Abstract
Background:
Human papillomavirus (HPV)–based cervical cancer screening requires triage markers to decide who should be referred to colposcopy. p16/Ki-67 dual stain cytology has been proposed as a biomarker for cervical precancers. We evaluated the dual stain in a large population of HPV-positive women.
Methods:
One thousand five hundred and nine HPV-positive women screened with HPV/cytology cotesting at Kaiser Permanente California were enrolled into a prospective observational study in 2012. Dual stain cytology was performed on residual Surepath material, and slides were evaluated for dual stain–positive cells. Disease endpoints were ascertained from the clinical database at KPNC. We evaluated the clinical performance of the assay among all HPV-positive women and among HPV-positive, cytology-negative women. We used internal benchmarks for clinical management to evaluate the clinical relevance of the dual stain assay. We evaluated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the dual stain compared with Pap cytology. All statistical tests were two-sided.
Results:
The dual stain had lower positivity (45.9%) compared with cytology at an ASC-US threshold (53.4%). For detection of CIN2+, the dual stain had similar sensitivity (83.4% vs 76.6%, P = .1), and statistically higher specificity (58.9% vs 49.6%, P < .001), PPV (21.0% vs 16.6%, P < .001), and NPV (96.4% vs 94.2%, P = .01) compared with cytology. Similar patterns were observed for CIN3+. Women with a positive test had high enough risk for referral to colposcopy, while the risk for women with negative tests was below a one-year return threshold based on current US management guidelines.
Conclusion:
Dual stain cytology showed good risk stratification for all HPV-positive women and for HPV-positive women with normal cytology. Additional follow-up is needed to determine how long dual stain negative women remain at low risk of precancer.
The understanding that infections with carcinogenic types of human papillomaviruses (HPV) are a necessary cause of almost all cervical cancers has led to development of new cervical cancer screening strategies (1). Cervical cancer screening guidelines in the United States recommend HPV testing with cytology cotesting as the primary screening option over cytology alone (2). Recently, the US Food and Drug Administration (FDA) approved an HPV DNA test for primary screening without concomitant cytology testing (3). HPV DNA testing has higher sensitivity and negative predictive value (NPV) for detection of cervical precancer and cancer compared with cytology, which gives reassurance that HPV-negative women are at very low risk of cervical cancer over multiple years and allows extending screening intervals (4–6). However, many HPV infections resolve spontaneously after a few months and pose very little cancer risk, reducing specificity of HPV testing.
Adoption and success of HPV-based screening depends on the effective management of HPV-positive women. It is neither feasible nor efficient to send all HPV-positive women to colposcopy, given that most do not have concurrent lesions. Current algorithms use HPV genotyping for HPV16 and HPV18 in conjunction with cytology to decide who among the HPV-positive women needs colposcopy (2). Other biomarkers have been proposed for triage of HPV-positive women, including p16 (7,8). A cytological p16 assay has been previously evaluated for triage of HPV-positive women, but it required morphological evaluation of p16-stained cells (9). Concurrent p16 and Ki-67 staining on cytological slides eliminates the need for morphological evaluation (10,11). The p16/Ki-67 dual stain has shown promise for detection of cervical precancer (12), but evaluation of the assay for triage of HPV-positive women in a large population is lacking. We evaluated p16/Ki-67 for detection of cervical precancer in women undergoing screening at Kaiser Permanente Northern California (KPNC), specifically for triage of: 1) all HPV-positive women and 2) HPV-positive/cytology-negative women.
Methods
Study Population
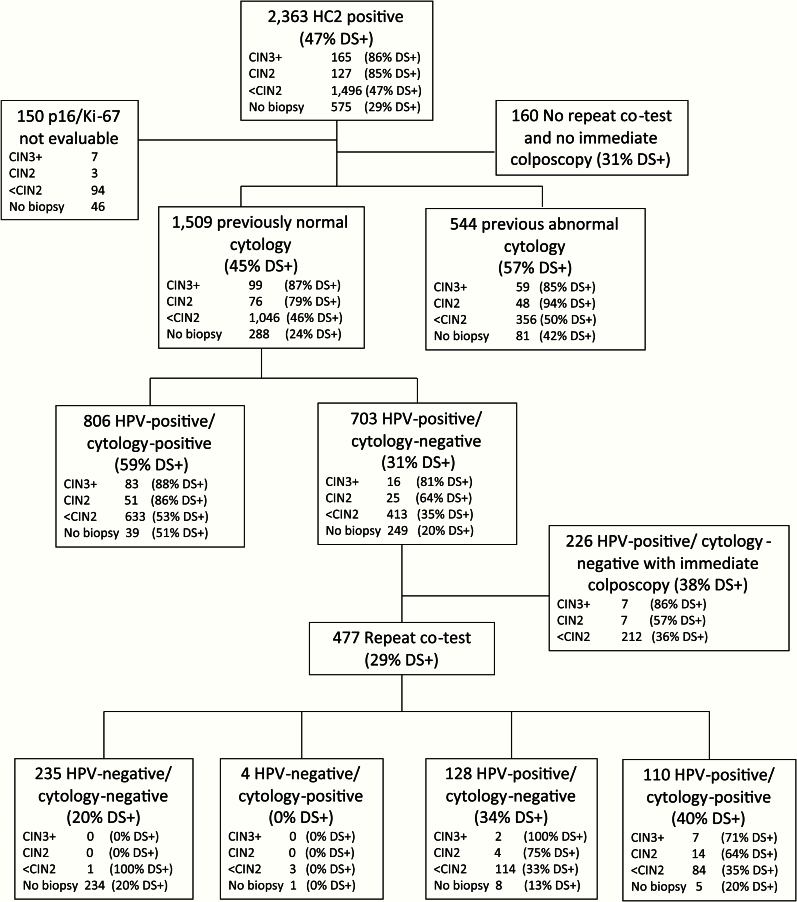
This prospective observational study was nested into routine cervical cancer screening at KPNC, where HPV and cytology cotesting was implemented in 2003 (13). At the time of this study, HPV cytology cotesting was restricted to women age 30 years and older. Women with negative HPV and cytology results are advised to return to regular screening after three years. Women with HPV-positive atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial lesions (LSIL), or more severe cytology are referred to colposcopy immediately. Women with HPV-positive but cytology-negative results undergo repeat cotesting after one year and are referred to colposcopy when either component of the test is positive. Management was not based on the dual stain results. Between March and May 2012, discard SurePath cytology specimens were collected at KPNC from 2363 HPV-positive women age 30 years and older. We followed women until they were referred to colposcopy or until they had a repeat cotest with normal results. Ascertainment of disease endpoints using electronic medical records was conducted through January 2014 to account for delayed colposcopy in some HPV-positive, cytology-negative women (Figure 1). We restricted the population to 1509 women who had an evaluable dual stain test, who had a follow-up result, and who had a previous negative Pap result, to exclude women managed for previous abnormal screening results or after treatment (Figure 1). The study was approved by the KPNC Institutional Review Board and was exempted from institutional review at the National Cancer Institute by the Office of Human Subjects Research.
Figure 1.
CONSORT diagram of the study population. DS+ = dual stain–positive subjects; HC2 = Hybrid Capture 2; HPV = human papillomavirus.
HPV Testing and Cytology
Hybrid Capture 2 (HC2; Qiagen Inc., Gaithersburg, MD) was conducted on specimen transport medium (STM) specimens per the manufacturer’s instructions. Pap samples were collected in SurePath (Becton Dickinson, Sparks, MD) fixative. SurePath slides were prepared, stained, and processed on the BD FocalPoint Slide Profiler. Medical history, HPV results, and FocalPoint results were transmitted to the cytotechnologists reviewing slides on Guided Screening (GS) microscopes. All HPV-positive women were evaluated by GS-assisted screening and full manual review. All abnormal slides were sent for pathology review. In addition, all negative Paps from HPV-positive women were rescreened manually.
p16/Ki-67 Dual Stain
Slides for p16/Ki-67 staining were produced at the manufacturer’s laboratory from the residual enriched cell pellet of SurePath specimens stabilized with CytoRich Fluid (BD) within one to four months of sample collection. The CINtec PLUS Cytology kit (Roche mtm Laboratories AG, Mannheim, Germany) was used according to the manufacturer’s instructions. Staining was performed on a Dako Autostainer using the staining program for SurePath slides, followed by hematoxylin counterstaining. Each staining run included at least two control specimens. All slides were evaluated by an expert cytotechnologist, using a semiquantitative assessment of number of positive cells (0, 1, 2–5, 6–50, >50). 150 slides were considered not evaluable, mostly because of background staining, which is typically related to extended storage.
Disease Endpoints
All women undergoing colposcopy at KPNC should have at least one biopsy taken, with the majority of women receiving multiple biopsies to improve ascertainment of cervical precancer (14). Histological evaluation was based on the cervical intraepithelial neoplasia (CIN) classification. We evaluated two clinical endpoints, detection of CIN2 or more severe diagnoses (CIN2+) and detection of CIN3, cervical adenocarcinoma in situ (AIS), or cervical cancer (CIN3+).
Statistical Analysis
Contingency tables and χ2 trend tests were used to evaluate dual stain positivity in cytology (negative, ASC-US, LSIL, atypical squamous cells, cannot rule out high-grade squamous intraepithelial lesion [ASC-H], and high-grade squamous intraepithelial lesions [HSIL])–histology (no biopsy, benign, CIN1, CIN2, CIN3/cancer) strata. McNemar’s test was used to evaluate the difference in referral rates between dual stain and cytology. We evaluated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), likelihood ratio positive, likelihood ratio negative, and odds ratios (ORs) of the dual stain compared with Pap cytology at an HPV-positive ASC-US threshold for detection of CIN2+ and CIN3+ during two years of follow-up. Differences in sensitivity and specificity were evaluated using an exact McNemar’s χ2. Differences in PPV and NPV were evaluated using the method developed by Leisenring and Pepe with the package ‘DTComPair’ in R (15). We evaluated increasing thresholds of dual stain–positive cells (1+, 2+, and 5+) for detection of CIN3+ and calculated the Youden’s index (YI = sensitivity+specificity-1) for different thresholds. We compared the risk of disease in dual stain–positive women (PPV) and the risk of disease in dual stain–negative women (complement of the NPV, cNPV) to internal risk benchmarks (8,16,17). A PPV higher than the established colposcopy referral thresholds suggests that women with a positive test result should be referred to colposcopy. A cNPV below the one-year return threshold suggests that women with a negative test result could return after an extended period of time. All analyses were run in Stata 13 (Statacorp, College Station, TX) and R version 3.1.1. All statistical tests were two-sided.
Results
Study Population
Of 1509 HPV-positive women, seven had cancer, six had AIS, 86 had CIN3, 76 had CIN2, 1046 had less than CIN2 histology, and 288 did not have a colposcopic biopsy (Table 1). Eight hundred and six women (53%) had a positive cytology result, while 703 women (47%) had a HPV-positive cytology-negative cotest result. Among these women, 226 underwent immediate colposcopy and 477 had a one-year repeat cotest (Figure 1). The repeat cotest was negative for 235 (49.3%), HPV positive with normal cytology for 128 (26.8%), cytology positive with negative HPV for four (0.8%), and HPV and cytology positive for 110 (23.1%) of the 477 women. One hundred sixty of the 175 CIN2 or greater were detected at the first colposcopy visit (91%).
Table 1.
p16/Ki-67 positivity by histology and cytology results*
| Cytology result | Total (n = 1509) | No biopsy (n = 288) | Benign (n = 417) | Atypical (n = 62) | CIN1 (n = 567) | CIN2 (n = 76) | CIN3/AIS (n = 92) | Cancer (n = 7) |
|---|---|---|---|---|---|---|---|---|
|
No.
(%) |
No.
(%) |
No.
(%) |
No.
(%) |
No.
(%) |
No.
(%) |
No.
(%) |
No.
(%) |
|
| Negative | 703 (46.59) |
249 (86.46) |
198 (47.48) |
20 (32.26) |
195 (34.39) |
25 (32.89) |
15 (16.30) |
1 (14.29) |
| p16/Ki-67+ | 271 (31.40) |
98 (23.96) |
67 (33.84) |
8 (40.00) |
69 (35.38) |
16 (64.00) |
12 (80.00) |
1 (100.00) |
| ASC-US | 383 (25.38) |
22 (7.64) |
136 (32.61) |
21 (33.87) |
165 (29.10) |
19 (25.00) |
20 (21.74) |
0 (0.00) |
| p16/Ki-67+ | 186 (48.56) |
8 (36.36) |
59 (43.38) |
8 (38.10) |
82 (49.70) |
15 (78.95) |
14 (70.00) |
0 (0.00) |
| LSIL | 319 (21.14) |
15 (5.21) |
65 (15.59) |
18 (29.03) |
181 (31.92) |
22 (28.95) |
17 (18.48) |
1 (14.29) |
| p16/Ki-67+ | 200 (62.70) |
10 (66.67) |
34 (52.31) |
10 (55.56) |
109 (60.22) |
20 (90.91) |
16 (94.12) |
1 (100.00) |
| ASC-H | 36 (2.39) |
1 (0.35) |
8 (1.92) |
1 (1.61) |
16 (2.82) |
5 (6.58) |
5 (5.43) |
0 (0.00) |
| p16/Ki-67+ | 27 (75.00) |
1 (100.00) |
5 (62.50) |
1 (100.00) |
11 (68.75) |
5 (100.00) |
4 (80.00) |
0 (0.00) |
| HSIL | 49 (3.25) |
0 (0.00) |
5 (1.20) |
1 (1.61) |
6 (1.06) |
5 (6.58) |
28 (30.43) |
4 (57.14) |
| p16/Ki-67+ | 45 (91.84) |
0 (0.00) |
4 (80.00) |
1 (100.00) |
5 (83.33) |
4 (80.00) |
27 (96.43) |
4 (100.00) |
| Other | 19 (1.26) |
1 (0.35) |
5 (1.20) |
1 (1.61) |
4 (0.71) |
0 (0.00) |
7 (7.61) |
1 (14.29) |
| p16/Ki-67+ | 14 (73.68) |
1 (100.00) |
4 (80.00) |
1 (100.00) |
1 (25.00) |
0 (0.00) |
6 (85.71) |
1 (100.00) |
| Total | 1,509 (100.0) |
288 (19.09) |
417 (27.63) |
62 (4.11) |
567 (37.57) |
76 (5.04) |
92 (6.10) |
7 (0.46) |
| p16/Ki-67+ | 694 (45.99) |
69 (23.96) |
173 (41.49) |
29 (46.77) |
277 (48.85) |
60 (78.95) |
79 (85.87) |
7 (100.00) |
* P trend % p16/Ki-67+ across cytology categories (excluding Other): P < .001; P trend % p16/Ki-67+ across histology categories (excluding Atypical): P < .001. AIS = cervical adenocarcinoma in situ; ASC-H = atypical squamous cells, cannot rule out high-grade squamous intraepithelial lesion; ASC-US = atypical squamous cells of undetermined significance; CIN = cervical intraepithelial neoplasia; LSIL = low-grade squamous intraepithelial lesions; HSIL = high-grade squamous intraepithelial lesions.
Dual Stain Positivity by Cytology and Histology Results
Among 1509 HPV-positive women with a previous normal cytology result, 703 (46.6%) had negative, 383 (25.4%) had ASC-US, 319 (21.1%) had LSIL, 36 (2.4%) had ASC-H, and 49 (3.3%) had HSIL cytology results (Table 1). Dual stain positivity increased with increasing severity of cytology from 31% in HPV-positive women with negative cytology to 92% in women with HSIL cytology (P < .001). Dual stain positivity increased from 24% in women without biopsy results to 86% in women with CIN3 (P < .001). All women with cancer were dual stain–positive.
Performance of Dual Stain and Cytology for Detection of CIN2+ and CIN3+
Fewer of these HPV-positive women were positive for the dual stain (694, 45.9%) compared with cytology at an ASC-US threshold (806, 53.4%, P < .001) (Table 2). For detection of CIN2+, the dual stain had significantly higher specificity (58.9% vs 49.6%, P < .001), PPV (21.0% vs 16.6%, P < .001), and NPV (96.4% vs 94.2%, P = .01) compared with cytology. The dual stain and cytology had similar sensitivity (83.4% vs 76.6%, P = .1). These patterns were similar for the CIN3+ endpoint, with higher specificity (56.9% vs 48.7%, P < .001) and PPV (12.4% vs 10.3%, P = .002) for the dual stain compared with cytology.
Table 2.
Clinical performance of dual stain and Pap cytology for detection of cervical precancer among 1509 HPV-positive women*
| Statistical measures | p16/Ki-67 dual stain 1+ (95% CI) |
Liquid-based cytology ASCUS+ (95% CI) | P |
|---|---|---|---|
| Positivity | 694 (46.0% [43.5% to 48.5%]) | 806 (53.4% [50.9% to 55.9%]) | <.001 |
| Detection of CIN2+ (n = 175) | |||
| Sensitivity | 83.4% (77.1% to 88.6%) | 76.6% (69.6% to 82.6%) | .1 |
| Specificity | 58.9% (56.2% to 61.6%) | 49.6% (46.9% to 52.3%) | <.001 |
| PPV | 21.0% (18.1% to 24.3%) | 16.6% (14.1% to 19.4%) | <.001 |
| NPV | 96.4% (94.9% to 97.6%) | 94.2% (92.2% to 95.8%) | .01 |
| LR+ | 2.0 (1.9 to 2.2) | 1.5 (1.4 to 1.7) | |
| LR- | 0.3 (0.2 to 0.4) | 0.5 (0.4 to 0.6) | |
| OR | 7.2 (4.8 to 11.0) | 3.2 (2.2 to 4.6) | |
| Detection of CIN3+ (n = 99) | |||
| Sensitivity | 86.9% (78.6% to 92.8%) | 83.8% (75.1% to 90.5%) | .7 |
| Specificity | 56.9% (54.2% to 59.5%) | 48.7% (46.1% to 51.4%) | <.001 |
| PPV | 12.4% (10.0% to 15.1%) | 10.3% (8.3% to 12.6%) | .002 |
| NPV | 98.4% (97.3% to 99.1%) | 97.7% (96.3% to 98.7%) | .3 |
| LR+ | 2.0 (1.8 to 2.2) | 1.6 (1.5 to 1.8) | |
| LR- | 0.2 (0.1 to 0.4) | 0.3 (0.2 to 0.5) | |
| OR | 8.7 (4.9 to 15.7) | 4.9 (2.9 to 8.5) | |
* P values for positivity, sensitivity, and specificity are based on McNemar’s Chi-square test. P values for positive predictive value and negative predictive value are based on the method developed by Leisenring and Pepe (14). All statistical tests were two-sided. LR+ = likelihood ratio–positive; LR- = likelihood ratio–negative; NPV = negative predictive value; PPV = positive predictive value; OR = odds ratio.
Dual Stain Performance at Increasing Thresholds of Dual Stain–Positive Cells
We evaluated the dual stain assay at higher thresholds of numbers of dual stain–positive cells and compared the performance for detection of CIN3+ to cytology and to the dual stain at the usual cutoff (Table 3). Increasing the threshold to two or more dual stain–positive cells led to a substantial reduction in test positivity compared with cytology and the dual stain assay at the usual cutoff. Sensitivity of the dual stain assay at the two-cell cutoff was almost identical to the sensitivity of cytology (82.8% vs 83.8%, P = 1.0), while the specificity was statistically increased (62.8% vs 48.7%, P < .001). There was a significant increase in PPV for the dual stain assay at the two-cell cutoff compared with cytology (13.5% vs 10.3%, P ≤ .001), while there was no significant difference in NPV (98.1% vs 98.4%, P = .5). Compared with the dual stain at the usual cutoff, the sensitivity at the two-cell cutoff was lower (82.8% vs 86.9%, P = .046), while both specificity (62.8% vs 56.9%, P < .001) and PPV (13.5% vs 12.4%, P = .001) were statistically significantly higher. At a cutoff of five or more dual stain–positive cells, the sensitivity for detection of CIN3+ was statistically significantly lower compared with both cytology and the dual stain assay at the usual cutoff, while the specificity and the PPV were statistically significantly increased.
Table 3.
Test performance of cytology and increasing thresholds of p16/Ki-67 positivity for the detection of CIN3+*
| CIN3+ (n = 99) | Positivity, % | P† | P‡ | Sensitivity, % (95% CI) |
P† | P‡ | Specificity, % (95% CI) |
P† | P‡ | PPV, % (95% CI) |
P† | P‡ | NPV, % (95% CI) |
P† | P‡ | Youden’s Index |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytology (n = 806) | 53.4 | Ref | NA | 83.8 (75.1 to 90.5) |
Ref | NA | 48.7 (46.1 to 51.4) |
Ref | NA | 10.3 (8.3 to 12.6) |
Ref | NA | 98.4 (97.3 to 99.1) |
Ref | NA | 0.33 |
| p16/Ki-67 1+ (n = 694) |
45.9 | <.001 | Ref | 86.9 (78.6 to 92.8) |
.7 | Ref | 56.9 (54.2 to 59.5) |
<.001 | Ref | 12.4 (10.0 to 15.1) |
.002 | Ref | 98.4 (97.3 to 99.1) |
.3 | Ref | 0.44 |
| p16/Ki-67, 2+ (n = 607) |
40.2 | <.001 | <.001 | 82.8 (73.9 to 89.7) |
1.0 | .046 | 62.8 (60.2 to 65.3) |
<.001 | <.001 | 13.5 (10.9 to 16.5) |
<.001 | .001 | 98.1 (97.0 to 98.9) |
.5 | .2 | 0.46 |
| p16/Ki-67, 5+ (n = 362) |
24.0 | <.001 | <.001 | 65.7 (55.4 to 74.9) |
.003 | <.001 | 78.9 (76.7 to 81.0) |
<.001 | <.001 | 18.0 (14.1 to 22.3) |
<.001 | <.001 | 97.0 (95.9 to 97.9) | .3 | <.001 | 0.45 |
* Cytology cutoff: ASCUS+. NPV = negative predictive value; PPV = positive predictive value.
† Comparison with cytology.
‡ Comparison with p16/Ki-67 1+. P values for positivity, sensitivity, and specificity are based on McNemar’s Chi-square test. P values for PPV and NPV are based on the method developed by Leisenring and Pepe (15). All statistical tests were two-sided.
Triage of HPV-Positive Women With Negative Cytology
We evaluated the performance of dual stain cytology to triage women with HPV-positive, cytology-negative results. Dual stain positivity among 703 HPV-positive, cytology-negative women was 31.6% (Table 4). During follow-up, 25 CIN2 and 16 CIN3 were diagnosed. The sensitivity and specificity of the dual stain assay for CIN2+ were 70.7% (95% CI = 54.3% to 83.4%) and 70.8% (95% CI = 67.2% to 74.3%), respectively. PPV and NPV were 13.1% (95% CI = 9.1% to 18.4%) and 97.5% (95% CI = 95.6% to 98.9%), respectively. For a CIN3+ endpoint, the sensitivity and specificity were 81.3% (95% CI = 53.7% to 95.0%) and 69.6% (95% CI = 66.0% to 73.0%), respectively. The PPV and NPV were 5.9% (95% CI = 3.3% to 10.0%) and 99.4% (95% CI = 98.0% to 99.8%), respectively.
Table 4.
Test performance of p16/Ki-67 for triage of HPV-positive/cytology-negative women*
| Test | Endpoint | Sensitivity, % (95% CI) |
Specificity, % (95% CI) |
PPV, % (95% CI) |
NPV, % (95% CI) |
Positivity, % |
|---|---|---|---|---|---|---|
| p16/Ki-67 (n = 703) | CIN2+ (n = 41) | 70.7 (54.3 to 83.4) |
70.8 (67.2 to 74.3) |
13.1 (9.1 to 18.4) |
97.5 (95.6 to 98.9) |
31.6 |
| CIN3+ (n = 16) | 81.3 (53.7 to 95.0) |
69.6 (66.0 to 73.0) |
5.9 (3.3 to 10.0) |
99.4 (98.0 to 99.8) |
* NPV = negative predictive value; PPV = positive predictive value.
Risk Stratification of the Dual Stain Assay Compared With Internal Risk Benchmarks
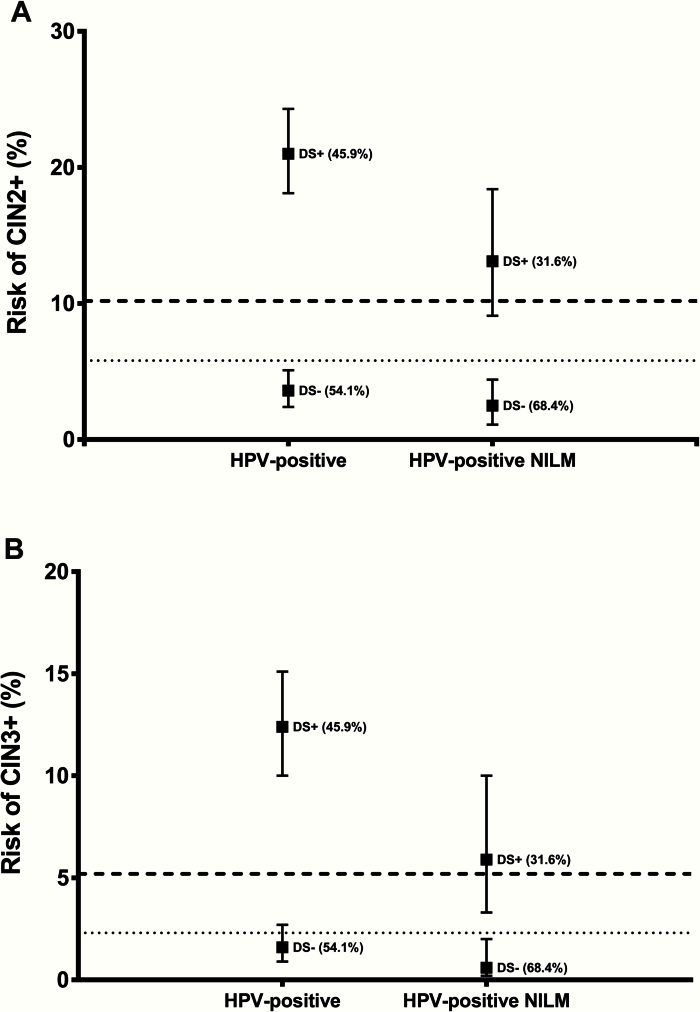
We compared the risk of precancer in women positive and negative for the dual stain with established management thresholds based on current US management guidelines (Figure 2). The risk of CIN2+ (10.2%) and CIN3+ (5.2%) in HPV-positive women with ASC-US in this population is used as benchmark for referral to colposcopy; the risk of CIN2+ (5.8%) and CIN3+ (2.3%) in HPV-positive women with normal cytology is used as a benchmark for a one-year return. Among all HPV-positive women, the risk of CIN2+ and CIN3+ in dual stain–positive women was clearly above the colposcopy referral threshold. The point estimates of the risk of CIN2+ and CIN3+ in dual stain–negative women were below the threshold for a one-year return. Among HPV-positive women with negative cytology, the risk in dual stain–positive women was above the colposcopy referral threshold for both endpoints, while the risk in dual stain negatives was clearly below the one-year return threshold.
Figure 2.
Risk of cervical precancer according to dual stain result in relation to clinical management thresholds. Risk of CIN2 or greater (A) and CIN3 or greater (B) in all HPV-positive women and in HPV-positive/cytology-negative women according to dual stain results. Point estimates of absolute risk (positive predictive value for DS-positive women, complement of the negative predictive value for DS-negative women) are shown as boxes with vertical lines indicating 95% confidence intervals. Percent estimates in parenthesis indicate the proportion of dual stain positive and dual stain negative women. The dashed line indicates the risk in women with HPV-positive ASC-US (threshold for referral to colposcopy), and the dotted line indicates the risk in HPV-positive/cytology-negative women (threshold for one-year return). DS+ = dual stain; HPV = human papillomavirus; NILM = negative for intraepithelial lesion or malignancy.
Discussion
Cervical cancer screening based on primary HPV testing, alone or in conjunction with cytology, has been successfully evaluated in clinical trials (18–21). Recently, the FDA approved a primary screening indication for an HPV assay (3). HPV-negative women are at lower risk of cervical precancer and cancer over several years compared with women with negative cytology results, allowing extended screening intervals (4,5). However, HPV testing doubles the screen-positive population compared with cytology-based screening. Successful implementation of primary HPV screening depends on triage strategies that reduce colposcopy referral while maintaining high sensitivity for cervical precancer (8).
We evaluated the clinical performance of the p16/Ki-67 dual stain assay for detection of cervical precancer in a large population of women undergoing HPV cytology cotesting. The dual stain assay was positive in 46% of all HPV-positive women, which is similar to currently accepted referral rates for HPV cytology cotesting (22) and to a previous evaluation of p16 cytology to triage HPV-positive women (9). The sensitivity of the dual stain assay for detection of CIN3 or greater was similar to sensitivity estimates of p16 or dual stain cytology from previous studies in other populations (9–12) and higher than the sensitivity reported for a combination of HPV16/18 genotyping and cytology (8,22).
In the KPNC population, the dual stain assay had a lower positivity and both a higher sensitivity and specificity for detection of precancer compared with cytology at an ASC-US threshold, which resulted in higher PPV and NPV of the dual stain assay compared with cytology. At KPNC, liquid-based cytology is evaluated using computer-assisted imaging, followed by cytotechnologist review with knowledge of HPV test results. In addition, quality control protocols require 100% rescreening of all HPV-positive slides with normal cytology results. As a result of the rigorous cytology evaluation, the sensitivity of cytology at KPNC is much higher compared with other settings. For example, the ATHENA trial reported an overall sensitivity of 53.3% for detection of CIN3+ (22), with sensitivity ranging from 41.5% to 73.4% for individual laboratories (23). Our data demonstrate that dual stain cytology could achieve equal or better performance compared with KPNC cytology, with shorter turnaround and potentially lower program cost. A formal cost-effectiveness analysis needs to account for the assay cost, the program cost for a specific technology, and the implications of the assay on downstream management, particularly repeat testing and colposcopy referral. It is likely that in other settings with worse cytology performance compared with KPNC, the incremental value of the dual stain over cytology is even higher, as suggested by Ikenberg et al. (10).
We evaluated the absolute risk of cervical precancer in women positive and negative for the dual stain assay against established US management thresholds (16,17). In the HPV-positive population, the risk of precancer in dual stain–positive women was much higher (12.4% for CIN3+) than the established risk threshold for referral to colposcopy (5.2% for CIN3+). The risk of precancer in dual stain–negative women was lower (1.6% for CIN3+) than the established threshold for a one-year return (2.3% for CIN3+). These data suggest that the dual stain assay can be used to triage HPV-positive women, sending dual stain–positive women to immediate colposcopy and retesting dual stain–negative women after up to two years. These estimates are similar to a recent report from Carozzi et al. (24).
In the United States, the only FDA-approved protocol for primary HPV screening includes HPV genotyping and cytology for triage. In this algorithm, women testing positive for HPV16/18 are referred to colposcopy, as are women testing positive for other HR types and ASC-US or greater. Women positive for other HR types and negative cytology are followed up after 12 months. In the current study, among women with an HPV-positive/cytology-negative result the risk of precancer in dual stain–positive women surpassed the colposcopy referral threshold, while the risk in dual stain–negative women was clearly below the threshold for a one-year return, suggesting that dual stain cytology could be used to triage HPV-positive/cytology- negative women. Larger studies in HPV-positive/cytology-negative women are needed for more precise risk estimates.
Our analysis has several important strengths: We evaluated a large population with uniform and well-organized screening and management procedures, excellent disease ascertainment and little loss to follow-up. Furthermore, the risk levels from KPNC for different combinations of cytology and HPV were the basis of current US screening and management guidelines, allowing us to directly compare dual stain results to established management thresholds. A potential weakness of our study is the differential follow-up of HPV-positive/cytology-positive and HPV-positive/cytology-negative women, with a one-year delay in the latter group. Also, management guidelines were not always followed exactly, reflecting practice in routine cervical cancer screening programs rather than in a tightly controlled clinical trial. However, we had a high completion of follow-up procedures over the two-year period, which captured most of the disease in this population during that interval. Follow-up is ongoing through the electronic medical records and will be important to determine how long a dual stain–negative test result predicts a low risk of cervical precancer. While absolute risk levels in KPNC differ from some clinical trials, it has been demonstrated that the risk benchmarking approach that we use (comparing dual stain results and their management to the current handling of cytology and HPV results with the principle of “similar management of similar risk”) is portable between populations. Indeed, the results from our study are in agreement with the Carozzi et al. study based on p16 alone, suggesting the possibility of extended retest intervals in p16-negative women (24).
In this study, p16/Ki-67 staining was performed in batches from residual Surepath material that were stored for one to four months before testing, possibly increasing unsatisfactory rates. We demonstrated in this large study that the dual stain assay works well in specimens collected in Surepath solution. We previously showed that the dual stain assay can be implemented with limited training and that it shows high reproducibility among cytotechnologists new to dual stain cytology (25). Additional precision and accuracy of dual stain cytology may be achieved by full slide scanning and automated evaluation of dual stain–positive cells (26).
In summary, we evaluated the dual stain for triage of HPV-positive women in a large population. Over two years of follow-up, we observed good risk stratification for all HPV-positive women and for the subgroup of women with HPV-positive/cytology-negative results. The observed risk levels would result in different management recommendations according to US management guidelines, suggesting that the assay could be useful in current cervical cancer screening programs.
References
- 1. Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103 (5):368–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62 (3):147–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. FDA approves first human papillomavirus test for primary cervical cancer screening. 2014. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394773.htm
- 4. Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gage JC, Schiffman M, Katki HA, et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst. 2014;106 (8):XXX-XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12 (7):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sahasrabuddhe VV, Luhn P, Wentzensen N. Human papillomavirus and cervical cancer: biomarkers for improved prevention efforts. Future Microbiol. 2011;6:1083–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wentzensen N. Triage of HPV-positive women in cervical cancer screening. Lancet Oncol. 2013;14 (2):107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carozzi F, Confortini M, Dalla PP, et al. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2008;9 (10):937–945. [DOI] [PubMed] [Google Scholar]
- 10. Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105 (20):1550–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res. 2012;18 (15):4154–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 Dual stained cytology. Gynecol Oncol. 2011;121 (3):505–509. [DOI] [PubMed] [Google Scholar]
- 13. Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Five-year experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol. 2009;113 (3):595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wentzensen N, Walker JL, Gold MA, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33 (1):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leisenring W, Alonzo T, Pepe MS. Comparisons of predictive values of binary medical diagnostic tests for paired designs. Biometrics. 2000;56 (2):345–351. [DOI] [PubMed] [Google Scholar]
- 16. Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S28–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27. [DOI] [PubMed] [Google Scholar]
- 18. Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370 (9601):1764–1772. [DOI] [PubMed] [Google Scholar]
- 19. Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119 (5):1095–1101. [DOI] [PubMed] [Google Scholar]
- 20. Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357 (16):1579–1588. [DOI] [PubMed] [Google Scholar]
- 21. Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360 (14):1385–1394. [DOI] [PubMed] [Google Scholar]
- 22. Castle PE, Stoler MH, Wright TC, Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011;12 (9):880–890. [DOI] [PubMed] [Google Scholar]
- 23. Wright TC, Jr, Stoler MH, Behrens CM, Sharma A, Sharma K, Apple R. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134 (8):1835–1843. [DOI] [PubMed] [Google Scholar]
- 24. Carozzi F, Gillio-Tos A, Confortini M, et al. Risk of high-grade cervical intraepithelial neoplasia during follow-up in HPV-positive women according to baseline p16-INK4A results: a prospective analysis of a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2013;14 (2):168–176. [DOI] [PubMed] [Google Scholar]
- 25. Wentzensen N, Fetterman B, Tokugawa D, et al. Interobserver reproducibility and accuracy of p16/Ki-67 dual stain cytology in cervical cancer screening. Cancer Cytopathol. 2014;122 (12):914–920. [DOI] [PubMed] [Google Scholar]
- 26. Grabe N, Lahrmann B, Pommerencke T, von Knebel DM, Reuschenbach M, Wentzensen N. A virtual microscopy system to scan, evaluate and archive biomarker enhanced cervical cytology slides. Cell Oncol. 2010;32(1–2):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]