Abstract
Streptococcus mutans is considered the principal cariogenic bacterium for dental caries. Despite the recognition of their importance for cariogenesis, the possible coordination among S. mutans’ main virulence factors, including glucan production, acidogenicity and aciduricity, has been less well studied. In the present study, using S. mutans strains with surface-displayed pH-sensitive pHluorin, we revealed sucrose availability- and Gtf functionality-dependent proton accumulation on S. mutans surface. Consistent with this, using a pH-sensitive dye, we demonstrated that both in vivo cell-produced and in vitro enzymatically synthesized insoluble glucans displayed proton-concentrating ability. Global transcriptomics revealed proton accumulation triggers the up-regulation of genes encoding functions involved in acid tolerance response in a glucan-dependent manner. Our data suggested that this proton enrichment around S. mutans could pre-condition the bacterium for acid-stress. Consistent with this hypothesis, we found S. mutans strains defective in glucan production were more acid sensitive. Our study revealed for the first time that insoluble glucans is likely an essential factor linking acidogenicity with aciduricity. The coordination of these key virulence factors could provide new insights on how S. mutans may have become a major cariogenic pathogen.
Dental caries is one of the most prevalent bacteria-related infectious diseases worldwide1,2. The primary etiological factor of caries is acid production from dietary carbohydrates by the cariogenic bacteria in dental biofilms3. As the principle causative organism of dental caries, Streptococcus mutans possesses many physiological traits relevant to its cariogenesis3. By rapid fermentation of carbohydrates, S. mutans is able to generate acidic end products (acidogenicity) which is not only the direct causative factor for demineralization of tooth surfaces, but also an environmental determinant that may impact the caries-related microbial flora during cariogenic process4. Meanwhile, S. mutans has also developed adaptive acid tolerance response (ATR) to combat the destructive nature of an acidic environment it produces (aciduricity)5. It is characterized by the induction of multiple cellular pathways upon exposure to mildly acidic conditions to allow cells to better adapt to acid challenge. For example, for maintenance of intracellular pH homeostasis, S. mutans alters its membrane composition to prevent proton from influx and increases proton extrusion via end-product efflux and acid-stable proton-translocating F1F0-H/ATPase activity6,7,8. S. mutans is able to alter its metabolic activity through enhancing glycolytic activity and synthesis of branched amino acids during acid stress9,10. It also up-regulates the expression of variety of chaperons, such as DnaK and DnaJ to protect and repair macromolecule damage due to the harmful effects of intracellular acidification5.
Another important virulence factor contributing to S. mutans’ cariogenecity is glucan, particularly insoluble glucan production11. In a dental biofilm, 10–20% of the dry weight is composed of EPS12 most of which is glucans synthesized by microbial glucosyltransferases (Gtfs) from substrate sucrose13. S. mutans is a major contributor to the production of glucans in dental biofilm14. In S. mutans, the synthesis of extracellular glucans from sucrose is catalyzed by three GTF enzymes. GtfB synthesizes mostly water-insoluble glucans; GtfC catalyzes the synthesis of a mixture of water-insoluble and soluble glucans; whereas GtfD exclusively synthesizes water-soluble glucans15,16. The insoluble glucans synthesized by surface-adsorbed GtfB and GtfC provide binding sites for the establishment of S. mutans on the solid surface as well as facilitate its coadherence with other bacterial cells17. It plays essential role in mediating the transition from initial cell attachment and clustering to microcolony formation and multi-microcolonies aggregates17. Deletion of both gtfB and gtfC caused the maximum reduction in S. mutans extracellular polysaccharide matrix production and biofilm formation, as well as decreased incidence of smooth surface caries in a rat model18 .
In addition to facilitating the biofilm formation, glucans have also been proposed to act as diffusion barrier and increase the sieving effect of dental biofilm, which could trap acid near the tooth surface and contribute to the demineralization process19. In a recent study, by using a surface-displayed pH sensitive green fluorescent protein (pHluorins), we revealed a much lower and well-maintained pH on cell surface than micro-environment surrounding the cells, suggesting the potential involvement of surface-associated component in influencing the accumulation of protons on cell surface20. Meanwhile, the frequent sucrose intake and microenvironmental acidification has been shown to induce gtf gene expression and enable S. mutans to accumulate glucans continuously under low pH conditions21. Taken together, these data suggested a possible interplay among acid production, acid tolerance and the glucans synthesis. In this study, we revealed an additional important role of glucans in cariogenic process by linking acidogenicity and aciduricity, two of the main virulence factors of S. mutans. Our data showed that S. mutans is able to concentrate protons via surface-associated insoluble glucans. The resultant low surface pH triggers ATR and allows S. mutans to better adapt to acid stress.
Results
Sucrose availability- and Gtf functionality-dependent proton accumulation on S. mutans cell surface
In a recent study, pH was monitored on the cell surface of S. mutans O87, a strain with cell surface-displayed pH sensitive green fluorescent protein (pHluorins), whose fluorescence signal intensity reduced corresponding to the decrease in surrounding pH20. The observation that a well-maintained, lower pH was monitored on the cell surface than in the macro-environment suggested the potential involvement of S. mutans surface-associated component, likely insoluble glucans in concentrating protons on cell surface. To further test this hypothesis, surface-displayed pHluorins was used to monitor the changes of pH value on the cell surface when S. mutans O87 cells were exposed to the medium with different pH values in a real time, in situ manner. The strain O87 cells grown in buffered minimal defined medium (MDM) (pH 7.5) in the presence of sucrose or glucose were switched to buffers with lower pH values (pH 7.0, 6.5, 6.0 and 5.5) and their surface fluorescence intensity was monitored after 10 minutes’ incubation. As shown in Fig. 1, when switched from pH 7.5 to each buffer with lower pH value, S. mutans cells pre-grown in the presence of sucrose exhibited significantly more reduction (p < 0.05) in the surface fluorescence intensity, indicating a greater cell surface pH drop compared with the cells grown in the presence of glucose. Meanwhile, cells experienced the most drastic surface pH drop (as indicated by more severe reduction in fluorescence signal) when external pH shifted from 7.5 to 5.5, suggesting that there existed a dose-effect relationship between proton amounts in surrounding environment and pH drop on the S. mutans surface.
Figure 1. Percentage changes of fluorescence intensity in S. mutans wild-type and gtfBC-deficient strains upon switching to different pH values.
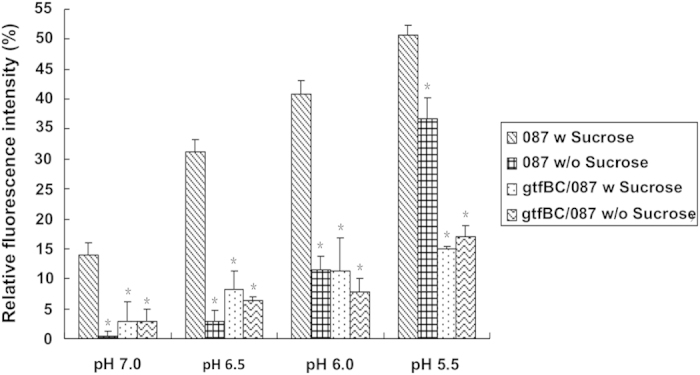
S. mutans strain O87 [O87] and its gtfBC-deficient strain [gtfBC/O87] were pre-grown in buffered pH7.5 medium and switched to the indicated pH values. The fluorescence signal reduction was calculated as the percentage of signal reduction relative to the initial signal at pH7.5. The plots show the average of triplicate samples, and the error bars correspond to the standard deviations. Student’s t-test was used to calculate the significance of the difference between the strain O87 supplemented with sucrose and that supplemented with glucose, and also between the gtfBC mutant and its parent strain. *Star indicates statistical significance between the value of indicated sample and that of strain O87 subjected to the same treatment (p < 0.05).
To further determine if GtfBC activity is required for the pH reduction on the cell surface, a mutant deficient in gtfBC while carrying cell surface-displayed ecliptic pHluorin was used in a similar experimental setup. Result showed that, in contrast to parent strain O87, GtfBC-defective strain grown in the presence or absence of sucrose exhibited a significantly less change (p < 0.05) in fluorescence intensity when switched from pH 7.5 buffer to each tested buffer with lower pH value (Fig. 1). These data showed that the pH reduction on the cell surface is associated with S. mutans’ GtfBC activity and dependent on the availability of sucrose.
S. mutans accumulates protons on cell surface by synthesized insoluble glucans
The above data showed that the observed pH reduction on the cell surface of S. mutans is dependent on both the availability of sucrose and the functionality of GtfBC. Since GtfB and GtfC are enzymes responsible for the production of insoluble glucans using sucrose as substrate13 it is likely that the surface-associated insoluble glucans synthesized by S. mutans GtfBC were responsible for the accumulation of protons and reducing cell surface pH value. To address this, we utilized pH-sensitive pHrodo Green dye, whose fluorescence signal intensity increases corresponding to the decrease in surrounding pH, to test the ability of surface-associated insoluble glucans to accumulate protons.
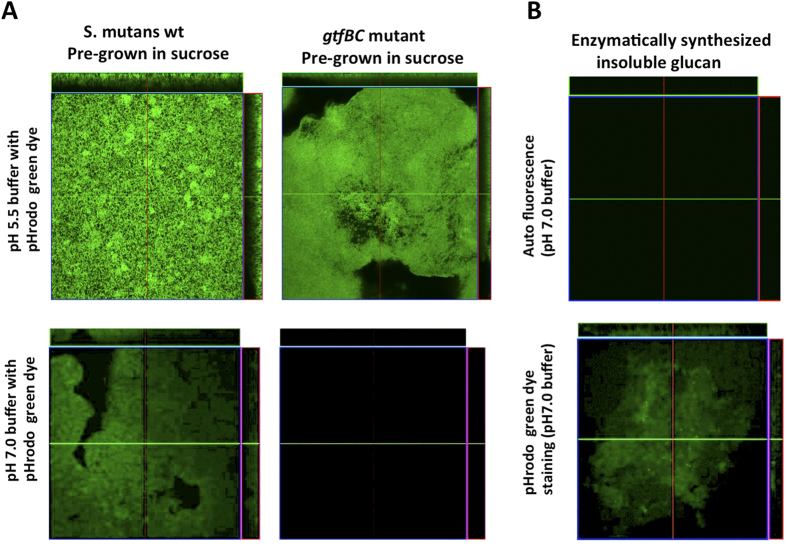
Results showed that when S. mutans wildtype or gtfBC mutant pre-grown in the buffered MDM (pH7.0) supplemented with sucrose was submerged in pH 5.5 buffer containing pHrodo Green dye, cells displayed bright fluorescence signal (Fig. 2A). However, when immersed in dye-containing pH 7.0 solution, wild type cells pre-grown in the presence of sucrose still displayed detectable green fluorescence signal, while no signal was observed for similarly cultured gtfBC mutant. Our data further implicated the involvement of surface-associated insoluble glucans in accumulating protons and achieved a cell surface pH lower than that in surrounding medium.
Figure 2. pHrodo Green STP Ester staining of S. mutans cells and in vitro prepared insoluble glucans.
(A) S. mutans UA 140 wildtype or gtfBC mutant cells pre-grown in the buffered (pH7.0) MDM supplemented with sucrose (20 mM) were harvested and stained with in pHrodo green dye in buffers with different pH (pH 5.5 and 7.0); and (B) in vitro prepared insoluble glucans were stained by pHrodo Green STP Ester in pH7 buffer.
To more directly determine if cell’s ability to concentrate protons is due to the presence of insoluble glucans on the cell surface, Gtfs were extracted and Gtf-derived insoluble glucans were prepared in vitro to test their ability to accumulate protons using pH-sensitive pHrodo Green dye. As shown in Fig. 2B, glucans did not display auto-fluorescence when immersed in pH 7.0 buffer. When incubated in pH 7.0 buffer containing pHrodo Green dye, the insoluble glucans pellets showed significant amount of fluorescence signal, indicating insoluble glucans were able to accumulate protons and decrease cell surface pH lower than that of the surrounding buffer (pH 7.0).
Low pH and insoluble glucans-dependent differential gene expression in S. mutans
Our data showed that insoluble glucans are able to concentrate protons, which could potentially precondition S. mutans against acid insults by inducing cellular acid-tolerance response. To test this, we further investigated the low pH-induced changes in the transcriptional profiles of S. mutans wild-type and gtfBC mutant cells pre-grown in the presence of sucrose or glucose using RNA-seq.
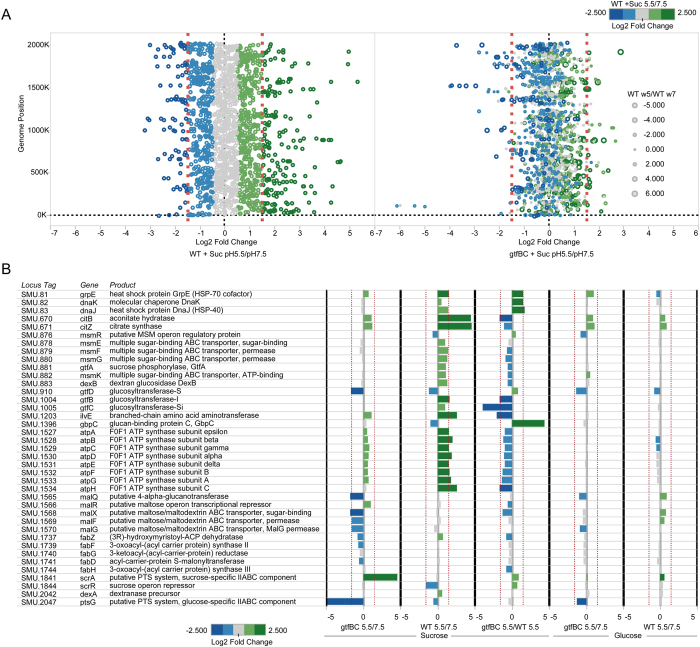
Our transcriptomic analysis revealed roughly 200 genes that were differentially regulated when wildtype S. mutans pregrown in buffered pH 7.0 MDM supplemented with sucrose was exposed to pH5.5 compared to pH7.5 solution; while this number reduced to about 100 when gtfBC mutant was tested (Fig. 3A, Figs S1–S4)(Table S2). In the wild-type background, the exposure of S. mutans cells pre-grown in the buffered (pH7.0), sucrose-containing MDM to pH5.5 solution induced the expression change of a multitude of genes spanning the whole genome and encoding diverse functions (Fig. 3B) (Fig. S1, S2). Many of the up-regulated genes observed in the WT have been implicated in acid tolerance response (Fig. 3), including msm and atp operon, which encode multiple sugar metabolism (MSM) transport system and F1F0-H/ATPase, as well as genes encoding chaperons (grpE, dnaK and dnaJ) and enzymes (aconitate hydratase and citrate synthase) involved in citrate metabolism. Most intriguingly, these genes were either not responsive or only induced to a very low level in the gtfBC background when cells were exposed to the same low pH (Fig. 3B) (Fig. S1). Meanwhile, the gtfB and gtfC genes were also up-regulated in response to lower pH in a similar gtfBC dependent manner. Furthermore, a subset of genes encoding functions such as fatty acid biosynthesis (fab operon) and putative maltose/maltodextrin ABC transporter (mal operon) did not respond to the low pH in the wildtype background, but displayed reduced expression in gtfBC mutant when exposed to same low pH. To further confirm the transcriptome data, real-time quantitative PCR was performed to monitor the expression of a panel of selected genes, including two of the F1F0-H/ATPase subunit-encoding genes, atpA and atpD; two multiple sugar binding ABC transporter component-encoding genes, msmK and dexB; as well as gtfB and gtfC. Overall, the qRT-PCR results were in agreement with our transcriptome findings (Table S3).
Figure 3. Global transcriptome profiling and differentially expressed genes previously observed in Acid Tolerance Response (ATR) and sugar metabolism in WT and gtfBC mutant detected through RNAseq.
(A) Differentially expressed genes between pH 5.5 and pH 7.5 across the genome in WT (upper left panel) and gtfBC deficient stain (upper right panel). Expression ratios are log2 fold changes. Expressed genes represented by open circles are colored and sized based on the log2 fold change in expression between WT at pH 5.5 and pH 7.5. Gene expression changes in the gtfBC mutant that show similar or opposing trends as the WT are then visible in the plot. (B) Differential expression of select key genes previously associated with ATR as well as sugar metabolism pathways and their log2 fold changes. Red dashed vertical lines indicate significant fold change.
Insoluble glucan-dependent acid tolerance in S. mutans
Our transcriptome analysis suggested that the accumulation of protons by surface associated insoluble glucans and the resultant pH drop on cell surface could allow S. mutans to pre-condition itself against acid-stress. To test this, UA140 wild-type and gtfBC deficient strain were cultivated in buffered (pH7.5) MDM supplemented with either sucrose or glucose. Cells were collected and their survival rates were compared after being exposed to the medium with a lethal pH (pH 2.8) for 0.5 h and 1 h. As shown in Fig. 4, exposure of S. mutans wild-type cells pre-grown in the presence of sucrose to pH 2.8 for 0.5 h did not greatly affect cell viability; however, the survival rate of cells pre-grown in the presence of glucose decreased dramatically to 0.52% (p < 0.05). After 1 h acid exposure, the difference in the survival rate between S. mutans cells pre-grown in the presence and absence of sucrose became even more drastic (20.38% vs. 0.002%) (p < 0.05). The significantly enhanced resistance to acid killing induced by sucrose suggested that sucrose metabolism plays a role in S. mutans’ acid resistance.
Figure 4. Acid killing assay of S. mutans wt and gtfBC strains pre-grown the presence or absence of sucrose.
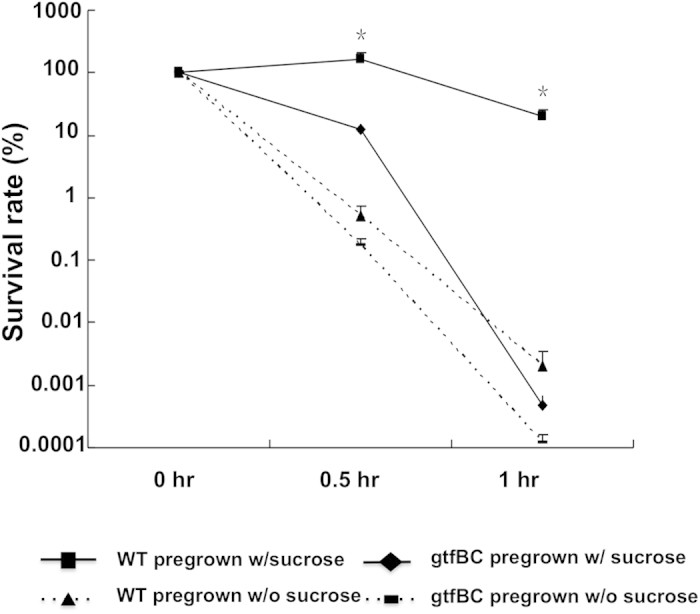
Cells of S. mutans wild-type [WT] and gtfBC-deficient strain [gtfBC] were pre-grown in the buffered minimal medium (pH 7.5) supplemented with glucose or sucrose (20 mM). The cultures were then rapidly acidified to pH 2.8 using glycine solution, and incubated for 0, 0.5 h and 1 h, respectively. The survivors were recovered by plating on TH agar. Survival rate was calculated as the CFU mL−1 at a given time divided by the CFU mL−1 at time zero. Values marked with an asterisk are statistically significantly different between groups (P < 0.05, ANOVA comparison for all pairs using Tukey-Kramer HSD).
Furthermore, compared to wild-type, the gtfBC mutant defective in synthesizing insoluble glucans suffered significantly more viability loss (p < 0.05) when exposed to the lethal pH (with survival rate of 12.67% and 0.0005% after 0.5 h and 1 h exposure, respectively) even after being pre-grown in the presence of sucrose (Fig. 4). The results further demonstrate the important role of insoluble glucans in linking S. mutans’ acidogenicity and aciduricity.
Discussion
Insoluble glucan production has been considered an important virulence factor of S. mutans, largely due to its ability to facilitate cell adherence and biofilm formation14,18. In this study, we demonstrated for the first time that insoluble glucans also play a crucial role in acid tolerance response by linking acidogenicity and aciduricity, two of the most significant physiological features of S. mutans relevant to its cariogenicity.
Previous studies have revealed the spatial distribution of pH within biofilms. Using a fluorescent pH indicator incorporated into the biofilm matrix, Xiao et al. detected low-pH microenvironments in the interior of the EPS-microcolony complexes and at the microcolony/sHA (saliva-coated hydroxyapatite) interface22. Our recent study corroborated these observations and further linked the pH heterogeneity of S. mutans microcolonies to the difference in the metabolic activity of the microcolonies20. Based on the S. mutans cell surface pH analysis, we speculated that insoluble glucans within S. mutans microcolonies might accumulate protons from its own acid production to create localized acidic microenvironment within the biofilms. In this study, we tested our hypothesis that S. mutans retains protons through surface-associated glucans and this results in up-regulation of acid tolerance response and leads to acid preconditioning for combating future acid challenge.
By measuring the change in fluorescence intensity of the surface-expressed pH-sensitive pHluorin, we revealed a significant positive correlation between sucrose-availability/GtfBC-activity and the cell’s ability to achieve pH drop on its surface when they were switched from neutral to acidic pH (Fig. 1). By performing an in vivo (cell-surface associated) and in vitro (enzymatically prepared) insoluble glucans pHrodo Green staining assay (Fig. 2), we confirmed that the surface component in S. mutans involved in protons recruitment is indeed the insoluble glucans synthesized by GtfBC enzyme.
Although the exact mechanism of proton accumulation is not known at this time, a few lines of evidence suggested that the ability of glucans to accumulate protons could be due to their chemical as well as physical properties. Insoluble glucans might chemically bind and recruit protons from its surroundings to the cell surface due to its negatively charged attribute. Employing a polarization circuit through platinum electrodes exposed in S. mutans suspension, Yamashita et al. showed that the number of cells adsorbed to the anode were much greater than the number of cells adsorbed to the cathode, indicating the cell surfaces of S. mutans are generally negatively charged23. Meanwhile, glucans-coated S. mutans cells adhered to positively charged DEAE-Sephadex, but not to uncharged Sephadex, suggesting the cell surface-adsorbed glucans contributed to the net negative charge on S. mutans cell surface23. The negatively charged nature of insoluble glucans could be attributed to the presence of acidic groups, e.g. phosphate or sulphate groups on the polysaccharides24. Meanwhile, the incorporation of lipoteichoic acid to glucans could also contribute to the negatively charged surface25. Furthermore, insoluble glucans could also act as ion exchanger in restricting the diffusion of a variety of molecules and then influence the pH distribution in biofilms22.
In addition to chemical binding, the accumulation of proton on the cell surface could also be achieved through physical entrapment. Insoluble glucans produced by GtfB composes mostly of α-(1, 3)-linked glucose moieties; while GtfC synthesizes a polymer with both α-(1, 3)-linked and α-(1, 6)-linked glucans. There is a marked increase in the number of α-(1, 3)-linkages and a higher percentage of 3-linker branch points in the surface-formed glucans compared with that formed in solution12. The molecular structure of insoluble glucans could decrease the porosity of the biofilm matrix, thus producing a strong sieving effect to physically “trap” proton-binding macromolecules19.
The “trapping” of proton by surface-associated insoluble glucans and the resultant pH drop in the cell surface could potentially triggers acid-adaptive response and allows S. mutans to prepare itself for further acid insult. Using global transcriptomic analyses, we revealed a set of genes encoding diverse functions in multiple cellular pathways whose expression was differently regulated by pH. Many of these genes are acid-inducible and, more importantly, the up-regulation of these genes under acidic condition is also dependent on the presence of insoluble glucans (Fig. 3). Further analysis assigned many of these genes to one of the following four previously proposed major mechanisms of acid tolerance in S. mutans:
Maintenance of internal pH homeostasis via proton pumping or intracellular proton consumption: Our data revealed F1F0-H/ATPase-encoding genes as one of the acid-induced operons which was clearly impacted by the loss of glucans (Fig. 3). In S. mutans, the membrane-bound, acid-stable, proton-translocating F1F0-H/ATPase is largely responsible for the maintenance of internal pH homeostasis, and plays an important role in acid tolerance7,8. Furthermore, we observed the induction of genes encoding enzymes involved in citrate metabolism, such as citrate synthase (citZ) and aconitate hydratase (citB). The citrate metabolism can also contribute to acid tolerance by converting citrate to oxaloacetate, which is then converted to pyruvate by oxaloacetate decarboxylase – a process that consumes an intracellular proton9,26.
Alteration of metabolic pathways: S. mutans employs the phosphoenolpyruvate:sugar phosphotransferase system (PTS)27 and multiple sugar metabolism (MSM) transport system28 for the internalization of carbohydrates. However, at acidic pH, S. mutans preferentially utilizes a non-PTS system29, such as MSM system. The MSM system is an ATP-binding cassette transporter involved in the uptake and metabolism of a wide range of sugars, including trisaccharides, disaccharides and monosaccharides30. Our finding is consistent with these reports, showing an up-regulated expression of msm operon when cells were exposed to low pH (Fig. 3). Our data implied that even under acidic microenvironment, S. mutans could still maintain its dietary carbohydrates in-take, and stay competitive within multispecies biofilms.
Protection and/or repair of macromolecules. A number of chaperon proteins displayed increased expression when cells encountered low pH, including GrpE, dnaK and dnaJ (Fig. 3). These chaperons intervene in a variety of stresses, including acid stress, for functions such as protein folding, re-naturation, protection of denatured proteins and evacuation of damaged ones5.
Cell-membrane change. Our study also revealed that when exposed to low pH, compared to wildtype, gtfBC mutant display an overall reduced expression of fab operon (although not statistically significant), which encodes membrane fatty acid biosynthesis function. The cell membrane fatty acid composition has been implicated to affect membrane proton permeability directly by changing the permeability of lipid bilayers to protons or indirectly by affecting the activity of the F1F0-ATPase31. Our resulted suggest the loss of insoluble glucans also impacts the regulation of membrane fatty acid synthesis during acid stress response.
In addition, transcriptomic analysis revealed insoluble-glucan dependent up-regulation of gtfBC under low pH condition, which would allow S. mutans to continuously produce insoluble glucans for biofilm accretion even under acidic environment. The exposure of S. mutans to sucrose and acidic environment has been shown to induce the expression of the gtfBC when growing under biofilm conditions21,32,33,34. While our results were consistent with above findings they contrasted with other reports where the expression of gtfBC was either reduced or remained unchanged when grown in the presence of sucrose35. The expression of gtfBC has been shown to be affected by multiple factors, including different bacteria strains, growth phase and mode, as well as environmental conditions such as medium pH, time of cultivation, and sugar concentration21,32,33,34,35. The observed discrepancy could potentially result from the combination of these factors.
The most intriguing finding of our transcriptomic and qRT-PCR analysis was that the induction of ATR genes is both low-pH and sucrose/glucan-dependent; while acidic environment alone was not sufficient to trigger their up-regulated gene expression as indicated by the lack of response in these gene sets when glucose was the only sugar available (Fig. 3). The transcriptome and acid killing data (Fig. 4) suggested that the insoluble glucans play a crucial role in S. mutans’ acid resistance, likely through trapping of protons which could allow S. mutans to achieve preconditioning against acid stress. These results further implicate a complex interplay between insoluble glucan biosynthesis, acid production and acid tolerance.
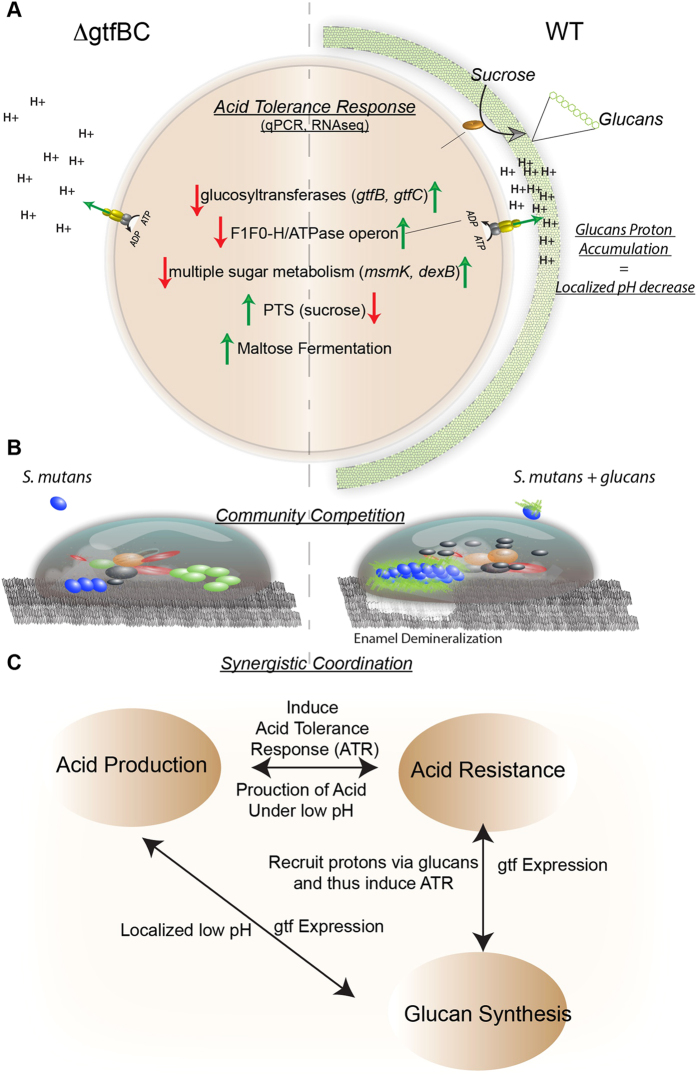
In conclusion, the results presented here deepen the understanding of the roles insoluble glucans play in the cariogenesis of S. mutans (summarized in Fig. 5). In addition to promoting S. mutans attachment and biofilm formation, our study revealed another important biological function of insoluble glucans produced by S. mutans. Our data suggested that S. mutans could retain protons from its own acid production via the surface-associated insoluble glucans. The resultant pH drop in the cell surface likely triggers acid-adaptive response and allows S. mutans to prepare itself for further acid insult ahead of other oral bacteria; while the absence of glucans could render cells to the instant acidic attack with detrimental effect. It has been postulated that pre-stress of bacteria within a biofilm community provides an insurance policy against future acid challenges, with ATR predicted to be the key cellular response for bacteria survival23. Meanwhile, the significantly enhanced resistance to acid killing induced by sucrose indicated that sucrose metabolism itself plays a role in S. mutans’ acid resistance. Sucrose utilization enables S. mutans to also survive acid stress better than many oral bacteria since it results in the synthesis of these glucans. It is tempting to speculate that this is probably the link between S. mutans becoming a major pathogen when refined sugar was introduced. Recently the evolution of these genes was investigated revealing a possible intra-genomic gene duplication event after the initial gene acquisition from other genera, which they suggest could conceivably be linked to selection pressure such as consumption of refined sugar36. Although that work was focused on GTFs and their role in biofilm formation, we show that it also allows S. mutans to achieve a low pH growth advantage. Our study demonstrated the positive correlation between sucrose-mediated glucans biosynthesis and the acid tolerance. More importantly, we revealed the crucial role of the insoluble glucans in linking acid production and acid tolerance, the two most important cariogenic factors relevant to cariogenesis of S. mutans.
Figure 5. Illustration of qRT-PCR and transcriptomic data comparing gtfBC deletion and WT revealing insoluble glucan linking acidogenicity and aciduricity in S. mutans.
(A) S. mutans establishes low localized pH via its surface-associated glucans and that low surface pH induces protective mechanisms for coping with acid-stress. The normal adaptive Acid Tolerance Responses (ATR) genes in WT are not induced when glucans are missing. (B) Illustration of how the lack of acid tolerance is likely to impact the overall persistence and growth of S. mutans within the community and (C) a model of how the virulence properties could be linked.
Experimental Procedures
Bacterial strains and growth conditions
S. mutans UA140 wild type, the UA140 gtfBC-deficient strain (kindly provided by H. Kuramitsu, University of Buffalo, NY, USA), as well as UA140 pHluorin-SpaP fusion strain20 and its corresponding gtfBC-deficient derivative were cultured in Todd-Hewitt (TH) media (Difco) or MDM37 at 37 °C in the presence of 5% CO2. The S. mutans expressing the pHluorin-SpaP fusion and the gtfBC-deficient strain were cultured in the same medium supplemented with 800 μg/mL spectinomycin and 15 μg/mL erythromycin, respectively (Table 1).
Table 1. Strains used in this study.
| Strain | Relevant characteristics | Reference |
|---|---|---|
| S. mutans UA140 | Wild-type S. mutans Kans Erms | 46 |
| S. mutans strain O87 | UA140::Φ(ldhp-leading sequence-gfp-linker-spaP), Specr | 20 |
| S. mutans strain gtfBC | UA140igtfBC, Ermr | 47 |
| S. mutans strain gtfBC/O87 | UA140::Φ:ldhp-leading sequence-gfp-linker-spaP)ΔgtfBC,Specr Ermr | This work |
Strain construction
S. mutans strain O87 with cell surface-displayed ecliptic pHluorin has been constructed previously20. Similar method was used for generating S. mutans strain gtfBC/O87, a UA140 gtfBC derivative carrying pHluorin on its cell surface.
Preparation of different S. mutans stains for fluorescence measurement/acid killing assay/transcriptome analysis
Overnight cultures (OD 600 nm = 1) of S. mutans wild-type and gtfBC-deficient strain grown in TH were diluted 1:100 in buffered (pH7.5) MDM supplemented with sucrose or glucose (20 mM)38. Two mL of bacterial suspension was added to the well of a 6-well flat-bottomed polystyrene microtiter plate (Corning, New York, NY) and incubated for 12 h at 37 °C in the presence of 5% CO2. After 12 h incubation, wild type S. mutans forms noticeable biofilms at the bottom of the well when sucrose was supplemented; while the rest of wells (with glucose supplement) showed bacterial growth but no significant biofilm formation. Six-well plates were centrifuged, the cells were either subjected to different pH treatment for fluorescence measurement, or collected for further acid killing assay. Alternatively, cells were washed with PBS and incubated in fresh sucrose or glucose-supplemented (20 mM) buffered MDM (phosphate buffer for the pH control; pH 7.5 or 5.5) for another 3 h, before they were harvested and processed for transcriptome analysis.
Fluorescence measurement
Our recent study showed that under biofilm growth conditions, S. mutans O87 cells responded to the shift in external pH from 7.5 to 5.5 with a drastic reduction (about 70%) in fluorescence intensity within 10 min, and the signal was almost fully restored ( > 90%) after 10 min exposure to a re-adjusted external pH of 7.5. To monitor the pH change on the cell surface, S. mutans strain O87 and gtfBC/O87 were pre-grown in buffered minimal defined medium (MDM) (pH 7.5) in the presence of sucrose or glucose as described above. Cells were then switched to buffers with lower pH values (pH 7.0, 6.5, 6.0 and 5.5) and their surface fluorescence intensity was monitored after 10 minutes’ incubation. The phosphate buffer solutions were prepared by mixing potassium phosphate monobasic anhydrous and sodium phosphate dibasic heptahydrate in different ratios to obtain the desired pH value. The pH value of the buffer solutions was determined using an AB15 pH meter (Fisher). The pHluorin fluorescence signal intensity of cells was measured at different pH values by a Cary Eclipse fluorescence spectrophotometer (Varian, Mulgrave, Victoria, Australia) using a bandpass filter with a center wavelength of 400 ± 5 nm. Meanwhile, viability counting (CFU/ml) on agar plates was performed for each sample and was factored in later to normalize the cell biomass.
Acid killing assay
To test their acid resistance ability, S. mutans wild-type and gtfBC-deficient strains were pre-grown as described above. Cells were immediately subjected to acid stress by being incubated in one mL of pH 2.8 glycine solution for 0, 0.5 h and 1 h39. The killing process was terminated by washing cells with one mL of PBS for three times. Collected cells were passed through a 26 gauge, 5/8 inches (15.9 mm) long needle 10 times to break cell clumps before being plated on non-selective agar plates. Plates were incubated at 37 °C and colonies were counted after 3 days. The acid resistance of different S. mutans strains was expressed as the survival rate of bacterial cells after being exposed to lethal pH, which was calculated as the percentage of number of surviving cells to the total initial cells (as the CFU mL−1) before the treatment.
Preparation of insoluble glucans
S. mutans strains were grown in TH medium at 37 °C for 16 h in the presence of 5% CO2. The cell-free GTF enzymes were prepared according to the method by Ebisu et al.40. The prepared enzymes were incubated with 100 mM sucrose in 0.1 M phosphate buffer, pH 6.8, at 37 °C for 48 h. The insoluble polysaccharide formed in the reaction mixture was collected by centrifugation at 45,000× g for 30 min and washed three times with excess distilled water. The total carbohydrate was estimated by the phenol sulphuric acid method41, and glucose was used as standard.
Confocal laser scanning microscope imaging
S. mutans UA 140 were grown in the MDM (buffered to pH 7.0) supplemented with glucose or sucrose (20 mM) for 16 h. The cells were collected by centrifugation and washed twice with PBS. Then the S. mutans cells and insoluble glucans prepared in vitro were spread onto cover glass (Fisher). To prevent glucans from detaching from cover glass, 1% agarose dissolved into phosphate buffer (pH 7.0 or 5.5) was poured onto the surface of glucans pellets. For visualization, 40 μl of phosphate buffer solution (pH 7.0 or 5.5) containing 40 μg/mL pHrodo Green STP Ester dye (Lifetech), which displays enhanced fluorescence intensity with the drop in pH, was added to the pellets and sample was incubated at room temperature for 30 min before imaging.
All images were collected with a Zeiss LSM 5 PASCAL confocal laser scanning microscope (CLSM) using LSM 5 PASCAL software (Zeiss, Jena, Germany). Excitation at 488 nm with an argon laser in combination with a 505 nm longpass emission filter was used for pH fluorescence imaging. The scanning module of the system was mounted onto an inverted microscope (Axiovert 200M). The 40 × (NA/1.3) numerical aperture oil-immersion objectives were used for imaging. Image stacks (1024 by 1024-pixel tagged image file format) were taken.
mRNA isolation and sequencing
S. mutans wild-type and gtfBC-deficient strain incubated under buffered pH 7.5 or 5.5 condition for 3 h in the presence of sucrose or glucose were collected by centrifugation as described and then frozen in liquid nitrogen. Total RNA extraction and purification was performed using the mirVana RNA extraction kit (Life Technologies) and the RNA Clean/Concentrator™ kit (Zymo Research, Irvine, CA). DNA was removed from the samples by adding 1 μl (2U) Turbo™ DNAse (Life Technologies) and incubating samples at 37 °C for 30 min. After DNA removal, 16S rDNA PCR was performed using the same protocol and primers as described in McLean et al.42 to verify that DNA was removed. To remove rRNA in total RNA extracts the RiboZero™ Magnetic Kit (Epicenter) was used according to manufacturer’s instruction. mRNA was purified by using the Zymo RNA Clean and Concentrator™ kit (Zymo Research). RNA concentration and integrity was monitored before and after rRNA removal by using the Agilent RNA 6000 Nano Kit (Agilent Technologies, Inc. Santa Clara, CA) and the Agilent RNA 6000 Pico Kit (Agilent Technologies), respectively. cDNA library from rRNA-depleted RNA was generated by using random-primed cDNA synthesis methods according to the ScriptSeq™ v2 RNA-Seq Library Preparation Protocol (Epicenter). Prior to second strand cDNA synthesis the di-tagged cDNA was purified using the Agencourt AMPure XP system (Beckman Coulter). Index-reads supplied with the ScriptSeq Kit were added to the libraries which then were PCR amplified for 15 cycles. RNA-Seq libraries were purified and quantified by using the Agencourt AMPure XP system (Beckman Coulter) and the Agilent DNA 1000 protocol (Agilent Technologies), respectively. Sequencing of cDNA libraries was performed by using an Illumina HiSeq 2000 platform (100 bp paired end reads) which has the capacity of 19 GB per lane providing high coverage of reads. Sequencing was carried out at the JCVI sequencing facility JTC. cDNA sample concentrations were normalized at JTC prior to sequencing. Using each sample’s individual barcodes, the Illumina data was deconvolved into the respective samples. After trimming the bar codes, low-quality and short sequences (<100 bp) were removed by using the CLC Genomics Workbench Software (CLCbio, Aahus, Denmark). The following CLC-parameters were applied during paired read sequence trimming and quality control: quality score setting: NCBI/Sanger or Illumina Pipeline 1.8 and later, minimum distance: 180, maximum distance: 250.
Read mapping of raw cDNA reads onto S. mutans 159 genome
Expression values for each mRNA sample were generated by BWA mapping43 of both filtered fragment and paired reads onto the well-annotated reference genome S. mutans UA159. Reads were mapped with the default BWA option (96% sequence identity). CLC RNAseq plugin software was used to normalize and determine statistical significance of expression. DESeq, which uses a model based on the negative binomial distribution with variance and mean linked by local regression44 was also employed to validate the expression observed with good agreement between the methods. Additional investigations of the transcription start sites, co-expressed genes, predicted small RNAs and normalized expression using by upper quartile normalization were performed with Rockhopper45 and manual curation. Genes with log2 1.5 fold changes in the WT or gtfBC mutant at pH 5.5 relative to pH 7.5 or between WT and gtfBC mutant at pH 5.5 are provided as Supplementary material (Fig. S1-S6).
Real-time quantitative PCR
Total RNA was extracted and purified according to above method. The RNA concentration was determined spectrophotometrically using a Nanodrop instrument (Nanodrop 2000; Thermo Scientific). The integrity of the RNA was assessed by agarose gel electrophoresis. cDNAs of S. mutans wild-type and gtfBC-deficient strain were synthesized according to the protocol of Transcriptor first strand cDNA sysnthesis kit (Roche).
Quantitative real-time PCR was performed using the iQ SYBR Green supermix (Bio-Rad Laboratories) on a Bio-Rad iQ5 real-time PCR detection system (Bio-Rad Laboratories, Inc., CA). The quantitative real-time PCR reaction mixture (20 μl) contained 1 × iQ SYBR Green supermix, 0.1μg cDNA, and 0.5 μM of the appropriate forward and reverse PCR primers (Table S1). The reactions were incubated at an initial denaturation at 95 °C for 10 min, followed by a 40-cycle amplification consisting of denaturation at 95 °C for 15 s, annealing at 55 °C for 15 s, and extension at 72 °C for 15 s. All primers pairs were checked for primer-dimer formation by using the dissociation curve analysis. A standard curve was plotted for each primer set with Ct values obtained from amplification of known quantities of S. mutans cDNAs. The expression level of gtfB, gtfC, atpA, atpD, msmK, and dexB gene was normalized using the S. mutans 16S rRNA gene, and the acid-induced fold changes of the expression levels in S. mutans wild-type or its gtfBC derivative were calculated by dividing the expression at pH 5.5 by that at pH 7.5. There was no significant difference in the expression of the 16S rRNA gene under the various conditions (data not shown). Each assay was performed with at least two independent RNA samples in triplicates.
Additional Information
How to cite this article: Guo, L. et al. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci. Rep. 5, 18015; doi: 10.1038/srep18015 (2015).
Supplementary Material
Acknowledgments
We thank H. Kuramitsu from the University of Buffalo (NY, USA) for kindly providing the gtfBC-deficient derivative of S. mutans UA140. And we thank Dr. Gero Miesenbőeck for the kind gift of the ecliptic pHluorin and Adam Hall for assistance with RNA-Seq library construction. This work was supported by grants from the National Institute of Health (NIH-1-R01-DE020102 , NIH-1-R01-DE023810 and 1-R01-GM095373), a grant from the Natural Sciences Foundation of China (30672322) and a grant from the International Science and Technology Cooperation Program of China (2011DFA30940).
Footnotes
The authors declare that W.S. is a part time Chief Science Officer of C3 Jian Inc., which has licensed technologies from UC Regents that could be indirectly related to this research project.
Author Contributions W.S. and X.H., conceived and designed the experiments. L.G. and J.S.M performed the experiments. J.S.M., L.G., R.L., X.H. and W.S. analyzed the data. L.G., J.S.M., R.L., X.H. and W.S. wrote the manuscript. The principle investigators are X.H. and W.S.
References
- Selwitz R. H., Ismail A. I. & Pitts N. B. Dental caries. Lancet 369, 51–59 (2007). [DOI] [PubMed] [Google Scholar]
- Marsh P. D. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 8, 263–271 (1994). [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 50, 353–380 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby S. M. & Russell R. R. Sugar metabolism by mutans streptococci. Soc Appl Bacteriol Symp Ser. 26, 80S–88S (1997). [PubMed] [Google Scholar]
- Cotter P. D. & Hill C. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev : MMBR 67, 429–453, table of contents (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivey R. G. Jr., Kuhnert W. L. & Hahn K. Adaptation of oral streptococci to low pH. Adv Microb Physiol 42, 239–274 (2000). [DOI] [PubMed] [Google Scholar]
- Hamilton I. R. & Buckley N. D. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol Immunol. 6, 65–71 (1991). [DOI] [PubMed] [Google Scholar]
- Dashper S. G. & Reynolds E. C. pH regulation by Streptococcus mutans. J Dent Res. 71, 1159–1165 (1992). [DOI] [PubMed] [Google Scholar]
- Matsui R. & Cvitkovitch D. Acid tolerance mechanisms utilized by Streptococcus mutans. Fut Microbiol. 5, 403–417 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. G. & Spatafora G. A. Gene regulation in S. mutans: complex control in a complex environment. J Dent Res. 91, 133–141 (2012). [DOI] [PubMed] [Google Scholar]
- Hamada S., Koga T. & Ooshima T. Virulence factors of Streptococcus mutans and dental caries prevention. J Dent Res. 63, 407–411 (1984). [DOI] [PubMed] [Google Scholar]
- Bowen W. H. & Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 45, 69–86 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paes Leme A. F., Koo H., Bellato C. M., Bedi G. & Cury J. A. The role of sucrose in cariogenic dental biofilm formation–new insight. Caries Res 85, 878–887 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H., Xiao J., Klein M. I. & Jeon J. G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol. 192, 3024–3032 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N. & Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 57, 2079–2085 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N. & Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 56, 1999–2005 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H., Falsetta M. L. & Klein M. I. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 92, 1065–1073 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Bowen W. H., Burne R. A. & Kuramitsu H. K. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 61, 3811–3817 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S. & Mayanagi H. Acid diffusion through extracellular polysaccharides produced by various mutants of Streptococcus mutans. Arch Oral Biol. 48, 431–438 (2003). [DOI] [PubMed] [Google Scholar]
- Guo L. et al. investigating acid production by Streptococcus mutans with a surface-displayed pH-sensitive green fluorescent protein. PloS one 8, e57182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. & Burne R. A. Regulation of the gtfBC and ftf genes of Streptococcus mutans in biofilms in response to pH and carbohydrate. Microbiol. 147, 2841–2848 (2001). [DOI] [PubMed] [Google Scholar]
- Xiao J. et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 8, e1002623 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Kunimori A. & Takehara T. Effect of calcium ions on cell surface electrostatics of Bacteroides gingivalis and other oral bacteria. Zentralblatt fur Bakteriologie: international journal of medical microbiology 275, 46–53 (1991). [DOI] [PubMed] [Google Scholar]
- Melvaer K. L., Helgeland K. & Rolla G. A charged component in purified polysaccharide preparations from Streptococcus mutans and Streptococcus sanguis. Arch Oral Biol. 19, 589–595 (1974). [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K., Wondrack L. & McGuinness M. Interaction of Streptococcus mutans glucosyltransferases with teichoic acids. Infect Immun. 29, 376–382 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimroth P., Jockel P. & Schmid M. Coupling mechanism of the oxaloacetate decarboxylase Na( + ) pump. Biochimica et biophysica acta 1505, 1–14 (2001). [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C. & Pelletier M. The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol Rev. 19, 187–207 (1997). [DOI] [PubMed] [Google Scholar]
- Russell R. R., Aduse-Opoku J., Sutcliffe I. C., Tao L. & Ferretti J. J. A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J Biol Chem. 267, 4631–4637 (1992). [PubMed] [Google Scholar]
- Buckley N. D. & Hamilton I. R. Vesicles prepared from Streptococcus mutans demonstrate the presence of a second glucose transport system. Microbiol. 140 (Pt 10), 2639–2648 (1994). [DOI] [PubMed] [Google Scholar]
- Tao L., Sutcliffe I. C., Russell R. R. & Ferretti J. J. Transport of sugars, including sucrose, by the msm transport system of Streptococcus mutans. J Dent Res. 72, 1386–1390 (1993). [DOI] [PubMed] [Google Scholar]
- Quivey R. G. Jr., Faustoferri R., Monahan K. & Marquis R. Shifts in membrane fatty acid profiles associated with acid adaptation of Streptococcus mutans. FEMS Microbiol Lette. 189, 89–92 (2000). [DOI] [PubMed] [Google Scholar]
- Hudson M. C. & Curtiss R. 3rd Regulation of expression of Streptococcus mutans genes important to virulence. Infect Immun. 58, 464–470 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M. I. et al. Streptococcus mutans protein synthesis during mixed-species biofilm development by high-throughput quantitative proteomics. PloS one 7, e45795 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne R. A., Chen Y. Y. & Penders J. E. Analysis of gene expression in Streptococcus mutans in biofilms in vitro. Advan Dental Res. 11, 100–109 (1997). [DOI] [PubMed] [Google Scholar]
- Goodman S. D. & Gao Q. Characterization of the gtfB and gtfC promoters from Streptococcus mutans GS-5. Plasmid 43, 85–98 (2000). [DOI] [PubMed] [Google Scholar]
- Hoshino T., Fujiwara T. & Kawabata S. Evolution of cariogenic character in Streptococcus mutans: horizontal transmission of glycosyl hydrolase family 70 genes. Sci Rep. 2, 518 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo C. Y., Corliss D. A. & Ganeshkumar N. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J Bacteriol. 182, 1374–1382 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J. et al. Quantitative analyses of Streptococcus mutans biofilms with quartz crystal microbalance, microjet impingement and confocal microscopy. Biofilms 1, 277–284 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. & Burne R. A. Multiple two-component systems modulate alkali generation in Streptococcus gordonii in response to environmental stresses. J Bacteriol. 191, 7353–7362 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisu S., Kato K., Kotani S. & Misaki A. Isolation and purification of Flavobacterium alpha-1,3-glucanase-hydrolyzing, insoluble, sticky glucan of Streptococcus mutans. J Bacteriol. 124, 1489–1501 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois M. Colorimetric method for determination of sugars and related substance. Analy Chemi. 28, 350–356 (1956). [Google Scholar]
- McLean J. S. et al. Identifying low pH active and lactate-utilizing taxa within oral microbiome communities from healthy children using stable isotope probing techniques. PloS one 7, e32219 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. & Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S. & Huber W. Differential expression analysis for sequence count data. Geno Biol. 11, R106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure R. et al. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res. 41, e140 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F., Chen P. & Caufield P. W. The group I strain of Streptococcus mutans, UA140, produces both the lantibiotic mutacin I and a nonlantibiotic bacteriocin, mutacin IV. Appl Environ Microbiol. 67, 15–21 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka K., Wang B., Sato Y. & Kuramitsu H. Regulation of sucrose-6-phosphate hydrolase activity in Streptococcus mutans: characterization of the scrR gene. Infect Immun. 66, 3736–3743 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.