Abstract
Lecithin:cholesterol acyltransferase (LCAT) deficiency is associated with hypoalphalipoproteinemia, generally a predisposing factor for premature coronary heart disease. The evidence of accelerated atherosclerosis in LCAT-deficient subjects is however controversial. In this study, the effect of LCAT deficiency on vascular tone and endothelial function was investigated in LCAT knockout mice, which reproduce the human lipoprotein phenotype. Aortas from wild-type (Lcatwt) and LCAT knockout (LcatKO) mice exposed to noradrenaline showed reduced contractility in LcatKO mice (P < 0.005), whereas acetylcholine exposure showed a lower NO-dependent relaxation in LcatKO mice (P < 0.05). Quantitative PCR and Western blotting analyses suggested an adequate eNOS expression in LcatKO mouse aortas. Real-time PCR analysis indicated increased expression of β2-adrenergic receptors vs wild-type mice. Aorta stimulation with noradrenaline in the presence of propranolol, to abolish the β-mediated relaxation, showed the same contractile response in the two mouse lines. Furthermore, propranolol pretreatment of mouse aortas exposed to L-NAME prevented the difference in responses between Lcatwt and LcatKO mice. The results indicate that LCAT deficiency leads to increased β2-adrenergic relaxation and to a consequently decreased NO-mediated vasodilation that can be reversed to guarantee a correct vascular tone. The present study suggests that LCAT deficiency is not associated with an impaired vascular reactivity.
Abbreviations: LCAT, lecithin:cholesterol acyltransferase; HDL, high density lipoproteins; NA, noradrenaline; ACh, acetylcholine; L-NAME, NG-nitro-l-arginine methyl ester; SNP, Sodium nitroprusside; NO, nitric oxide; eNOS, endothelial NO synthase; LcatKO, LCAT knockout mice
Keywords: Lecithin:cholesterol acyltransferase, Adrenergic receptors, Endothelial NO synthase, Mouse model, Aorta
Graphical abstract
1. Introduction
Lecithin:cholesterol acyltransferase (LCAT) is a 63 kDa glycoprotein which catalyzes the transfer of a fatty acid from the sn-2 position of phosphatidylcholine to the 3-hydroxyl group of cholesterol, generating cholesteryl esters and lysolecithin [1]. In blood, LCAT preferentially binds to high density lipoproteins (HDL) and, by esterifying free cholesterol, it converts discoidal preβ-HDL into mature, spherical α-migrating HDL, thus playing a key role in the metabolism of this lipoprotein class [2].
Loss-of-function mutations at both alleles of the human LCAT gene result either in familial LCAT deficiency or in fish eye disease, two very rare metabolic disorders characterized by severe hypoalphalipoproteinemia [3]. HDL deficiency is generally expected to be associated with increased coronary heart disease risk [4], [5]. However, premature coronary heart disease is not a consistent finding among individuals with LCAT deficiency [6], [7], [8]. Moreover, the measurement of carotid intima media thickness in LCAT-deficient subjects, compared to matched controls, suggests that LCAT deficiency has a modest if no effect on atherosclerosis development [9], [10], [11].
The impact of LCAT in atherosclerosis has also been investigated in genetically modified models with inconclusive results. Targeted deletion of LCAT in mice reproduces the lipoprotein phenotype found in LCAT-deficient subjects, with extremely low HDL-cholesterol levels and increased pre-β HDL vs control mice [12], [13]. When, however, for atherosclerosis studies, LCAT knockout mice were cross-bred into atherosclerosis-prone mouse lines, such as apoE knockout or LDLR-deficient mice, contradictory results were obtained [14], [15]. A possible explanation resides in the fact that, depending on the genetic background or the dietary treatment adopted, LCAT deletion differently affected apoB-containing lipoprotein levels, leading to reduced or increased atherosclerosis development.
Several in vitro and in vivo studies have highlighted the ability of HDL to preserve the vascular tone by inducing the release of endothelial vasoactive molecules, thus protecting from atherosclerosis development [16], [17], [18]; however, the impact of hypoalphalipoproteinemia on endothelial function has been poorly addressed [19] and a possible effect of the hypoalphalipoproteinemia driven by LCAT deficiency on the vascular tone has not been explored yet. In the present study, the effect of LCAT deletion on vascular reactivity was investigated in LCAT knockout mice fed chow diet. Interestingly, differences in vascular reactivity to both contractile and relaxant stimuli were detected in LCAT knockout mouse aortas.
2. Materials and methods
2.1. Materials
Norepinephrine hydrochloride, Angiotensin II, NG-nitro-l-arginine methyl ester, acetylcholine, sodium nitroprusside, and d,l-propranolol were purchased from Sigma-Aldrich. All compounds were freshly dissolved in distilled H2O.
2.2. Animals
Procedures involving animals and their care were conducted in accordance with institutional guidelines that are in compliance with national (D.L. No. 26, March 4, 2014, G.U. No. 61 March 14, 2014) and international laws and policies (EEC Council Directive 2010/63, September 22, 2010: Guide for the Care and Use of Laboratory Animals, United States National Research Council, 2011).
C57BL/6J male mice, aged 4–6 months, wild type (Lcatwt) or homozygous for a targeted disruption of LCAT gene (LcatKO) (kindly provided by Prof. J.S. Parks) [13], and mice deficient of murine apoA-I (A-IKO) [20] have been enrolled for the study. Mice were housed at constant temperature and relative humidity, and fed a commercial standard diet (Mucedola, Italy).
2.3. Vascular reactivity study
Mice were sacrificed by cervical dislocation. Thoracic aortas were carefully excised, cleaned of fat and connective tissue, and cut into 2–3 mm rings. Ring segments were suspended in 5-ml organ baths containing Krebs' solution at 37 °C continuously bubbled with 95% O2:5% CO2. The Krebs' solution had the following composition (mM): NaCl 118, KCl 4.7, KH2PO4 1.2, MgSO4 1.1, CaCl2 2.5, NaHCO3 25 and glucose 5.5; pH 7.4. Ring segments were mounted horizontally in the organ bath by inserting 2 tungsten wires (50 μm) through the lumen of the vessel, one wire attached to a rigid support and the other to an isometric force transducer (Fort 10, World Precision Instruments, Sarasota, FL) for tension recording. Mechanical activity was displayed and recorded with a digital recording system (PowerLab 8SP — AD Instruments, Basile, Comerio, Italy). Tissues were equilibrated for 1 h, and loaded to a tension of 0.8 g. A slight reduction in this value occurred after the equilibration period.
At the end of the equilibration period, each preparation was exposed to noradrenaline EC60 (NA 10− 7 M) until two reproducible contractions were obtained. Cumulative concentration–response curves to NA (10− 9–10− 5 M) and to Angiotensin II (Ang-II, 10− 9–10− 5 M) were obtained at resting tone. Contractile response to NA was expressed as mN/mg tissue. Cumulative concentration–response curves to: acetylcholine (ACh, 10− 9–3 × 10− 6 M), NG-nitro-l-arginine methyl ester (L-NAME, 10− 6–10− 4 M) and Sodium nitroprusside (SNP, 3 × 10− 11–3 × 10− 7 M) were run in preparations precontracted with NA (10− 7 M).
After each concentration–response curve, tissues were washed several times with fresh Krebs' solution and left in the bath (for about 1 h) until the resting tone had recovered. Contractile response to NA and cumulative concentration–response curves to L-NAME were also assessed in aortic rings pretreated with the selective β-adrenergic receptor antagonist d,l-propranolol (10− 6 M).
2.4. Aorta histology
After the ex-vivo evaluation of vascular reactivity, the same aortic rings were embedded in OCT compound (Sakura Finetek Europe B.V., Alphen aan den Rijn, The Netherlands). Serial cryosections (7 μm thick) of the aorta were cut and stained with hematoxylin and eosin (Bio-Optica, Milano, Italy). The Aperio ScanScope GL Slide Scanner (Aperio Technologies, Vista, CA, USA) equipped with a Nikon 20 ×/0.75 Plan Apochromat objective producing a 0.25 μm/pixel scanning resolution with a 40 × magnification was used to acquire images. The Aperio ImageScope software (version 8.2.5.1263) was used to evaluate tunica media area and thickness, internal and external elastic lamina perimeter as well as the number of elastic laminae. An operator blinded to genotype acquired and evaluated the images.
2.5. qPCR analyses
Aortas (thoracic to iliac arteries bifurcation) were collected from anesthetized animals (isoflurane, Baxter, Italy) after perfusion with PBS, cleaned of excess fat and snap-frozen in liquid nitrogen. Total RNA was isolated using the NucleoSpin RNA extraction kit (Macherey-Nagel, Duren, Germany) according to the manufacturer's instructions. RNA concentration and purity were estimated evaluating the ratio of optical density at 260 and 280 nm (Nanodrop 1000, ThermoScientific, Wilmington, DE). RNA integrity was checked by electrophoresis in a 1.7% TAE gel stained with ethidium bromide (Sigma-Aldrich, Seelze, Germany). Total RNA (1 μg) was reverse transcribed with random hexamer primers and MultiScribe reverse transcriptase (Life Technologies, Carlsbad, CA) following the manufacturer's instructions. cDNA (20 ng) was quantified by qPCR on a CFX Connect 96 thermal cycler by using an iTaq Universal SYBR® Green Supermix (Biorad, Segrate, Italy) and specific primers indicated in Table 1. Efficiency and melting curve were calculated for each primer pair. Expression data are relative to housekeeping gene cyclophilin, used as reference. Fold changes were calculated with the ΔΔCt method.
Table 1.
Sequences of the primers used for real-time PCR analysis.
| Gene | Forward primer (from 5′ to 3′) | Reverse primer (from 5′ to 3′) |
|---|---|---|
| α1a | TGCGAGGACTGAAGGTCCGC | CAGGGACGCTGGGCGAATGG |
| α2a | TGCTGGTTGTTGTGGTTGTT | GGGGGTGTGGAGGAGATAAT |
| β1 | GAAGGCGCTCAAGACACTGG | CCAGGTCGCGGTGGAA |
| β2 | TCTGTCTGTCTGTCTGGATGATG | CCAGGTCGCGGTGGAA |
| β3 | GGCAACCTGCTGGTAATCAT | TCCACTGACGTCCACAGTTC |
| AKT | GATCAAGATGACAGCATGGAGTGT | GGCAATGCAGAGGAGCGT |
| CAT | CCTCGTTCAGGATGTGGTTT | TCTGGTGATATCGTGGGTGA |
| Cyclophilin | AGCACTGGGGAGAAAGGATT | AGCCACTCAGTCTTGGCAGT |
| Edn1 | CCTGGACATCATCTGGGTC | TGTGGCCTTATTGGGAAG |
| eNOS | ACAAATAGAGGCAATCTTCGTTCA | CTATAGCCCGCATAGCGTATCA |
| GTPCH1 | GCAGCGAGGAGGAAAACCA | CCAGCGAGAGCAGAATGGA |
| HMOX1 | GCTAGCCTGGTGCAAGATACT | GCCAACAGGAAGCTGAGAGTG |
| iNOS | CAGCTGGGCTGTACAAACCTT | CATTGGAAGTGAAGCGTTTCG |
| M3 | CACGGCTGCCAGATATGACC | TGGTCACTTGGTCAGAACGC |
| NOX1 | CTGACAAGTACTATTACACGAGAG | CATATATGCCACCAGCTTATGGAAG |
| NOX2 | AACTGTATGCTGATCCTGCTGC | GTTCTCATTGTCACCGATGTCAG |
| NOX4 | TGAGGAGTCACTGAACTATGAAGTTAATC | TGACTGAGGTACAGCTGGATGTTCACA |
| p47PHOX | ACCGGCTATTTCCCATCC | TGGATCCTCTGTGCGTTG |
| p67PHOX | CTCTACTACAGAATGGAGAAGTACG | GCCCCAGGATCTTGTAGTCTAT |
| Scarb1 | AGCGTGGACCCTATGTCTACA | CCATGCGACTTGTCAGGCT |
| SOD1 | ACCAGTGCAGGACCTCATTTTAA | TCTCCAACATGCCTCTCTTCATC |
| SOD2 | CACATTAACGCGCAGATCATG | CCAGAGCCTCGTGGTACTTCTC |
| SOD3 | GCTTCGACCTAGCAGACAGG | GTCGTCCTAGCTCCATCCAG |
| XDH | AAAGGACCAGACGATTGCTCC | TCACACGTTCCCCTTCAAAAC |
2.6. Western blotting analyses
PBS-perfused aortas were collected from anesthetized animals (isoflurane, Baxter, Italy), cleaned of excess fat and lysed using an Ultra-Turrax T25 in ice-cold RIPA buffer (50 mM Tris–Cl pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) supplemented with 2✕ Protease Inhibitor Cocktail (Sigma-Aldrich, Seelze, Germany) and 2✕ HaltTM Phosphatase Inhibitor Cocktail (Thermo Scientific, Waltham, MA). Lysates were sonicated with short bursts for 1 min on ice, then cleared by two rounds of centrifugation at 12,000 rpm for 10 min at 4 °C; the protein concentration was determined using the BCA protein assay method. 100 μg of proteins were fractionated by SDS-PAGE in 12% acrylamide gels and then transferred onto Hybond-C extra nitrocellulose membranes (Amersham, Buckinghamshire, UK). The following antibodies (Cell Signaling, Danvers, MA) were used at 1:1000 dilution for overnight hybridization at 4 °C: Akt (#4691), Phospho-Akt Ser473 (#4060), eNOS (#9570) and Phospho-eNOS Ser1177 (#9570). Membranes were then washed and further incubated with 1:5000 horseradish peroxidase-conjugated anti-rabbit IgG (#7074). The signal was detected with the Pierce ECL Western Blotting Substrate (Thermo Scientific). Bands were quantified with ImageJ (http://rsbweb.nih.gov).
2.7. Statistical analysis
Vascular reactivity data are expressed as mean ± SEM of at least six experiments, and represent unpaired data. Concentration–response curves were calculated by the software GraphPad Prism 3.0 and compared by means of analysis of variance. All the other results are expressed as mean ± SD. Group differences were tested for statistical significance by unpaired Student's t-test. A value of P < 0.05 was considered statistically significant. The statistical analysis was performed using the SYSTAT software (Version 13; Systat Software, Inc., Chicago, IL).
3. Results
3.1. Vascular reactivity of Lcatwt and Lcatko aortic rings
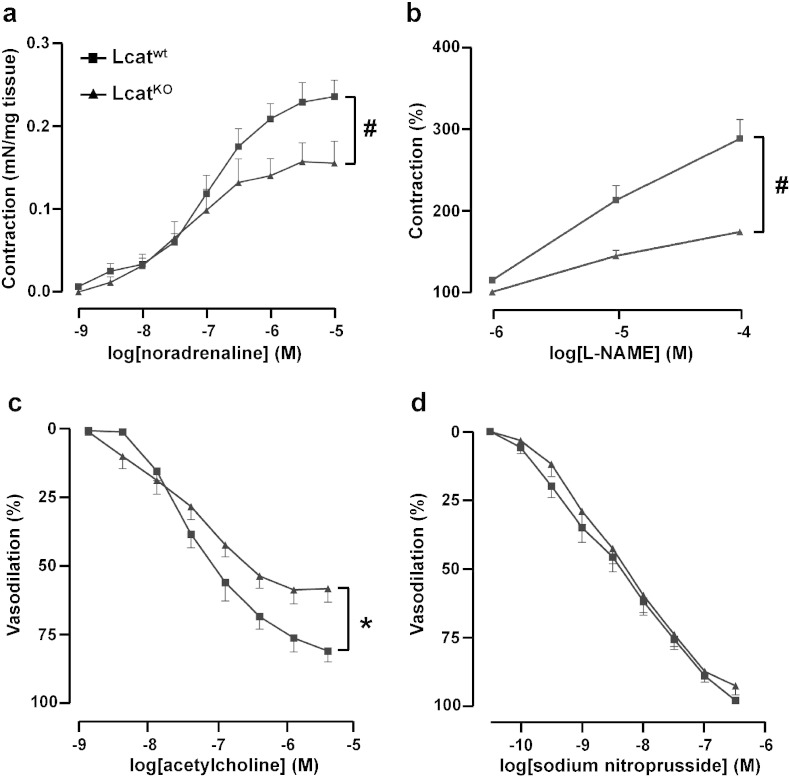
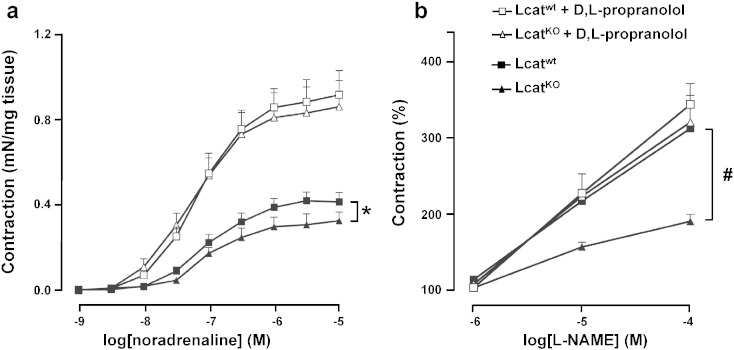
Contractions elicited in response to cumulative concentrations of NA (10− 9 M to 10− 5 M) were significantly impaired in LcatKO aortic rings as compared with Lcatwt (P < 0.005) (Fig. 1a). Sensitivity to NA was similar in both groups with pD2 values of 6.94 ± 0.13 in Lcatwt and 7.35 ± 0.29 in LcatKO mouse aortas (P = 0.22). Maximal response was significantly attenuated in LcatKO (0.16 ± 0.02 mN/mg) when compared to that of Lcatwt aortic rings (0.24 ± 0.02 mN/mg) (P = 0.01).
Fig. 1.
Concentration–response curves to noradrenaline (a), L-NAME (b), acetylcholine (c) and sodium nitroprusside (d) in aortic rings isolated from Lcatwt and LcatKO mice. For panels b, c and d, tissues were precontracted with noradrenaline (10− 7 M), and then incubated with increasing concentrations of L-NAME, acetylcholine or sodium nitroprusside (n = 6). #P < 0.005 and *P < 0.05 vs Lcatwt. L-NAME = NG-nitro-l-arginine methyl ester.
The response curves to L-NAME of aortas precontracted with (EC60)-NA (10− 7 M) showed a reduced increase in tone of aortic rings from LcatKO mice when compared with those from Lcatwt mice (P < 0.005, Fig. 1b), suggesting that reduced amounts of NO were modulating the final tone in LcatKO mice.
As expected, ACh caused a dose-dependent relaxation of aortic rings precontracted with (EC60)-NA (10− 7 M), but the two concentration-response curves were significantly different (P < 0.05) (Fig. 1c): while the tissue sensitivity to ACh was similar in both groups (pD2 values were 7.49 ± 0.17 in LcatKO and 7.45 ± 0.11 in Lcatwt, P > 0.05), the maximal vasodilation was significantly attenuated in LcatKO vs Lcatwt aortic rings (61.3 ± 4.8% vs 81.2 ± 4.5%, respectively, P = 0.01), again suggesting that reduced amounts of NO were released in response to ACh in LcatKO mice.
Finally, the aortic relaxation caused by SNP on aortas precontracted with (EC60)-NA (10− 7 M) did not differ between the two mouse lines (Fig. 1d), suggesting that the ability to relax in response to exogenous NO was preserved in LcatKO mice.
3.2. Endothelial NO synthase expression in Lcatwt and LcatKO mouse aortas
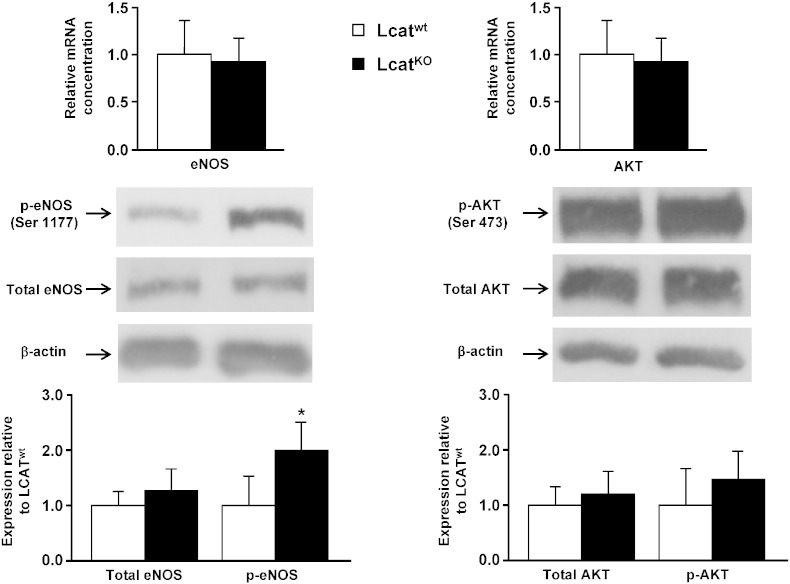
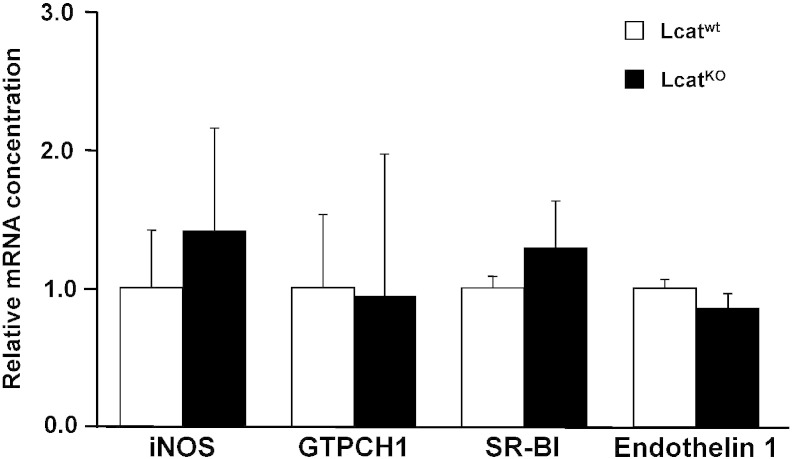
In order to understand if the reduced responsivity to ACh in LCATKO mice could relate to an impaired expression of endothelial NO synthase (eNOS), we studied the expression of eNOS in aortic tissues from LCATKO and LCATwt mice. Gene expression of eNOS and serine/threonine protein kinase Akt was not different between the two mouse lines (Fig. 2, top). Inducible NO synthase and GTP Cyclohydrolase 1, an essential enzyme for NO synthase activity and NO generation (Fig. 3), failed to show differences between groups, as well as SR-BI which mediates phosphorylation of endothelial nitric oxide synthase at Ser1177 upon binding to HDL (Fig. 3).
Fig. 2.
mRNA and protein expression of eNOS and AKT in aortas of Lcatwt and LcatKO mice. Top, relative mRNA concentrations of eNOS and AKT normalized to reference gene cyclophilin. Center, representative Western blots of total eNOS, Ser 1177 phosphorylated eNOS (p-eNOS), total AKT, and AKT phosphorylated at Ser 473 (p-AKT). Bottom, mean data of total eNOS, p-eNOS, AKT, and p-AKT expression values. Data are relative to the levels in Lcatwt animals set as 1 and expressed as mean ± SD (n = 6). *P < 0.005 vs Lcatwt.
Fig. 3.
Relative mRNA concentrations of inducible NO synthase (iNOS), GTP Cyclohydrolase 1 (GTPCH1), scavenger receptor class B type I (SR-BI) and Endothelin 1 in Lcatwt and LcatKO mouse aortas, normalized to reference gene cyclophilin. Data are relative to the levels in Lcatwt animals set as 1 and expressed as mean ± SD (n = 8).
Whereas total eNOS protein levels were comparable between groups, eNOS phosphorylation at serine residue 1177 was increased by about twofold in LcatKO aortas (P < 0.005, Fig. 2). Akt protein levels were unchanged between Lcatwt and LcatKO aortas, as well as Akt phosphorylation at serine residue 473 (Fig. 2).
3.3. Aortic expression of genes related to oxidative stress
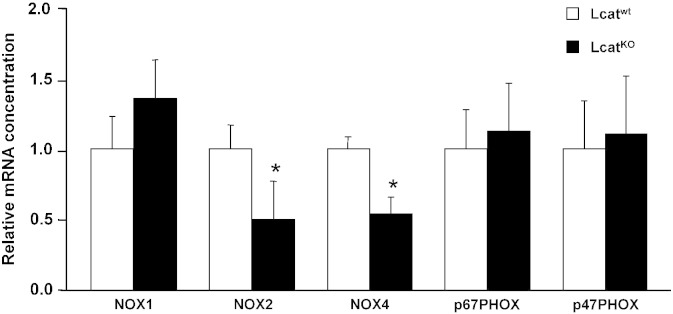
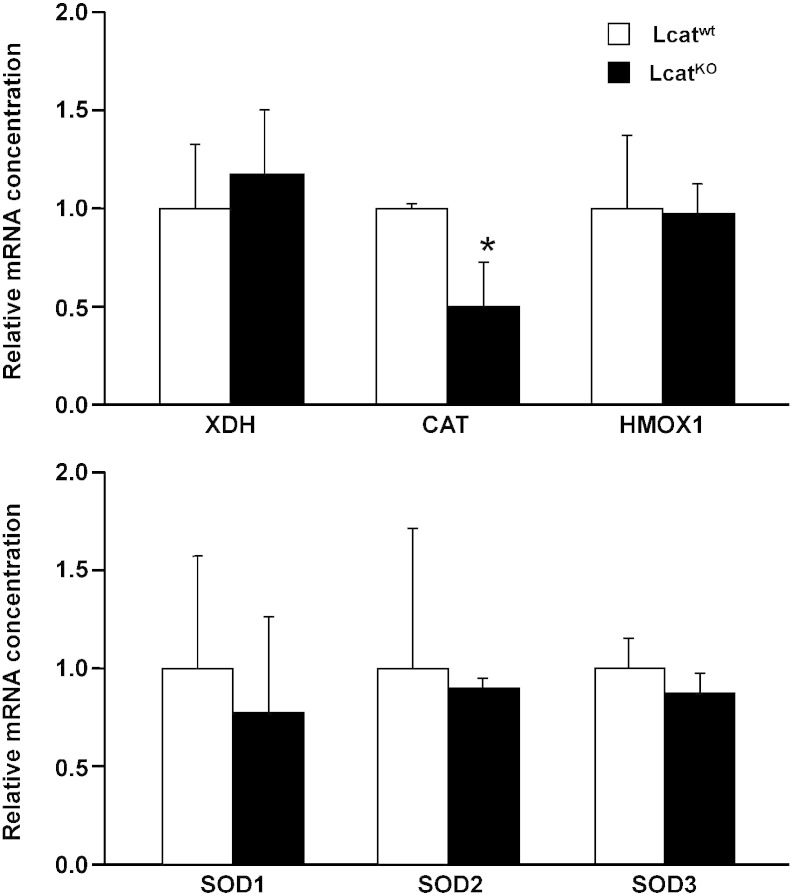
Aorta expression of the following oxidative stress-related genes, that could affect the final tissue availability of NO, was investigated by real-time PCR: the core catalytic subunits of NADPH oxidase 1, 2 and 4 together with the cytosolic activator p67phox and the cytosolic organizer p47phox; Xanthine dehydrogenase; Catalase; Heme oxygenase; Superoxide Dismutase 1, 2 and 3 (Fig. 4, Fig. 5).
Fig. 4.
Relative mRNA concentrations of NADPH oxidase isoform 1 (NOX1), 2 (NOX2), 4 (NOX4), cytosolic activator p67phox (p67PHOX), and cytosolic organizer p47phox (p47PHOX) in Lcatwt and LcatKO mouse aortas, normalized to reference gene cyclophilin. Data are relative to the levels in Lcatwt animals set as 1 and expressed as mean ± SD (n = 6 to 8). *P < 0.05 vs Lcatwt.
Fig. 5.
mRNA expression in aortas of Lcatwt and LcatKO mice, normalized to reference gene cyclophilin. Top, relative mRNA concentrations of Xanthine dehydrogenase (XDH), Catalase (CAT) and Heme Oxygenase 1 (HMOX1). Bottom, relative mRNA levels of Superoxide Dismutase 1 (SOD1), 2 (SOD2), and 3 (SOD3). Data are relative to the levels in LcatKO animals set as 1 and expressed as mean ± SD (n = 6 to 8). *P < 0.05 vs Lcatwt.
NADPH oxidase 2 and 4 were found down-regulated in LcatKO aortas (P < 0.05) and catalase was also down-regulated in LcatKO mice (P < 0.05). The expression of all the other genes was comparable between the two mouse lines (Fig. 4, Fig. 5).
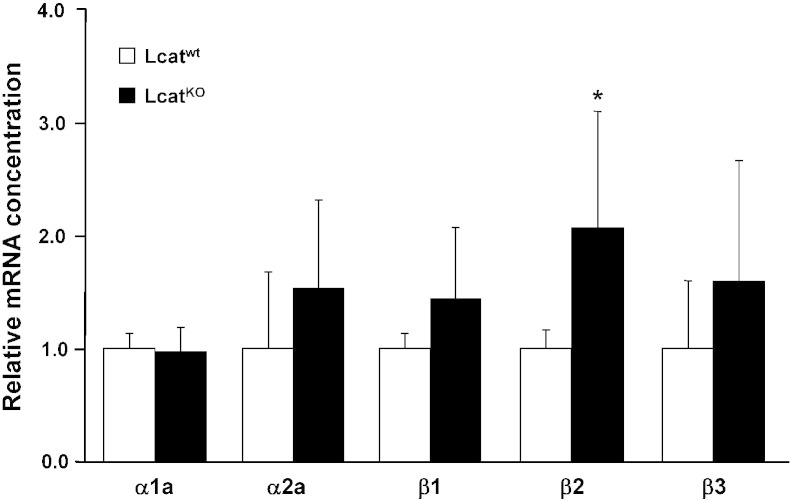
3.4. Adrenergic and muscarinic receptor expression in Lcatwt and LcatKO mouse aortas
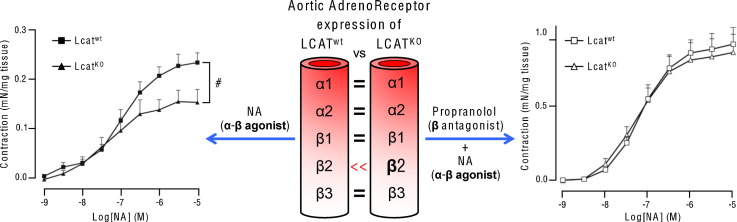
Expression of adrenergic receptor genes (α1a, α2a, β1, β2 and β3) was evaluated on total RNA extracted from mouse aortas (Fig. 6). Whereas α1a, α2a, β1 and β3 gene expression was unchanged between groups, β2-adrenergic receptor gene expression was significantly higher in LcatKO vs Lcatwt aortas (P < 0.01).
Fig. 6.
Relative mRNA concentrations of α1a, α2a, β1, β2 and β3 adrenergic receptors in Lcatwt and LcatKO mouse aortas, normalized to reference gene cyclophilin. Data are relative to the levels in Lcatwt animals set as 1 and expressed as mean ± SD (n = 6 to 8). *P < 0.01 vs Lcatwt.
M3-muscarinic receptor expression, also assessed by real-time PCR, was comparable in Lcatwt and LcatKO mice (1 ± 0.53 vs 1.31 ± 0.66, P > 0.05).
3.5. Effect of propranolol on vascular reactivity of Lcatwt and LcatKO aortic rings
In consideration of the gene expression results, which suggested a potential role of β2-adrenergic receptor in the different vascular responses of the two mouse lines, the contractile response to increasing concentrations of NA was assessed in the absence and in the presence of the selective β-adrenergic receptor antagonist d,l propranolol (10− 6 M). As already shown in Fig. 1, in the presence of NA a reduced contractility of LcatKO aortic rings was observed. Propranolol pretreatment caused an increased contractility in aortic rings of both genotypes, with a significantly larger effect on LcatKO mice, resulting in comparable dose–response curves to NA (Fig. 7a). Indeed, maximal response to NA in the presence of propranolol was 0.92 ± 0.06 mN/mg in Lcatwt and 0.87 ± 0.08 mN/mg in LcatKO (P = 0.22). Tissue sensitivity to NA in the presence of propranolol was again similar in both groups with pD2 values of 7.27 ± 0.22 in LcatKO and 7.17 ± 0.15 in Lcatwt (P = 0.21).
Fig. 7.
Concentration–response curves to noradrenaline (a), and L-NAME (b) in aortic rings isolated from Lcatwt and LcatKO mice. Responses of arterial rings to increasing concentrations of noradrenaline or L-NAME were obtained in the absence or presence of d,l-propranolol (10− 6 M) (n = 6). *P < 0.05 and #P < 0.01 vs Lcatwt. L-NAME = NG-nitro-l-arginine methyl ester.
The pretreatment with propranolol of aortic rings pre contracted with 10− 7 M NA and challenged with increasing doses (10− 6 M to 10− 4 M) of NOS inhibitor L-NAME also abolished the differences between the two genotypes, leading to similar responses (Fig. 7b).
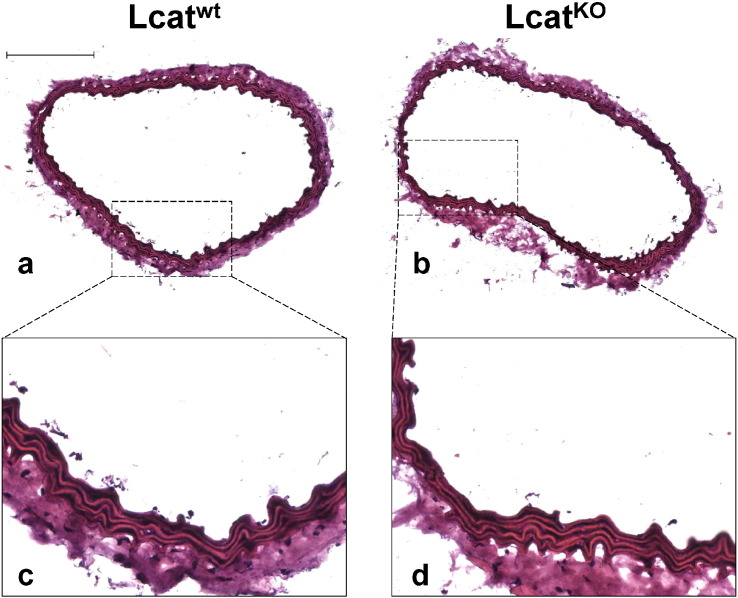
3.6. Lcatwt and LcatKO mouse aorta histology
Aortic rings used for ex vivo experiments were embedded in OCT compound, cut, stained with hematoxylin/eosin and evaluated for histology (Fig. 8). Each Lcatwt and LcatKO aortas showed 5 elastic laminae, and had a similar inner and external elastic laminae perimeter and area, thus medial area was also similar between the two groups. (Table 2).
Fig. 8.
Representative images of aorta cryosections isolated from Lcatwt and LcatKO mice (a, b), and relative 4 × magnification (c, d). Bar in top-left panel = 200 μm.
Table 2.
Histological parameters.
| Lcatwt | LcatKO | |
|---|---|---|
| IEL perimeter (μm) | 2255 ± 120 | 2087 ± 169 |
| EEL perimeter (μm) | 2350 ± 126 | 2134 ± 184 |
| IEL area (μm2) | 297,704 ± 64,155 | 255,473 ± 48,207 |
| EEL area (μm2) | 350,233 ± 68,952 | 300,875 ± 51,850 |
| Medial area (μm2) | 52,529 ± 4797 | 45,402 ± 3642 |
Data are expressed as mean ± SD (n = 5). IEL, inner elastic lamina; EEL, external elastic lamina. Media area is calculated as external elastic lamina area minus inner elastic lamina area.
4. Discussion
With the aim of understanding the impact of LCAT deficiency on the onset of atherosclerosis, the vascular reactivity of aortas from LcatKO mice was investigated ex vivo, by exposing aortic rings to different vasoactive molecules. Interestingly, the experiments highlighted an altered response to both contractile and relaxant stimuli. First of all, vessel contractility induced by NA was reduced in LcatKO vs Lcatwt mice. NA acts, with different affinity, as an agonist of different adrenergic receptors located in the arterial wall. Specifically, the contractile response to NA is mediated by the interaction with α-adrenergic receptors located in the smooth muscle cells (SMC) of the vessel [21], [22], [23], [24], and it is partially counterbalanced by relaxant stimuli associated with α2- and β-receptor activation. Alpha- and β-receptors expressed by endothelial cells mediate vasorelaxation through NO release via eNOS activation [24], [25], but β adrenergic receptors are also present in SMC, where they cause relaxation through a reduction in intracellular calcium concentration [26]. Based on these assumptions, the impaired NA-mediated contraction of LcatKO mouse aortas could be attributed to a lower α-mediated contraction, or to an increased α- or β-mediated relaxation.
The addition to NA of the non-selective eNOS inhibitor L-NAME did not overcome the lower contractility of LcatKO mouse aortas vs control mice, indicating that the impaired contraction is not due to an increased NO release, but, possibly, to a lower contractile α activity or to a higher relaxant β activity in SMC. Indeed, real-time PCR of adrenergic receptors showed a significantly increased β2 expression in LcatKO mouse aortas vs control, suggesting an increased β2 activity in SMC of LcatKO mice. To verify this hypothesis, vascular response to NA was assessed in the presence of the selective β-adrenergic receptor antagonist d,l propranolol that increased the contractility of LcatKO mouse aortas to values comparable to those of controls. Altogether, the results suggest that β2-receptor activity in SMC plays an important role in modulating the vascular tone in LcatKO mouse aortas. We do not have a mechanistic explanation for the observed increase of β2-receptor activity in LcatKO mice, but we can exclude that this effect is simply consequent to the hypoalphalipoproteinemic status of this mouse line. In preliminary experiments comparing LcatKO animals with wild-type and apolipoprotein A-I deficient mice, the latter were characterized by dramatically reduced HDL levels [27]. NA stimulation of aortas caused a contraction that was not different between wild-type and apolipoprotein A-I deficient mice. In LcatKO mice, NA-driven contraction was instead significantly lower compared to that of both wild-type and apolipoprotein A-I deficient mice (Fig. 1, Supplementary data).
An anomalous response of LcatKO mouse aortas was also observed when pre contracted aortic rings were exposed to increasing concentrations of ACh, i.e. a lower NO-mediated relaxation was detected vs that of control mice. This lower relaxation was neither associated to an altered response of SMC to NO dilatory stimulus, as shown by the vasodilatory response to a direct NO-donor, nor it was consequent to morphological abnormalities of the SMC layers of the vessel, as assessed by histology. It is therefore possible that the lower response to ACh of LcatKO mouse vessels could be the result of a reduced NO production/availability in LcatKO vs control aortas. We tested several hypotheses that could explain the reduced NO production/availability observed in LcatKO mice: considering that ACh interacts with endothelial M3-muscarinic receptors to enhance NO release and thereby cause vasodilation [28], we assessed M3 expression by real-time PCR, and found no difference between LcatKO and control mice. Impaired eNOS activity in LcatKO mice could be consequent to the severe hypoalphalipoproteinemia associated with this genetic defect [3], [12], [13]; HDL can indeed modulate eNOS activity through the endothelial SR-BI receptor [29] or through the HDL-associated sphingosine 1 Phosphate [30], both mechanisms leading to phosphorylation of eNOS Ser1177 and activation of the enzyme [17], [31], [32]. SR-BI expression was not different between the two mouse lines. Moreover, Western blot analyses of total eNOS and Ser1177 phosphorylated protein showed that total eNOS expression was comparable between the two mouse lines, whereas phosphorylated eNOS was even increased in LcatKO mice. Finally, no differences between LcatKO mice and controls were observed for mRNA levels, as well as total and phosphorylated protein expression of serine/threonine protein kinase Akt, a relevant kinase responsible for eNOS phosphorylation [33]. Taken together, all these experimental evidences seem to exclude the possibility of an impaired NO production in LcatKO mouse aorta.
The hypothesis of an increased oxidative stress was also explored, since this condition can lead to lower NO availability and impaired relaxation [34], [35], [36]. A previous report on this same genetically modified mouse line indicated an increased O2• production in LcatKO aortic rings. In the present study, real-time PCR performed on mouse aortas on the major oxidative or anti-oxidant enzyme [36] did not highlight a pro-oxidative status in LcatKO mouse aortas. Although an increased oxidative stress affecting ACh-mediated relaxation cannot be excluded, it should be noted that the pretreatment with propranolol was able to completely abolish the differences between LcatKO and Lcatwt. Interestingly, propranolol also normalized the response of LcatKO aortas pre contracted with NA and exposed to L-NAME, suggesting that, once corrected for the altered β2-adrenergic responsiveness, a similar NO production was clearly present in the two mouse lines.
Based on our experimental observation, we therefore hypothesize that NO production in LcatKO mouse aortas is modulated to provide a balanced vascular tone. In particular, LcatKO mouse aortas, being characterized by an elevated β2-mediated relaxation, maintain a low NO production in normal conditions, yielding a relatively normal vascular tone, but are able to increase NO production whenever required, i.e. when the β2-receptor activity is blocked and the vasocontraction is therefore enhanced.
5. Conclusions
In conclusion, the results indicate that LCAT deficiency, in a genetically modified mouse model, leads to an altered vascular reactivity, characterized by an increased β2-adrenergic relaxation and by a decreased NO-mediated vasodilation that can be reversed, whenever required, to guarantee a correct vascular tone. Altogether, it can be concluded that LCAT deficiency and its associated hypoalphalipoproteinemia do not seem to impair the endothelial function. It cannot be excluded that other routes of vascular tone regulation may be altered by LCAT deficiency. Interestingly, in preliminary experiments, a lower angiotensin II-mediated contraction was observed in LcatKO mouse aortas compared with Lcatwt mouse aortas (Fig. 2, Supplementary data). Further investigations will be required to explore the biological causes and consequences of the observed results.
The following are the supplementary data related to this article.
Concentration–response curves to NA in aortic rings isolated from Lcatwt, LcatKO and apoA-I deficient (apoA-IKO) male mice of 4–6 months of age. Data are shown as mean ± S.E.M. (n = 6). *P < 0.005 vs Lcatwt and apoA-IKO mice.
Concentration–response curves to angiotensin II (Ang-II) in aortic rings isolated from Lcatwt and LcatKO male mice of 6 months of age. Data are shown as mean ± S.E.M. (n = 3). *P < 0.001 vs Lcatwt mice.
Conflict of interest
The authors declare that no conflicts of interest exist.
Acknowledgments
We deeply acknowledge Mrs. Loredana Bonacina for her careful assistance in animal maintenance. This work was supported by a grant from Telethon (L. C., project # GGP07132).
Contributor Information
S. Manzini, Email: stefano.manzini@gmail.com.
C. Pinna, Email: christian.pinna@unimi.it.
M. Busnelli, Email: marco.busnelli@gmail.com.
P. Cinquanta, Email: paola.cinquanta84@gmail.com.
E. Rigamonti, Email: rigamonti.elena@hsr.it.
G.S. Ganzetti, Email: giuliaganzetti@gmail.com.
F. Dellera, Email: dellera.federica@gmail.com.
A. Sala, Email: angelo.sala@unimi.it.
L. Calabresi, Email: laura.calabresi@unimi.it.
G. Franceschini, Email: guido.franceschini@unimi.it.
C. Parolini, Email: cinzia.parolini@unimi.it.
G. Chiesa, Email: Giulia.Chiesa@unimi.it.
References
- 1.Jonas A. Lecithin cholesterol acyltransferase. Biochim. Biophys. Acta. 2000;1529:245–256. doi: 10.1016/s1388-1981(00)00153-0. [DOI] [PubMed] [Google Scholar]
- 2.Rousset X., Shamburek R., Vaisman B. Lecithin cholesterol acyltransferase: an anti- or pro-atherogenic factor? Curr. Atheroscler. Rep. 2011;13:249–256. doi: 10.1007/s11883-011-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calabresi L., Simonelli S., Gomaraschi M., Franceschini G. Genetic lecithin:cholesterol acyltransferase deficiency and cardiovascular disease. Atherosclerosis. 2012;222:299–306. doi: 10.1016/j.atherosclerosis.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Gordon T., Castelli W.P., Hjortland M.C., Kannel W.B., Dawber T.R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 5.Assmann G., Schulte H., von Eckardstein A., Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124(Suppl.: S11-S20) doi: 10.1016/0021-9150(96)05852-2. [DOI] [PubMed] [Google Scholar]
- 6.Kuivenhoven J.A., Pritchard H., Hill J., Frohlich J., Assmann G., Kastelein J. The molecular pathology of lecithin:cholesterol acyltransferase (LCAT) deficiency syndromes. J. Lipid Res. 1997;38:191–205. [PubMed] [Google Scholar]
- 7.Tietjen I., Hovingh G.K., Singaraja R., Radomski C., McEwen J., Chan E. Increased risk of coronary artery disease in Caucasians with extremely low HDL cholesterol due to mutations in ABCA1, APOA1, and LCAT. Biochim. Biophys. Acta. 1821;2012:416–424. doi: 10.1016/j.bbalip.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Kunnen S., Van Eck M. Lecithin:cholesterol acyltransferase: old friend or foe in atherosclerosis? J. Lipid Res. 2012;53:1783–1799. doi: 10.1194/jlr.R024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayyobi A.F., McGladdery S.H., Chan S., John Mancini G.B., Hill J.S., Frohlich J.J. Lecithin:cholesterol acyltransferase (LCAT) deficiency and risk of vascular disease: 25 year follow-up. Atherosclerosis. 2004;177:361–366. doi: 10.1016/j.atherosclerosis.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Hovingh G.K., Hutten B.A., Holleboom A.G., Petersen W., Rol P., Stalenhoef A. Compromised LCAT function is associated with increased atherosclerosis. Circulation. 2005;112:879–884. doi: 10.1161/CIRCULATIONAHA.105.540427. [DOI] [PubMed] [Google Scholar]
- 11.Calabresi L., Baldassarre D., Castelnuovo S., Conca P., Bocchi L., Candini C. Functional lecithin:cholesterol acyltransferase is not required for efficient atheroprotection in humans. Circulation. 2009;120:628–635. doi: 10.1161/CIRCULATIONAHA.108.818143. [DOI] [PubMed] [Google Scholar]
- 12.Sakai N., Vaisman B.L., Koch C.A., Hoyt R.F., Jr., Meyn S.M., Talley G.D. Targeted disruption of the mouse lecithin:cholesterol acyltransferase (LCAT) gene. Generation of a new animal model for human LCAT deficiency. J. Biol. Chem. 1997;272:7506–7510. doi: 10.1074/jbc.272.11.7506. [DOI] [PubMed] [Google Scholar]
- 13.Ng D.S., Francone O.L., Forte T.M., Zhang J., Haghpassand M., Rubin E.M. Disruption of the murine lecithin:cholesterol acyltransferase gene causes impairment of adrenal lipid delivery and up-regulation of scavenger receptor class B type I. J. Biol. Chem. 1997;272:15777–15781. doi: 10.1074/jbc.272.25.15777. [DOI] [PubMed] [Google Scholar]
- 14.Ng D.S., Maguire G.F., Wylie J., Ravandi A., Xuan W., Ahmed Z. Oxidative stress is markedly elevated in lecithin:cholesterol acyltransferase-deficient mice and is paradoxically reversed in the apolipoprotein E knockout background in association with a reduction in atherosclerosis. J. Biol. Chem. 2002;277:11715–11720. doi: 10.1074/jbc.M112320200. [DOI] [PubMed] [Google Scholar]
- 15.Furbee J.W., Jr., Sawyer J.K., Parks J.S. Lecithin:cholesterol acyltransferase deficiency increases atherosclerosis in the low density lipoprotein receptor and apolipoprotein E knockout mice. J. Biol. Chem. 2002;277:3511–3519. doi: 10.1074/jbc.M109883200. [DOI] [PubMed] [Google Scholar]
- 16.Calabresi L., Gomaraschi M., Franceschini G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler. Thromb. Vasc. Biol. 2003;23:1724–1731. doi: 10.1161/01.ATV.0000094961.74697.54. [DOI] [PubMed] [Google Scholar]
- 17.Kratzer A., Giral H., Landmesser U. High-density lipoproteins as modulators of endothelial cell functions: alterations in patients with coronary artery disease. Cardiovasc. Res. 2014;103:350–361. doi: 10.1093/cvr/cvu139. [DOI] [PubMed] [Google Scholar]
- 18.Luscher T.F., Landmesser U., von Eckardstein A., Fogelman A.M. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Cir. Res. 2014;114:171–182. doi: 10.1161/CIRCRESAHA.114.300935. [DOI] [PubMed] [Google Scholar]
- 19.Calabresi L., Gomaraschi M., Simonelli S., Bernini F., Franceschini G. HDL and atherosclerosis: insights from inherited HDL disorders. Biochim. Biophys. Acta. 1851;2015:13–18. doi: 10.1016/j.bbalip.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Marchesi M., Parolini C., Caligari S., Gilio D., Manzini S., Busnelli M. Rosuvastatin does not affect human apolipoprotein A-I expression in genetically modified mice: a clue to the disputed effect of statins on HDL. Br. J. Pharmacol. 2011;164:1460–1468. doi: 10.1111/j.1476-5381.2011.01429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrier G.O., White R.E. Enhancement of alpha-1 and alpha-2 adrenergic agonist-induced vasoconstriction by removal of endothelium in rat aorta. J. Pharmacol. Exp. Ther. 1985;232:682–687. [PubMed] [Google Scholar]
- 22.Alosachie I., Godfraind T. The modulatory role of vascular endothelium in the interaction of agonists and antagonists with alpha-adrenoceptors in the rat aorta. Br. J. Pharmacol. 1988;95:619–629. doi: 10.1111/j.1476-5381.1988.tb11684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topouzis S., Schott C., Stoclet J.C. Participation of endothelium-derived relaxing factor and role of cyclic GMP in inhibitory effects of endothelium on contractile responses elicited by alpha-adrenoceptor agonists in rat aorta. J. Cardiovasc. Pharmacol. 1991;18:670–678. doi: 10.1097/00005344-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Vanhoutte P.M. Endothelial adrenoceptors. J. Cardiovasc. Pharmacol. 2001;38:796–808. doi: 10.1097/00005344-200111000-00016. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald A., McLean M., MacAulay L., Shaw A.M. Effects of propranolol and L-NAME on beta-adrenoceptor-mediated relaxation in rat carotid artery. J. Auton. Pharmacol. 1999;19:145–149. doi: 10.1046/j.1365-2680.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka Y., Horinouchi T., Koike K. New insights into beta-adrenoceptors in smooth muscle: distribution of receptor subtypes and molecular mechanisms triggering muscle relaxation. Clin. Exp. Pharmacol. Physiol. 2005;32:503–514. doi: 10.1111/j.1440-1681.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- 27.Chiesa G., Parolini C., Canavesi M., Colombo N., Sirtori C.R., Fumagalli R. Human apolipoprotein A-I and A-II in cell cholesterol efflux: studies with transgenic mice. Arterioscler. Thromb. Vasc. Biol. 1998;18:1417–1423. doi: 10.1161/01.atv.18.9.1417. [DOI] [PubMed] [Google Scholar]
- 28.Khurana S., Chacon I., Xie G., Yamada M., Wess J., Raufman J.P. Vasodilatory effects of cholinergic agonists are greatly diminished in aorta from M3R −/− mice. Eur. J. Pharmacol. 2004;493:127–132. doi: 10.1016/j.ejphar.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Yuhanna I.S., Zhu Y., Cox B.E., Hahner L.D., Osborne-Lawrence S., Lu P. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 30.Nofer J.R., van der Giet M., Tolle M., Wolinska I., von Wnuck Lipinski K., Baba H.A. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mineo C., Yuhanna I.S., Quon M.J., Shaul P.W. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J. Biol. Chem. 2003;278:9142–9149. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- 32.Gonzales E., Kou R., Michel T. Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositide 3-kinase/Akt signaling pathways in vascular endothelial cells. J. Biol. Chem. 2006;281:3210–3216. doi: 10.1074/jbc.M510434200. [DOI] [PubMed] [Google Scholar]
- 33.Fulton D., Gratton J.P., McCabe T.J., Fontana J., Fujio Y., Walsh K. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou M.H., Shi C., Cohen R.A. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landmesser U., Dikalov S., Price S.R., McCann L., Fukai T., Holland S.M. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. - Eur. J. Physiol. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Concentration–response curves to NA in aortic rings isolated from Lcatwt, LcatKO and apoA-I deficient (apoA-IKO) male mice of 4–6 months of age. Data are shown as mean ± S.E.M. (n = 6). *P < 0.005 vs Lcatwt and apoA-IKO mice.
Concentration–response curves to angiotensin II (Ang-II) in aortic rings isolated from Lcatwt and LcatKO male mice of 6 months of age. Data are shown as mean ± S.E.M. (n = 3). *P < 0.001 vs Lcatwt mice.