manufacturer. When combining all data, we found a moderate positive correlation (r = 0.367, p <0.01) that was driven both by the different charge values across companies, and that the company with the highest mean charge values only offers straight electrode arrays. When grouped by electrode type, however, we found a weak correlation (r = 0.12, p <0.01) for perimodiolar array electrodes only. When considering a single array type from any one manufacturer, only one was observed where distance mildly predicted charge.
Conclusion
Our results suggest that electrode distance minimally contributes to the current level required for suprathreshold stimulation.
Cochlear implant electrode arrays are in a constant state of evolution. From the number of electrodes, to the length and shape of the arrays, various designs are currently implemented across and within implant manufacturers. Cochlear implant teams are tasked with making a decision as to which electrode style will be best suited for a given patient. One variable to consider is whether to select a pre-curved electrode array or a straight array. Two of the three implant manufacturers holding FDA approval in the United States currently offer both pre-curved and straight electrode arrays. Early cochlear implant designs utilized straight arrays, while pre-curved arrays were approved in the United States in the late 1990’s.
Shepherd et al. (1) measured electrically-evoked auditory brainstem responses (EABR) in cats with the electrode array in four positions – with the two extreme positions including a lateral wall placement and a modiolar hugging placement. They reported that EABR thresholds decreased significantly as the array was positioned progressively closer to the modiolus in 9 of the 10 cats in this sample. Growth rates of the EABR function were also steeper when arrays were positioned along the lateral wall. A number of early human studies also confirmed that so-called perimodiolar electrode array designs resulted in reduced electrode-to-modiolus distances and lower programmed threshold and comfortable levels than comparable arrays designed for lateral wall placement (2–3). Balkany et al. (4) measured modiolar proximity in perimodiolar electrode arrays from all three FDA approved manufacturers in 15 adult human temporal bones using videofluoroscopic imaging and computer morphometrics. They found that all three designs were effective at positioning electrodes in close proximity to the modiolus, with average distance across all three array designs being ≤ 0.5 mm. Thus there is no doubt that pre-curved designs are effective in positioning electrodes in a perimodiolar orientation, as designed.
The majority of current literature evaluating effects of charge on array type reports that perimodiolar placements result in reduced charge for equivalent stimulation at threshold and at suprathreshold comfort levels (3, 5–6) and lower EABR thresholds (7–8). There are, however, a few published studies that have reported contrasting results. Hughes & Abbas (9) evaluated behavioral thresholds in ten adult Nucleus 24 recipients, half of which received a straight array while the other five received the perimodiolar Contour array. They found no significant difference in behavioral threshold between the two electrode types. While a fairly small sample size was used in this study, these findings (9) call into question the extent to which charge levels can be predicted by modiolar proximity and/or electrode types. Van Weert et al. (10) compared intra-operatively-obtained electrically evoked compound action potential (ECAP) data in 14 subjects with a perimodiolar electrode array before and after the surgical stylet was removed. No significant difference was found in ECAP responses before and after the stylet was removed.
Studies evaluating relationships between electrode type and an objective neural response (e.g. eSRT, EABR, ECAP, etc.) are certainly of value as they can demonstrate how modiolar proximity relates to neural stimulation. Indeed, the majority of studies show that decreased modiolar distance results in increased neural responses for the same stimulation. However, electrophysiological measures such as EABR and ECAP do not necessarily correlate with behavioral threshold or comfort levels. Several studies have evaluated the relationship between eSRT, EABR, and ECAP and behavioral threshold and comfort levels (11–13) and found that ECAP threshold data were highly variable and tended to underpredict behavioral comfort levels. EABR was found to better predict behavioral threshold levels than comfort levels.
Although there is consensus in the literature that perimodiolar electrode arrays are effective in placing individual electrodes closer to the modiolus, it is unclear to what extent this relationship holds across implant manufacturers. Also, there still remains some question as to the influence of electrode array type (and by extension, modiolar distance) on behavioral charge levels. Based on the findings that pre-curved arrays are associated with lower behavioral threshold and comfort levels as well as EABR thresholds, it would not be unreasonable to assume that modiolar distance is correlated with charge. But this relationship has yet to be explicitly reported in the literature. Thus the primary goal of this study was to directly measure electrode-to-modiolar distance and compare to charge levels in a large sample of all three FDA approved manufacturers and several electrode types. Our hypothesis was that electrode-to-modiolus distance would be positively correlated with charge units calculated from clinically programmed stimulation levels.
Methods
Subjects
Subject information is provided in Table 1. Analysis was conducted on 137 implanted ears. 80 subjects (58%) received Cochlear brand electrodes, 27 had Advanced Bionics devices (20%), and 30 ears were implanted with MED-EL devices (22%). Seventy-three arrays (53%) in this study were pre-curved, which encompasses all designs not intended to be positioned along the lateral wall. For example, Advanced Bionics produces both the helix and mid-scala array. The helix and mid-scala are shown in Table 1 and for analysis purposes here, both were characterized as pre-curved. One subject received a CII HiFocus device with positioner and was included in this precurved group, as well. Balkany and colleagues (4) showed that this device achieved modiolar proximity in line with other perimodiolar array designs. There were no efforts to specifically recruit subjects with a specific electrode type or manufacturer. A total of 2,375 electrodes were initially analyzed for this study. However, 10 electrodes were rejected as they were determined to be extracochlear. After discarding those 10 electrodes, 2,365 electrodes remained for analysis. Many subjects came to Vanderbilt from other cities and were implanted in other centers.
Table 1.
Number of implanted ears with each electrode type
| Number of Ears | |
|---|---|
| Cochlear | |
| Straight | |
| CI422 | 11 |
| CI24RE (ST) | 4 |
| Pre-Curved | |
| CI512 | 13 |
| Freedom CI24RE(CA) | 52 |
| SubTotal | 80 |
| Advanced Bionics | |
| Straight | |
| 1j | 19 |
| Pre-Curved | |
| Mid-Scala | 3 |
| Helix | 4 |
| CII HiFocus | 1 |
| SubTotal | 27 |
| MED-EL | |
| Straight | |
| Flex 28 | 15 |
| Flex 24 | 3 |
| Standard | 10 |
| Medium | 2 |
| SubTotal | 30 |
| Total | 137 |
Distance Calculation
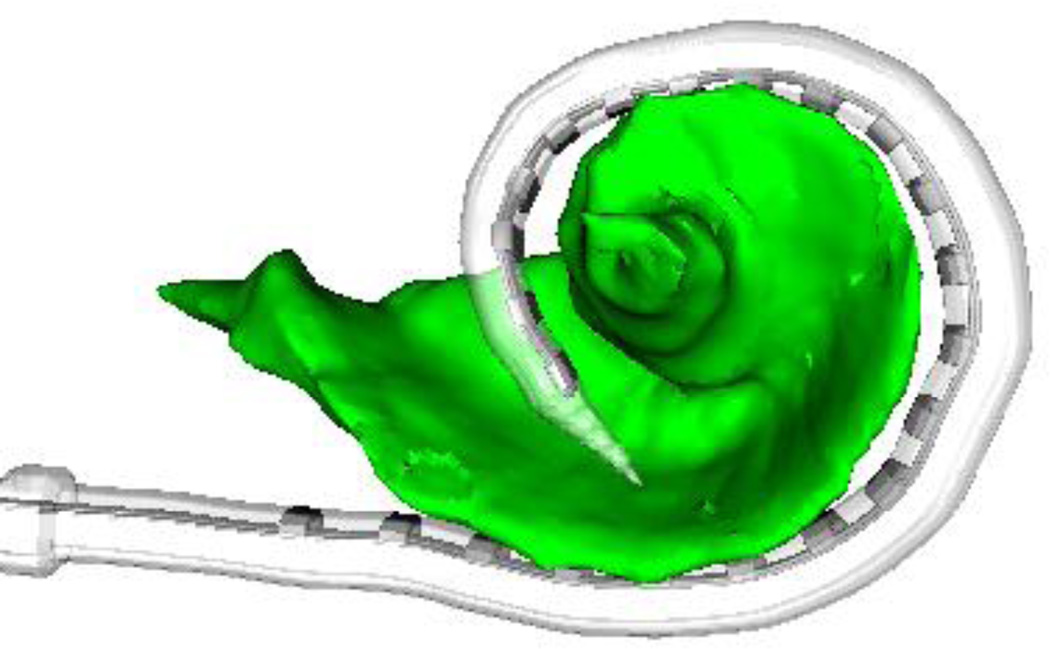
Methods for calculating electrode-to-modiolar distance are explained in detail in two previous studies (14–15). Briefly, pre- and post-implantation CT scans were processed using a CI image analysis software suite developed by Noble et al. to identify the location of individual electrodes relative to the modiolar wall. CT scan analysis consists of three steps: (i) the electrodes are first localized in post-implantation CT using the previously reported techniques (15), (ii) the modiolus is accurately localized in the pre-implantation CT where there are no implant related artifacts distorting the cochlea using the automated processing techniques (14), and (iii) the two CTs are aligned so that the location of the electrodes can be quantified relative to the modiolus. This approach for quantifying electrode position has been validated in a histological study (16). An example result of this process is shown in Figure 1. Once the electrodes and modiolus are localized, the distance from each electrode to the closest point on the modiolar wall is computed.
Figure 1.
Example image analysis result showing position of electrode array relative to modiolus (green) extracted from CT images.
Charge Calculation
Currently, two implant manufacturers (Advanced Bionics and Cochlear) do not directly report charge levels in the programming software. Rather, the value shown is in clinical units/levels. The third company (MED-EL) provides charge level information in the programming software. Since the reported values in the programming software vary from company to company, clinical programming units for Advanced Bionics and Cochlear were converted to charge [nanoCoulombs (nC)] using formulas provided by the manufacturers, as shown in Table 2. Clinical C/M levels were collected from each subject’s clinical MAPs that were routinely used when they enrolled in the study. No modifications or measurements were made to any clinical MAPs as part of this study.
Table 2.
| Formulas for converting clinical units to charge |
|---|
| Advanced Bionics |
| Clinical unit/0.0128/1,000 |
| Cochlear |
| (100*(clinical level/255) * 17.5) * (pulse width/1,000) |
All protocols were approved by the Vanderbilt University Institutional Review Board (IRB# 090155).
Results
All Electrodes Combined
A total of 2,365 electrodes were analyzed from 137 implanted ears. Combining all electrodes of both array types across all three manufacturers, there was a moderate, significant correlation between charge and distance (Pearson r = .367, p < 0.001). We are using Cohen’s operational definitions for determining correlations strength (17).The mean electrode-to-modiolus distance was .729 mm (sd = .45), and the mean charge was 14.258 nC (sd = 7.45). Correlations for all electrode and manufacturer groups are shown in Table 3.
Table 3.
Correlations between electrode distance and charge (nC) for each electrode type and several other groupings. Significant correlations (p < 0.05) are shown in bold. “C” denotes pre-curved arrays and “S” denotes straight arrays.
| Category | n | r | P |
|---|---|---|---|
| Cochlear C | 1349 | .118 | <0.001 |
| Cochlear S | 288 | 0.030 | .613 |
| All Cochlear | 1637 | .106 | <0.001 |
| Advanced Bionics C | 123 | −0.018 | .847 |
| Advanced Bionics S | 271 | .090 | 0.141 |
| All Advanced Bionics | 394 | .141 | 0.005 |
| MED-EL | 334 | .058 | 0.289 |
| All C | 1472 | 0.117 | <0.001 |
| All S | 893 | .070 | 0.037 |
| Full | 2365 | .367 | <0.001 |
Array Type
Separating the data into two general electrode types, straight and pre-curved, yielded groups of 893 and 1,472 electrodes respectively. Although the number of straight vs pre-curved arrays in this sample were very similar, there were many more electrodes on pre-curved arrays in this analysis given the high number of pre-curved arrays from Cochlear which has 22 intracochlear electrodes as compared to 16 and 12 for Advanced Bionics and MED-EL, respectively. The combined pre-curved group contains electrodes from both Cochlear and Advanced Bionics. A total of 1,349 electrodes on 65 Cochlear arrays were analyzed. Of these, 52 were CI24RE (Contour Advance) and the remaining 13 were CI512 arrays. Mean distance and charge for the Cochlear pre-curved arrays was .511 mm (sd = .45; range: 0.1 – 1.68) and 11.43 nC (sd = 5.14; range: 2.43 – 36.33). There was a small but significant correlation between distance and charge for the Cochlear pre-curved array electrodes (n= 1,349; r= 0.118, p<0.001). There were 123 electrodes on 8 pre-curved arrays from Advanced Bionics in this study and the arrays were primarily helix (n= 4) and mid-scala (n= 3). One Clarion CII HiFocus array with positioner was also included in the analysis. Mean distance and charge for the Advanced Bionics pre-curved array electrodes was 0.601 mm (sd = .31; range: .02 – 1.40) and 15.93 nC (sd = 5.14; range: 6.64 – 27.34). There was no observed correlation between charge and distance for Advanced Bionics pre-curved array electrodes (r= −0.018, p = 0.85). However, a small but highly significant positive correlation was found between charge and distance when combining pre-curved electrode arrays from both Cochlear and Advanced Bionics (n= 1,472; r= 0.117, p<0.001).
All three manufacturers are represented in the straight array-only analysis. Advanced Bionics was represented by 271 electrodes on 19 electrode arrays. All straight arrays from Advanced Bionics were 1j. Mean distance and charge estimates were 1.077 mm (sd = .26; range: 0.38 – 1.77) and 18.11 nC (sd = 6.99; range: 3.75 – 43.36), respectively. Straight arrays from Cochlear included 288 electrodes from 15 implanted ears. Eleven were CI422, and 4 were CI24RE(ST) arrays. Mean distance and charge for the Cochlear straight array electrodes was 1.175 mm (sd = .32; range: 0.17 – 2.06) and 12.27 nC (sd = 5.13; range: 6.81 – 31.61), respectively. The entirety of the MED-EL electrodes in this study are included in this portion of the analysis given that MED-EL only offers straight electrode arrays. Four MED-EL electrode types were included (Flex28 = 15, Flex24 = 3, Standard = 10, Medium = 2). In all, 334 electrodes from 30 arrays from MED-EL were included in the analysis. Mean distance and charge for MED-EL electrodes was 1.201 mm (sd= 0.28; range: 0.04 – 2.02) and 23.64 nC (sd= 8.82; range: 8.93 – 76.50), respectively. Altogether, 893 electrodes on straight arrays are included here. The correlation for the combined straight array electrodes (n= 893, r = 0.07, p = 0.037) did not meet Cohen’s (17) criteria for a small correlation (r = 0.10–0.29) so although it reached statistical significance, it is not considered to be meaningful in this context. None of the three manufacturer-specific correlation analyses for straight array electrodes were significant on their own.
Manufacturer
Looking just at Advanced Bionics electrode arrays and combining straight and pre-curved, 394 total electrodes were compared. A small, but significant correlation was found between charge and distance (r = 0.141, p = 0.005). Advanced Bionics produces two pre-curved arrays with different intended scalar locations. The helix electrode is intended to be modiolar-hugging, while the mid-scala is intended to rest approximately in the center of the scala tympani. Although the sample size of each array is small in this study, a post-hoc t-test found that the electrode-to-modiolar distance not significantly differ for the helix and mid-scala electrodes (p = .765).
Electrodes from Cochlear comprised 69% of the total data set and 81% of these arrays were pre-curved. Combining both array types, a small but significant correlation was observed (r= 0.106, p < 0.001). The correlation for the combined array types was essentially the same as for pre-curved arrays alone. Again, no significant correlation was observed for MED-EL electrodes which are all straight in design.
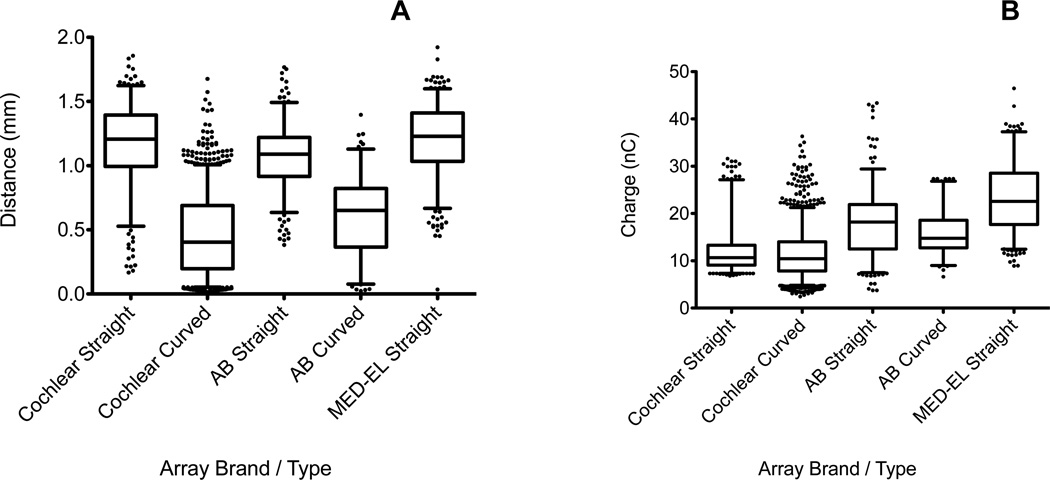
Distance
Distance data are shown for all electrode array types and manufacturers in Figure 2A. Mean distance for pre-curved arrays (mean = 0.471 mm) was significantly smaller than straight arrays (mean = 1.15 mm, t = 53.27, df = 2363, p < 0.0001). A one-way ANOVA for straight electrode arrays showed a highly significant effect of manufacturer on both charge (F2,890 = 15.130, p<0.001) and distance (F2,890 = 191.137, p<0.001). Post-hoc Tukey HSD tests found distances were significantly lower for Advanced Bionics straight array electrodes than either of the other manufacturers (p< 0.01), and Cochlear and MED-EL were not significantly different from each other (p = 0.50). For pre-curved arrays, an unpaired t-test found Advanced Bionics arrays were significantly further from the modiolus, on average, than Cochlear pre-curved arrays (p<0.001).
Figure 2.
(A) Electrode to modiolar surface distances are shown for each array type and manufacturer. (B) Charge values (converted from C/M levels) are shown for each array type and manufacturer. Horizontal lines are median values, boxes represent 25th and 75th percentiles, whiskers denote 5th and 95th percentiles, and dots indicate outliers.
Charge
Charge data for each array type and manufacturer are shown in Figure 2B. Charge varied significantly within a manufacturer and across manufacturers. Because of the large sample of Cochlear arrays, a small difference in mean charge between straight and pre-curved array electrodes was found to be significant (mean difference = 0.84 nC, p = 0.006). Advanced Bionics straight array electrodes were associated with significantly higher charge than pre-curved array electrodes (mean difference = 2.18 nC, p = 0.002). Highly significant differences were observed between manufacturers (p < 0.001). Five outliers from MED-EL straight array electrodes were removed from Figure 2B but are shown in Figure 3E.
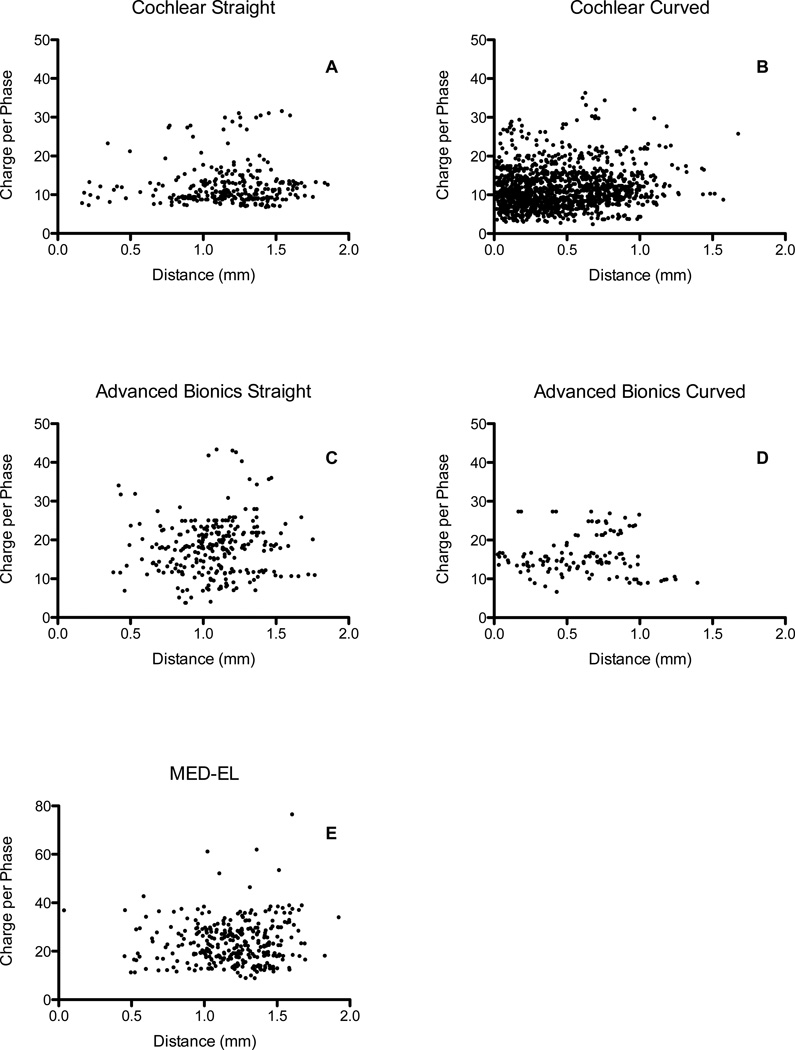
Figure 3.
Scatter plots of distance and charge for each electrode array type and manufacturer.
Discussion
The results of this study show that pre-curved arrays from both manufacturers tend to have significantly reduced electrode-to-modiolar distances compared to straight arrays. This finding is in agreement with several previous studies (2–4). To quantify or estimate differences in electrode-to-modiolus distance, previous studies have largely relied on either histology (2,4) or surrogate in-situ measures such as EABR (8) or ECAP (10) which do not directly measure distance. Studies using surrogate markers of electrode-to-modiolus distance have suggested that pre-curved arrays—and the presumed reduced distance to the modiolus—yield lower EABR thresholds. Our results confirm that pre-curved arrays were positioned closer to the modiolus and were associated with lower charge levels. However, significant correlations between distance and charge were not observed for straight arrays alone. When evaluating pre-curved arrays in isolation, only Cochlear arrays demonstrated such a correlation, though it was rather small (r = .12) When electrodes were combined across manufacturers, other correlations emerged, which are due, in part, to systematic differences in charge requirements across companies.
In a typical clinical setting, behavioral comfort levels are programmed for each patient and for each active electrode. These settings are known as “C” levels for Cochlear, and “M” levels for Advanced Bionics and MED-EL. Manufacturers have slightly different recommended criteria for patient instruction during C/M level programming. For example, Cochlear instructs that C levels should be set at the level for which the patient perceives a stimulus as “loud but comfortable,” while MED-EL instructs that M levels should be set to the patient’s upper limit of comfort. Indeed, charge levels for MED-EL electrodes were the highest of any group in this study. Mean manufacturer charge levels in this study for Advanced Bionics and MED-EL were very similar to previously reported charge levels (18), while mean Cochlear charge levels were lower in this study.
Scatter plots of all data are shown in Figure 3. It is evident from these figures that distance is generally a poor predictor of clinically-programmed C/M levels for any particular electrode array manufacturer and type. Even when nearly 1,400 electrodes from a single manufacturer were compiled, only a small correlation was observed (r = 0.12). It is further evident from the scatter plots that for a given charge, electrode geometry can vary from essentially 0.1 mm to as much as 2.0 mm—which is nearly the entire possible range of distances expected for intracochlear electrodes. Pre-curved array electrodes from Cochlear were, on average, closest to the modiolus and needed the lowest charge for C/M level stimulation. MED-EL array electrodes were, on average, the furthest from the modiolus and required the greatest charge. These two groups together drive the overall moderate correlation of the full data set. When only these two groups of arrays are combined, the observed correlation increases (r = 0.486).
The correlations observed when grouping electrodes across manufacturers should therefore be interpreted carefully. Distance does not predict charge for any group of straight electrode arrays. Only pre-curved arrays from Cochlear showed a small but significant correlation between distance and charge. The combined straight and pre-curved array comparison of Cochlear arrays showed a significant correlation but was weakened slightly by the addition of the straight arrays, compared to the pre-curved arrays alone. In contrast, for the combined group of Advanced Bionics arrays, the small but significant correlation (r = 0.14) is likely due to the greater range of charge in the straight array group which had significantly greater mean distance. This finding is more in line with conventional thinking since neither the straight nor pre-curved groups showed significant correlations on their own, but when combined a trend emerged. Given the relatively weak or absent correlations observed for a given electrode type, clinically-programmed charge levels should be taken only as a limited resource in estimating electrode-to-modiolus geometry.
There are notable differences between this study and previous reports of a significant relationship between distance and charge. Saunders et al. (2002) reported that C levels were 12 CL lower for pre-curved arrays, and that comfort levels were predictive of radial distance in 10/18 participants with Nucleus Contour electrode arrays. However, a relationship was not reported for straight electrode arrays in that study and only one manufacturer was included. While Parkinson et al. (2002) reported a significant reduction in C levels for Contour arrays compared to straight CI24M, electrode to modiolus distances were not evaluated. Neither study included a large sample of straight and pre-curved arrays from all three manufacturers, or used a precise measure, if any, of electrode to modiolus distance. Shepherd et al. (1993) used a precise measurement of electrode position in the scala tympani of cats. They reported a significant relationship between distance and charge, but only for threshold values. It is important to note that many of the previously cited reports of a significant relationship between electrode to modiolus distance and charge levels evaluated behavioral threshold levels and EABR thresholds, rather than comfort levels. It remains unclear to what extent threshold and comfort levels may be differentially affected by electrode distance. Clinically programmed threshold levels were not considered in this study as such values are not routinely obtained in the clinic for two of the three included manufacturers.
In addition to electrode to modiolus distance, there are several other possible variables that may contribute to the electrical current needed to stimulate neural structures. These variables include the density of surviving spiral ganglion cells, fibrous tissue growth around the electrodes, and bone impedances. In addition to these physiological variables, variability in the clinical assessment of suprathreshold levels may also contribute to the weak relationship observed here between distance and charge. It is possible that some of these variables may have contributed to the generally weak relationship observed in this study between charge and distance. We hope to continue this line of research and assess to what extent threshold charge values may predict distance, as well as how electrode distance and other electrode and demographic variables may predict various clinical outcomes.
Conclusion
This study measured distances between implanted electrodes and the modiolar surface and compared these values to behavioral C/M levels. Straight electrode arrays showed no significant correlation between distance and charge. Pre-curved arrays from one company and the combined array designs from each of two companies showed a small but highly significant correlation between distance and charge. Observed correlations when combining electrode arrays from multiple manufacturers were likely driven, in part, by systematic differences in charge levels across companies. When considering only a single array type from one manufacturer, only one instance was observed where distance mildly predicted charge.
Acknowledgments
R.F.L. is a consultant for Advanced Bionics, Ototronix, and Medtronic. R.H.G. sits on the advisory committee for Cochlear Corporation, Advanced Bionics, and MED-EL.
This research was made possible by funding from the National Institutes of Health grants R01DC014037 and R21DC012620 from the National Institute of Deafness and other Communications Disorders. The content is solely the responsibility of the authors and does not necessarily reflect the views of this institute.
Footnotes
VU Institutional Review Board Approval: 090155
The authors have no other funding, financial relationships or conflicts of interest to disclose.
References
- 1.Shepherd RK, Hatsushika S, Clark GM. Electrical stimulation of the auditory nerve: The effect of electrode position on neural excitation. Hear Res. 1993;66:108–120. doi: 10.1016/0378-5955(93)90265-3. [DOI] [PubMed] [Google Scholar]
- 2.Tykocinski M, Cohen LT, Pyman BC, et al. Comparison of Electrode Position in the Human Cochlea Using Various Perimodiolar Electrode Arrays. Am J Otol. 2000;21:205–211. doi: 10.1016/s0196-0709(00)80010-1. [DOI] [PubMed] [Google Scholar]
- 3.Saunders E, Cohen L, Aschendorff A, et al. Threshold, Comfortable Level and Impedance Changes as a Function of Electrode-Modiolar Distance. Ear Hear. 2002;23:28S–40S. doi: 10.1097/00003446-200202001-00004. [DOI] [PubMed] [Google Scholar]
- 4.Balkany TJ, Eshraghi AA, Yang N. Modiolar Proximity of Three Perimodiolar Cochlear Implant Electrodes. Acta Otolaryngol. 2002;122:363–369. doi: 10.1080/00016480260000021. [DOI] [PubMed] [Google Scholar]
- 5.Parkinson AJ, Arcaroli J, Staller SJ, Arndt PL, Cosgriff A, Ebinger K. The Nucleus 24 Contour Cochlear Implant System: Adult Clinical Trial Results. Ear Hear. 2002;23:41S–48S. doi: 10.1097/00003446-200202001-00005. [DOI] [PubMed] [Google Scholar]
- 6.Jeong J, Kim M, Heo JH, et al. Intraindividual Comparison of Psychophysical Parameters Between Perimodiolar and Lateral-type Electrode Arrays in Patients With Bilateral Cochlear Implants. Otol Neurotol. 2015;36:228–234. doi: 10.1097/MAO.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 7.Wackym PA, Firszt JB, Gaggl W, Runge-Samuelson CL, Reeder RM, Raulie JC. Electrophysiologic Effects of Placing Cochlear Implant Electrodes in a Perimodiolar Position in Young Children. Laryngoscope. 2004;114:71–76. doi: 10.1097/00005537-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Runge-Samuelson C, Firszt JB, Gaggl W, Wackym PA. Electrically Evoked Auditory Brainstem Responses in Adults and Children: Effects of Lateral to Medial Placement of the Nucleus 24 Contour Electrode Array. Otol Neurotol. 2009;30:464–470. doi: 10.1097/MAO.0b013e31819fe7ea. [DOI] [PubMed] [Google Scholar]
- 9.Hughes ML, Abbas PJ. Electrophysiologic channel interaction, electrode pitch ranking, and behavioral threshold in straight versus Perimodiolar cochlear implant electrode arrays. J Acoust Soc Am. 2006;119:1538–1547. doi: 10.1121/1.2164969. [DOI] [PubMed] [Google Scholar]
- 10.Van Weert S, Stokroos RJ, Rikers MMJG, Van Dijk P. Effect of peri-modiolar cochlear implant positioning on auditory nerve responses: A neural response telemetry study. Acta Oto-Laryngol. 2005;125:725–731. doi: 10.1080/00016480510028492. [DOI] [PubMed] [Google Scholar]
- 11.Gordon KA, Papsin BC, Harrison RV. Toward a Battery of Behavioral and Objective Measures to Achieve Optimal Cochlear Implant Stimulation Levels in Children. Ear Hear. 2004;25:447–463. doi: 10.1097/01.aud.0000146178.84065.b3. [DOI] [PubMed] [Google Scholar]
- 12.Polak M, Hodges AV, King JE, Payne SL, Balkany TJ. Objective methods in postlingually and prelingually deafened adults for programming cochlear implants: ESR and NRT. Cochlear Implants Int. 2006;7:125–141. doi: 10.1179/cim.2006.7.3.125. [DOI] [PubMed] [Google Scholar]
- 13.Muhaimeed HA, Anazy FA, Hamed O, Shubair E. Correlation between NRT measurement level and behavioral levels in pediatrics cochlear implant patients. Int J Pediatr Otorhinolaryngol. 2010;74:356–360. doi: 10.1016/j.ijporl.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Noble JH, Gifford RH, Labadie RF, Dawant BM. Statistical Shape Model Segmentation and Frequency Mapping of Cochlear Implant Stimulation Targets in CT. In: Ayache N, et al., editors. MICCAI 2012, Part II, LNCS 7511. 2012. pp. 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble JH, Labadie RF, Gifford RH, Dawant BM. Image-guidance enables new methods for customizing cochlear implant stimulation strategies. IEEE Trans Neural Syst Rehabil Eng. 2013;21:820–829. doi: 10.1109/TNSRE.2013.2253333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuman TA, Noble JH, Wright CG, Wanna GB, Dawant B, Labadie RF. Anatomic Verification of a Novel Method for Precise Intrascalar Localization of Cochlear Implant Electrodes in Adult Temporal Bones Using Clinically Available Computed Tomography. Laryngoscope. 2010;120:2277–2283. doi: 10.1002/lary.21104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New Jersey: Erlbaum; 1988. pp. 79–81. [Google Scholar]
- 18.Zwolen TA, O’Sullivan MB, Fink NE, Niparko JK. Electric Charge Requirements of Pediatric Cochlear Implant Recipients Enrolled in the Childhood Development After Cochlear Implantation Study. Otol Neurotol. 2008;29:143–148. doi: 10.1097/MAO.0b013e318161aac7. [DOI] [PubMed] [Google Scholar]