Abstract
Background
Rickettsia spp. belonging to the spotted fever group (SFG) cause infections in humans, domestic animals and wildlife. At least five SFG rickettsial species have been reported in China, but the occurrence of Rickettsia aeschlimannii and R. massiliae in ticks has not been characterized to date.
Findings
A total of 114 adult ticks were collected from sheep in Yining County, Xinjiang Uygur Autonomous Region, in northwest China. The ticks were identified from morphological and molecular characteristics. All samples were examined by polymerase chain reaction (PCR), and six genetic markers were used to determine the Rickettsia spp. in the ticks. The ticks collected were identified as Rhipicephalus turanicus. Three different lineages of Rh. turanicus from Yining County were discovered on phylogenetic analysis of 16S rDNA and cox1. Twenty-one of the 114 samples (18.42%) were positive for rickettsial agents. Phylogenetic analysis based on six genetic sequences showed that three rickettsial species were present, namely: R. aeschlimannii (19.05%, 4/21), R. massiliae (19.05%, 4/21) and R. sibirica variant (61.90%, 13/21), which is clustered in the clade of R. sibirica subsp. sibirica.
Conclusions
This is the first description of R. aeschlimannii and R. massiliae in China. R. massiliae, R. aeschlimannii and R. sibirica variant co-circulate in the region of the China-Kazakhstan border, in northwest China. Rickettsial agents in ticks of the genus Rhipicephalus from migrant birds, transported livestock, wildlife and human beings should be investigated further in the region of the China–Central Asian border.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-015-1242-2) contains supplementary material, which is available to authorized users.
Keywords: Rickettsia aeschlimannii, Rickettsia massiliae, Rhipicephalus turanicus ticks, Northwest China
Findings
Background
Rickettsia spp. belonging to the spotted fever group (SFG) cause infections in animals and humans worldwide [1, 2]. To date, at least five validated SFG rickettsial species have been detected in ticks in China, including R. heilongjiangii, R. sibirica, R. raoultii, R. slovaca and R. felis [3]. Molecular evidence of the first four species was reported in northeastern and northwestern China, mainly in Dermacentor and Haemaphysalis ticks [4–6], and the last was found in Rhipicephalus sanguineus from Jiangsu Province [7].
Xinjiang Uygur Autonomous Region (XUAR), the largest province in China, occupies one-sixth of China, borders eight countries with a 5,600-km frontier, and there are 29 trading ports. In the present study, we assessed the occurrence of rickettsial agents in Rh. turanicus ticks in Yining County, the location of Yining Port, which is adjacent to Kazakhstan.
Methods
Tick sampling and identification
A total of 114 ticks were collected from sheep in Yining County (928 m above sea level, at 44°003681′N 81°558182′E). All of the ticks were identified morphologically according to previous reports, and 23 representative ticks underwent molecular analysis based on partial mitochondrial (16S rDNA and cox1) gene sequences [8].
Ethical approval
This study was approved by the Animal Ethics Committee of Shihezi University (Approral No. AECSU2014-6).
PCR amplification and sequence analysis
For genetic detection of Rickettsia spp., the genomic DNA of all the ticks was extracted from individual specimens using the TIANamp Genomic DNA Kit (Tiangen, Beijing, China). All samples were examined by polymerase chain reaction (PCR), and six genetic markers [434-, 1332-, 1060-, 488-, 491-, and 812-bp products of the genes encoding the 17 kilodalton antigen (17-kDa), 16S rRNA (rrs), citrate synthase (gltA), surface cell antigen 1 (sca1), and outer membrane proteins A and B (ompA and ompB)] were amplified using previously described primers [3]. The amplication products were purified using the TIANgel Midi Purification Kit (TIANGEN, Beijing, China) and then cloned into the pGEM-T Easy vector and subjected to sequencing. A phylogenetic tree was constructed using the maximum likelihood and neighbor-joining algorithms with MEGA 6.0 software [9].
Results
The ticks were identified morphologically as Rh. turanicus. Sequencing data from the 23 representative ticks indicated three different lineages of Rh. turanicus from Yining County on the basis of phylogenetic analysis of 16S rDNA and cox1 (shown in Additional file 1). Six nucleotide sequences from our study have been deposited in the GenBank database (16S rDNA: KF547984, KF547987, and KF547989; cox1: KF188136–KF188138).
Twenty-one of the 114 samples (18.42%) were positive by PCR for products of six rickettsial genetic markers. Out of the 21 positive samples, four were confirmed as R. aeschlimannii, four were identified as R. massiliae, and the remaining thirteen were R. sibirica variant based on phylogenetic tree of the representative makers (ompA gene and gltA gene) and the 17-kDa-ompA-gltA-rrs-sca1-ompB concatenated sequence (shown in Additional file 2; Fig. 1). There were no differences in the DNA sequences of six responding genetic markers for R. aeschlimannii, with sequence similarities of 99.74% (1,169bp/1,172bp), 100% (1,048bp/1,048bp), 98.49% (458bp/465bp), 98.77% (722bp/731bp) and 99.33% (593bp/597bp) for the rrs, gltA, ompA, ompB and sca1 genes, respectively, and 99.19% (366 bp/369bp) to R. raoultii strain Alashankou-99 for the 17k-Da gene (KT261761). Except the sca1 gene, which has two different sequences with sequence similarities of 99.13% (573bp/578bp) and 99.48% (576bp/579bp) to R. massiliae MTU5 (CP000683), and the ompB gene, which has two different sequences with sequence similarities of 100% (765bp/765bp) and 98.56% (754bp/765bp) to R. massiliae MTU5 (CP000683), the DNA sequences of four genetic markers for R. massiliae were the same, with sequence similarities of 100% (383bp/383bp), 100% (1,162bp/1,162bp), 99.90% (1,022bp/1,023bp), 100% (434bp/434bp) for the 17k-Da, rrs, gltA, ompA genes, respectively. However, for the R. sibirica variant, except the gltA gene, which has two different sequences with sequence similarities of 99.54% (1,075bp/1,080bp) and 99.63% (1,076bp/1,080bp) to R. sibirica subsp. sibirica (KM28871), respectively, the sequences of the other five responding genetic markers have different levels of divergences, with sequence similarities of 100% (385bp/385bp) to R. raoultii strain Alashankou-131(KT261760) for the 17k-Da gene, 99.82% (1,121bp/1,123bp) to R. raoultii isolate BL029-2 (KJ410261) for the rrs gene, 99.58% (469bp/471bp) to Rickettsia sp. Tselentii (EU194445) for the ompA gene, 99.48% (772bp/776bp) to R. parkeri str. Portsmouth (CP003341) for the ompB gene and 99.34 (598/602) to R. africae ESF-5 (CP001612) for the sca1 gene. The similarities and divergences of the sequences in this study are shown in Additional file 3: Table S1. All the sequences obtained from our study have been deposited in the GenBank database [17 kDa: KT318742, KT588057, KT588065; rrs: KT318741, KT588056, KT588064; gltA: KT318743, KT588058, KT588066, KT588070; sca1: KT318746, KT588061, KT588063, KT588069; ompA: KT318744, KT588059, KT588067; ompB: KT318745, KT588060, KT588062, KT588068].
Fig. 1.
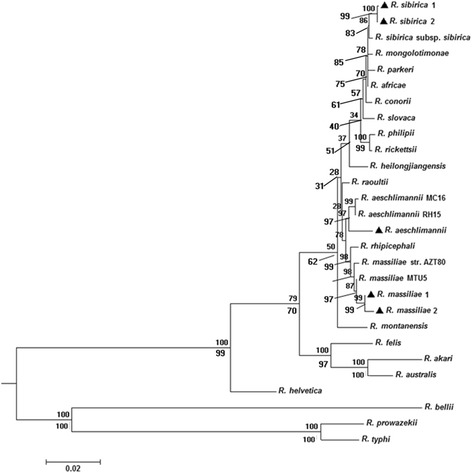
Phylogenetic tree of the 17-kDa-ompA-gltA-rrs-sca1-ompB concatenated sequence of rickettsial agents in Rhipicephalus turanicus (). The tree was constructed on the basis of maximum likelihood (ML; bootstrap replicates: 500) and neighbor-joining (NJ; bootstrap replicates: 500) analyses of concatenated sequence data for six genes (17-kDa, ompA, gltA, rrs, sca1, ompB) using MEGA6. The scale bar represents the inferred substitutions per nucleotide site. The relative support for clades in the tree produced from the ML and NJ analyses are indicated above and below branches, respectively
Discussion
R. massiliae, R. rhipicephali and R. aeschlimannii are grouped phylogenetically into a clade in the family Rickettsiaceae [10]. R. massiliae was first isolated in 1990 from a Rh. turanicus tick in an area near Marseille, France [11]. Since then, this pathogen has been identified from other Rhipicephalus ticks in regions of Europe, North and Central Africa, and the United States [12]. Furthermore, cases showed that it can cause human infection. R. aeschlimannii was first described from Hyalomma marginatum in Morocco in 1997 [13]. The presence of R. aeschlimannii has been demonstrated in Hyalomma ticks from Europe (e.g. France, Croatia, Spain, Italy), Asia (e.g. Israel, Turkey) and Africa (e.g. Mali, Algeria, Egypt) [14–16] and from Haemaphysalis ticks in Spain and Kazakhstan [17]. Furthermore, Ixodes ricinus, H. punctata, Rh. bursa, and Rh. sanguineus isolated from human Spanish patients were shown to contain DNA from R. aeschlimannii [14], and there is a report of R. aeschlimannii from Rh. turanicus infecting a man in Greece in 2013 [18]. In this study, we report the first molecular evidence that R. aeschlimannii and R. massiliae are present in Rh. turanicus from sheep in the region of the China-Kazakhstan border, in the northwest of China.
To date, R. sibirica is known to contain two subspecies [19], R. sibirica subsp. sibirica and R. sibirica subsp. mongolotimonae. The former was first isolated in Russia but it has subsequently been found in northern China [5]. In contrast, R. sibirica subsp. mongolotimonae was first isolated in Inner Mongolia and then found in Europe and Africa [20, 21]. Here, the R. sibirica variant found in the region of the China–Kazakhstan border appeared divergent in the ompA, ompB and sca1, used to differentiate Rickettsia species, although it was closest to R. sibirica subsp. sibirica, on the basis of the gltA gene and the phylogenetic tree of the 17-kDa-ompA-gltA-rrs-sca1-ompB concatenated sequence. Further genomic analysis should be carried out to confirm the classification of the R. sibirica variant found in this study.
The Rh. turanicus tick is widely distributed throughout the Mediterranean subregion, Africa, and Asia, including China, especially in XUAR [22], and it has been implicated as a vector of several human and veterinary pathogens, including Rickettsia spp. [18]. Here, R. massiliae, R. aeschlimannii and R. sibirica variant were found in the same area, Yining County, which suggests that several SFG Rickettsia spp. co-circulate in Rh. turanicus as a potential vector near the China-Kazakhstan border.
In 2004, Shpynov et al detected R. aeschlimannii in the Alma-Ata region, east of Kazakhstan [17]. Here we found that Rh. turanicus in the region of the China-Kazakhstan border showed genetic divergence in the loci of 16S rDNA and cox1, which indicates that these ticks collected from sheep may come from different lineages. At present, it is unknown whether these ticks are imported from the Chinese hinterland or abroad through migrant birds, or with internationally transported livestock. This topic needs to be further investigated.
Conclusions
This is the first report of the molecular analysis of R. aeschlimannii and R. massiliae in China. The findings of the study suggest that R. massiliae, R. aeschlimannii and R. sibirica variant co-circulate in Rh. turanicus in the region of the China–Kazakhstan border, in northwest China. The origin of the Rhipicephalus genus (such as migrant birds, transported livestock, wildlife and human beings) and the epidemiology of tick-borne pathogens should be further investigated in the region of the China–Central Asian border.
Acknowledgements
This research was supported in part by grants from the National Natural Science Foundation of China (Granted No. 81560338), the National Science & Technology Pillar Program (No. 2013BAI05B05) and Co-innovation Center for the High Incidence of Zoonotic Disease Prevention and Control in Western China (No. 2013-179).
Additional files
The photo of Rhipicephalus turanicus and Phylogenetic tree of Rhipicephalus turanicus 16S rDNA and CO1 gene. (DOC 198 kb)
The single gene Phylogenetic tree of Rickettsia spp. (DOC 863 kb)
Closest relative sequences to the partial 17-kDa, 16S, gltA, ompA, ompB and sca1 genes, sequences of the Rickettsia aeschlima nnii (Table S1A), Rickettsia massiliae (Table S1B) and Rickettsia sibirica (Table S1C) detected in the Rhipicephalus turanicus ticks, Northwest of China. (DOCX 22 kb)
Footnotes
Qing-Qing Wei, Li-Ping Guo and An-Dong Wang contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YZW conceived and designed the study, and critically revised the manuscript. QQW, LPG, ADW and YZW performed the experiments, analyzed the data and drafted the manuscript. LMM, YZW CFC and WJZ performed the tick collection. KZ and QQW conducted the morphological and molecular analyses. All authors read and approved the final manuscript.
Contributor Information
Qing-Qing Wei, Email: 549818072@qq.com.
Li-Ping Guo, Email: liping-guo@qq.com.
An-Dong Wang, Email: 417534504@qq.com.
Lu-Meng Mu, Email: 734234401@qq.com.
Ke Zhang, Email: 1500441019@qq.com.
Chuang-Fu Chen, Email: ccf-xb@163.com.
Wan-Jiang Zhang, Email: zwj1117@sina.com.
Yuan-Zhi Wang, Email: wangyuanzhi621@126.com.
References
- 1.Maina AN, Jiang J, Omulo SA, Cutler SJ, Ade F, Ogola E, et al. High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural western Kenya: implications for human health. Vector Borne Zoonotic Dis. 2014;14(10):693–702. doi: 10.1089/vbz.2014.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Velez R, Palomar AM, Oteo JA, Norman FF, Pérez-Molina JA, Portillo A. Novel Candidatus rickettsia species detected in nostril tick from human, Gabon, 2014. Emerg Infect Dis. 2015;21(2):325–7. doi: 10.3201/eid2102.141048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo LP, Mu LM, Xu J, Jiang SH, Wang AD, Chen CF, et al. Rickettsia raoultii in Haemaphysalis erinacei from marbled polecats. China-Kazakhstan border. Parasit Vectors. 2015;8:461. doi: 10.1186/s13071-015-1065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang JZ, Fan MY, Wu YM, Fournier PE, Roux V, Raoult D. Genetic Classification of “Rickettsia heilongjiangii” and “Rickettsia hulinii”, two Chinese Spotted Fever Group Rickettsiae. J Clin Microbiol. 2000;38:3498–501. doi: 10.1128/jcm.38.9.3498-3501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X, Jin Y, Fan M, Xu G, Liu Q, Raoult D. Genotypic and antigenic identification of two new strains of spotted fever group rickettsiae isolated from China. J Clin Microbiol. 1993;31(1):83–8. doi: 10.1128/jcm.31.1.83-88.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian ZC, Liu GY, Shen H, Xie JR, Luo J, Tian MY. First report on the occurrence of Rickettsia slovaca and Rickettsia raoultii in Dermacentor silvarum in China. Parasit Vectors. 2012;5(19):1–4. doi: 10.1186/1756-3305-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Lu G, Kelly P, Zhang Z, Wei L, Yu D, et al. First report of Rickettsia felis in China. BMC Infect Dis. 2014;14:682. doi: 10.1186/s12879-014-0682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dantas-Torres F, Latrofa MS, Annoscia G, Giannelli A, Parisi A, Otranto D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasit Vectors. 2013;6:213. doi: 10.1186/1756-3305-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol NLM. 2011;28(10):2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitale G, Mansuelo S, Rolain JM, Raoult D. Rickettsia massiliae human isolation. Emerg Infect Dis. 2006;12(1):174–5. doi: 10.3201/eid1201.050850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beati L, Raoult D. Rickettsia massiliae sp. nov., a new spotted fever group Rickettsia. Int J Syst Bacteriol. 1993;43(4):839–40. doi: 10.1099/00207713-43-4-839. [DOI] [PubMed] [Google Scholar]
- 12.Fernández de Mera IG, Zivkovic Z, Bolaños M, Carranza C, Pérez-Arellano JL, Gutiérrez C, et al. Rickettsia massiliae in the Canary Islands. Emerg Infect Dis. 2009;15(11):1869–70. doi: 10.3201/eid1511.090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarih M, Socolovschi C, Boudebouch N, Hassar M, Raoult D, Parola P. Spotted fever group rickettsiae in ticks, Morocco. Emerg Infect Dis. 2008;14:1067–73. doi: 10.3201/eid1407.070096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Soto P, Encinas-Grandes A, Perez-Sanchez R. Rickettsia aeschlimannii in Spain: molecular evidence in Hyalomma marginatum and five other tick species that feed on humans. Emerg Infect Dis. 2003;9:889–90. doi: 10.3201/eid0907.030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabriela K, Gad B, Kosta Y. Molecular Detection of Rickettsia africae, Rickettsia aeschlimannii, and Rickettsia sibirica mongolitimonae in Camels and Hyalomma spp. Ticks from Israel. Vector-borne and Zoonotic Dis. 2013;13:851–6. doi: 10.1089/vbz.2013.1330. [DOI] [PubMed] [Google Scholar]
- 16.Orkun Ö, Karaer Z, Çakmak A, Nalbantoğlu S. Identification of tick-borne pathogens in ticks feeding on humans in Turkey. PLoS Negl Trop Dis. 2014;8(8):1–11. doi: 10.1371/journal.pntd.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shpynov S, Fournier PE, Rudakov N, Tankibaev M, Tarasevich I, Raoult D. Detection of a Rickettsia closely related to Rickettsia aeschlimannii, “Rickettsia heilongjiangensis”, Rickettsia sp. strain RpA4, and Ehrlichia muris in ticks collected in Russia and Kazakhstan. J Clin Microbiol. 2004;42:2221–3. doi: 10.1128/JCM.42.5.2221-2223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germanakis A, Chochlakis D, Angelakis E, Tselentis Y, Psaroulaki A. Rickettsia aeschlimannii infection in a man, Greece. Emerg Infect Dis. 2013;19:1176–7. doi: 10.3201/eid1907.130232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fournier PE, Zhu Y, Yu X, Raoult D. Proposal to create subspecies of Rickettsia sibirica and an emended description of Rickettsia sibirica. Ann N Y Acad Sci. 2006;1078:597–606. doi: 10.1196/annals.1374.120. [DOI] [PubMed] [Google Scholar]
- 20.Fournier PE, Gouriet F, Brouqui P, Lucht F, Raoult D. Lymphangitis-associated rickettsiosis, a new rickettsiosis caused by Rickettsia sibirica mongolotimonae: seven new cases and review of the literature. Clin Infect Dis. 2005;40(10):1435–44. doi: 10.1086/429625. [DOI] [PubMed] [Google Scholar]
- 21.Ramos JM, Jado I, Padilla S, Masiá M, Anda P, Gutiérrez F. Human infection with Rickettsia sibirica mongolitimonae, Spain, 2007-2011. Emerg Infect Dis. 2013;19(2):267–9. doi: 10.3201/eid1902.111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chochlakis D, Ioannou I, Papadopoulos B, Tselentis Y, Psaroulaki A. Rhipicephalus turanicus: from low numbers to complete establishment in Cyprus. Its possible role as a bridge-vector. Parasit Vectors. 2014;7:11. doi: 10.1186/1756-3305-7-S1-P11. [DOI] [Google Scholar]


