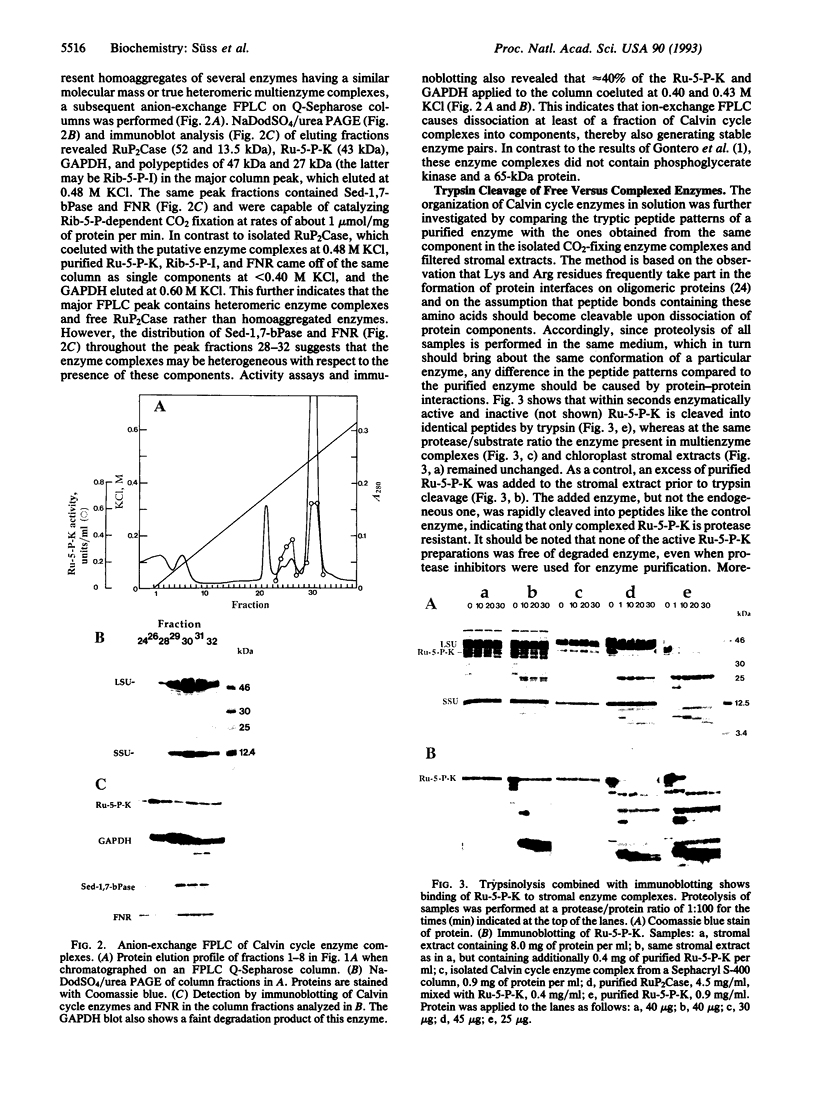
Abstract
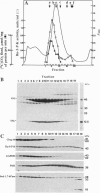
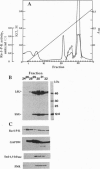
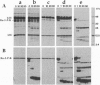
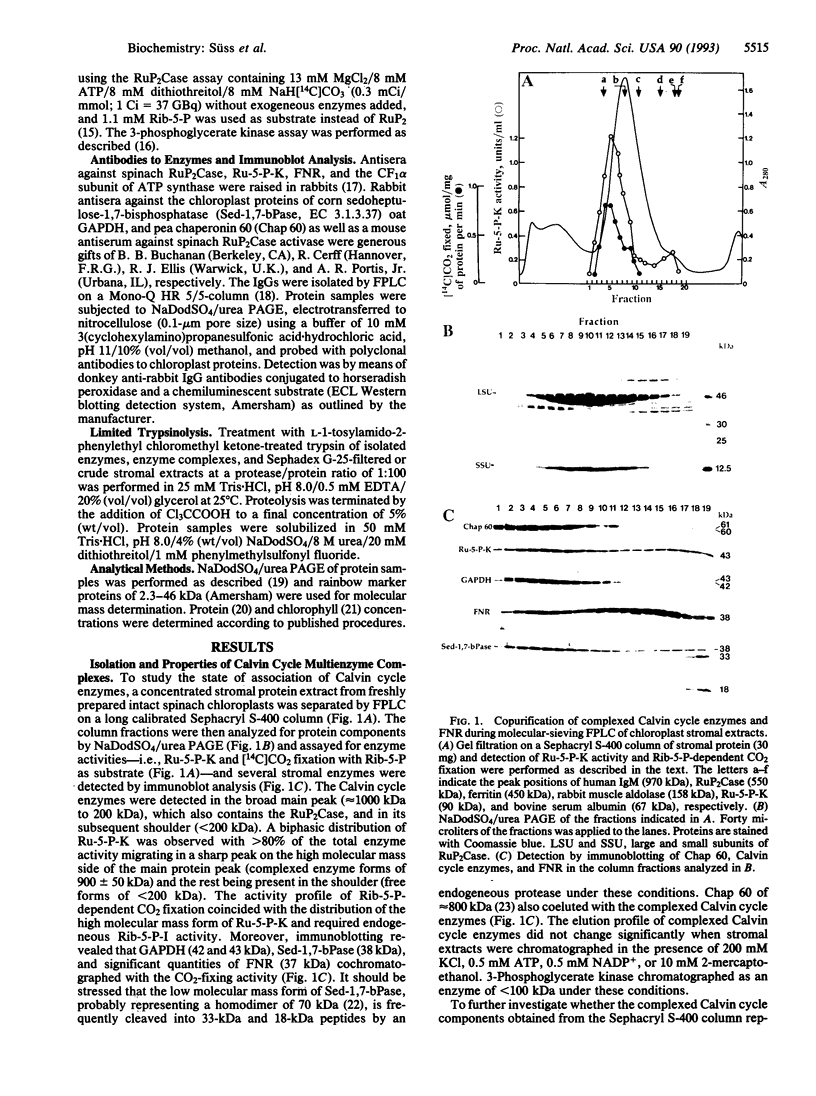
Further evidence is provided that the Calvin cycle enzymes ribose-5-phosphate isomerase (EC 5.3.1.6), ribulose-5-phosphate kinase (Ru-5-P-K, EC 2.7.1.19), ribulose-1,5-bisphosphate carboxylase (RuP2Case, EC 4.1.1.39), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, EC 1.2.1.12), sedoheptulose-1,7-bisphosphatase (Sed-1,7-bPase, EC 3.1.3.37), and electron transport protein ferredoxin-NADP+ reductase (FNR, EC 1.18.1.1) are organized into stable CO2-fixing multienzyme complexes with a molecular mass of 900 kDa. Limited trypsinolysis combined with immunoblotting revealed that all of chloroplast stromal Ru-5-P-K and GAPDH is located in enzyme complexes. The Calvin cycle enzyme complexes remain intact indefinitely at lower ionic strength but dissociate into components at KCl concentrations >250 mM. Immunoelectron microscopy showed that Ru-5-P-K, GAPDH, Sed-1,7-bPase, and FNR are bound to stroma-faced thylakoid membranes in situ, whereas RuP2Case and RuP2Case activase are randomly distributed throughout chloroplasts. The results indicate that membrane-bound enzyme supercomplexes may play an important role in photosynthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berzborn R. J. Untersuchungen über die Oberflächenstruktur des Thylokoidystems der Chloroplasten mit Hilfe von Antikörpern gegen die Ferredoxin-NADP-Reduktase. Z Naturforsch B. 1969 Apr;24(4):436–446. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cadet F., Meunier J. C., Ferté N. Isolation and purification of chloroplastic spinach (Spinacia oleracea) sedoheptulose-1,7-bisphosphatase. Biochem J. 1987 Jan 1;241(1):71–74. doi: 10.1042/bj2410071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasper S., Easterby J. S., Powls R. Properties of two high-molecular-mass forms of glyceraldehyde-3-phosphate dehydrogenase from spinach leaf, one of which also possesses latent phosphoribulokinase activity. Eur J Biochem. 1991 Dec 18;202(3):1239–1246. doi: 10.1111/j.1432-1033.1991.tb16496.x. [DOI] [PubMed] [Google Scholar]
- Gontero B., Cárdenas M. L., Ricard J. A functional five-enzyme complex of chloroplasts involved in the Calvin cycle. Eur J Biochem. 1988 Apr 15;173(2):437–443. doi: 10.1111/j.1432-1033.1988.tb14018.x. [DOI] [PubMed] [Google Scholar]
- Hermoso R., de Felipe M. R., Vivó A., Chueca A., Lázaro J. J., Gorge J. L. Immunogold localization of photosynthetic fructose-1,6-bisphosphatase in pea leaf tissue. Plant Physiol. 1989 Jan;89(1):381–385. doi: 10.1104/pp.89.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J., Miller S., Chothia C. Surface, subunit interfaces and interior of oligomeric proteins. J Mol Biol. 1988 Nov 5;204(1):155–164. doi: 10.1016/0022-2836(88)90606-7. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz G. W., Krietsch W. K. Phosphoglycerate kinase from animal tissue. Methods Enzymol. 1982;90(Pt E):103–110. doi: 10.1016/s0076-6879(82)90114-8. [DOI] [PubMed] [Google Scholar]
- Lazaro J. J., Sutton C. W., Nicholson S., Powls R. Characterisation of two forms of phosphoribulokinase isolated from the green alga, Scenedesmus obliquus. Eur J Biochem. 1986 Apr 15;156(2):423–429. doi: 10.1111/j.1432-1033.1986.tb09599.x. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Mangeney E., Hawthornthwaite A. M., Codd G. A., Gibbs S. P. Immunocytochemical Localization of Phosphoribulose Kinase in the Cyanelles of Cyanophora paradoxa and Glaucocystis nostochinearum. Plant Physiol. 1987 Aug;84(4):1028–1032. doi: 10.1104/pp.84.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson L. O., Johansson G. Studies of protein-protein interaction using countercurrent distribution in aqueous two-phase systems. Partition behaviour of six Calvin-cycle enzymes from a crude spinach (Spinacia oleracea) chloroplast extract. Biochem J. 1989 May 1;259(3):863–870. doi: 10.1042/bj2590863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Streusand V. J., Chatfield J. M., Portis A. R. Purification and assay of rubisco activase from leaves. Plant Physiol. 1988 Dec;88(4):1008–1014. doi: 10.1104/pp.88.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalbach G., Jung R., Kunze G., Saalbach I., Adler K., Müntz K. Different legumin protein domains act as vacuolar targeting signals. Plant Cell. 1991 Jul;3(7):695–708. doi: 10.1105/tpc.3.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainis J. K., Harris G. C. The association of ribulose-1,5-bisphosphate carboxylase with phosphoriboisomerase and phosphoribulokinase. Biochem Biophys Res Commun. 1986 Sep 30;139(3):947–954. doi: 10.1016/s0006-291x(86)80269-8. [DOI] [PubMed] [Google Scholar]
- Sainis J. K., Merriam K., Harris G. C. The Association of d-Ribulose- 1,5-Bisphosphate Carboxylase/Oxygenase with Phosphoribulokinase. Plant Physiol. 1989 Jan;89(1):368–374. doi: 10.1104/pp.89.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere P. A. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]