Abstract
A transient but prominent increase in the level of “silent synapses”—a signature of immature glutamatergic synapses that contain only NMDA receptors without stably expressed AMPA receptors—has been identified in the nucleus accumbens (NAc) following exposure to cocaine. As the NAc is a critical forebrain region implicated in forming addiction-associated behaviors, the initial discoveries have raised speculations about whether and how these drug-induced synapses mature and potentially contribute to addiction-related behaviors. Here, we summarize recent progress in recognizing the pathway-specific regulations of silent synapse maturation, and its diverse impacts on behavior. We provide an update of the guiding hypothesis—the “neural rejuvenation hypothesis”—with recently emerged evidence of silent synapses in cocaine craving and relapse.
Keywords: cocaine, addiction, silent synapse, NMDA receptor, synaptic plasticity, accumbens
Drug addiction has long been conceptualized as a form of memory, development of which involves synaptic plasticity and other adaptive cellular processes that are important for learning and memory (Hyman and others 2006). Compared with other forms of memories, addiction-related memories are exceedingly robust and durable, suggesting that a set of highly efficient plasticity mechanisms are used.
Over the past few years, we and others summarize several frequently reported observations that certain developmental molecular and cellular neuronal substrates are reexpressed or reactivated in brain regions that are critically involved in forming addiction-related memories following exposure to drugs of abuse (Creed and Luscher 2013; Huang and others 2013; Lee and Dong 2011). These notions were recently summarized as the “neural rejuvenation hypothesis” (Dong and Nestler 2014), which suggests that exposure to drugs of abuse temporarily reopens juvenile plasticity to certain addiction-related brain regions, resulting in robust and long-lasting plastic changes related to drug addiction. Among the rejuvenated neuronal substrates, AMPA receptor (AMPAR)-silent glutamatergic synapses are the focus of this article (Fig. 1; Box 1). In this article, we will provide an update of this hypothesis by incorporating important findings that are recently published and discuss a number of questions evoked by these findings.
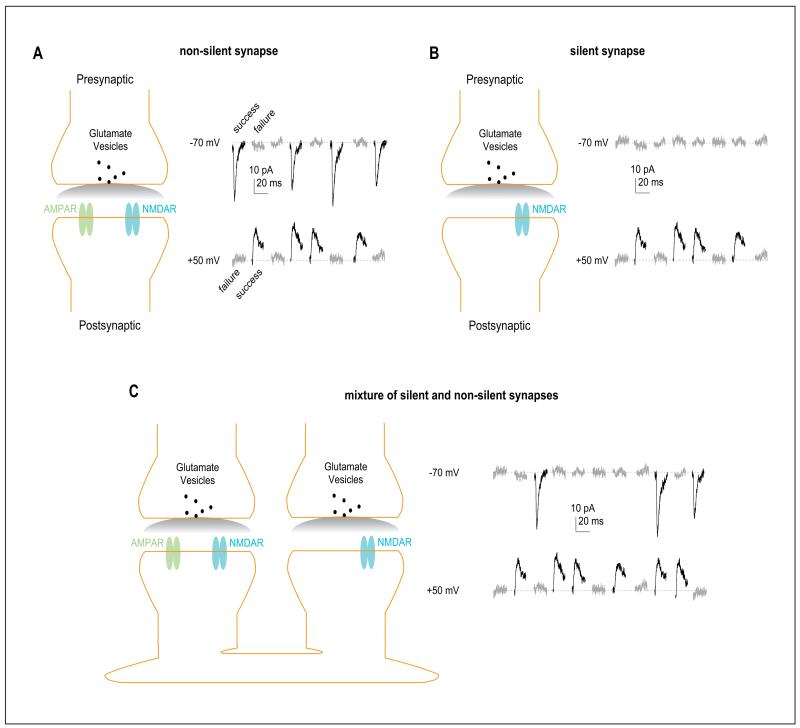
Figure 1.
AMPAR-silent nascent synapses. (A) Diagram (left) showing a typical glutamatergic synapse at which both AMPARs and NMDARs are present on the postsynaptic membrane. Example EPSCs (from real recordings in the nucleus accumbens; right) showing that on presynaptic activation, small EPSCs are elicited at both depolarized and hyperpolarized membrane potentials with a similar success or failure rate. The success rate is determined by, and equal to, the release probability. (B) Diagram (left) showing an AMPAR-silent synapse at which only stable NMDARs are present on the postsynaptic membrane (AMPARs are absent). Example traces (right) showing that on presynaptic activation, no synaptic responses are evoked at hyperpolarized membrane potentials, at which the only responding glutamatergic receptors NMDARs are blocked by Mg2+. (C) Diagram (left) showing that a set of synapses, mixed with one nonsilent synapse and one silent synapse, are recorded together in one experimental setup through the minimal stimulation assay. Example traces (right) showing that at hyperpolarized membrane potentials, presynaptic releases can only elicit postsynaptic responses at nonsilent synapses, whereas at depolarized membrane potentials, presynaptic releases elicit postsynaptic responses at both silent and nonsilent synapses. Through the trials, the success rate at depolarized membrane potentials is higher than at hyperpolarized membrane potentials. In other word, the failure rate at depolarized membrane potentials is lower than at hyperpolarized membrane potentials. AMPAR = α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor; EPSC = excitatory postsynaptic currents; NMDAR = N-methyl-d-aspartate receptor.
Box 1. Silent Excitatory Synapses.
Silent excitatory synapses are normally referred to synapses with fully functional presynaptic release, which can trigger reliable N-methyl-d-aspartate receptor (NMDAR)-mediated responses postsynaptically, but AMPAR-mediated responses are minimal. Because of their magnesium-mediated blockade, NMDARs conduct very little current at resting membrane potentials. As such, these synapses are often silent when the postsynaptic neurons dwell in their resting state.
Silent synapses can be generated by insertion of NMDARs to newly formed synaptic structures via synaptogenesis process (Hanse and others 2013; Kerchner and Nicoll 2008). Silent synapses may present newly generated synaptic contacts; they are extremely abundant in the developing brain but diminish in adulthood (Durand and others 1996). Alternatively, silent synapses can also be generated by removal or destabilization of AMPARs from preexisting synapses (Xiao and others 2004), which may turn stable synapses into an instable state.
Silent synapses are highly unstable and plastic; they can either recruit AMPARs and become mature, fully functional synapses, or be pruned away via metabolic turnover. As such, they can serve as intermediate neuronal substrates to form new neurocircuits or eliminate old neurocircuits.
Cocaine-generated silent synapses in the nucleus accumbens (NAc) exhibit several core features of nascent synapses. They are generated concurrently with insertion of new, GluN2B NMDARs, and the generation requires activation of several pro-synaptogenesis signaling molecules (Dong and Nestler 2014). These and other results suggest, but not unequivocally prove, that cocaine-induced silent synapses are formed in an NMDAR-driven manner rather than via internalization of AMPARs from preexisting synapses. Additional results show that a large portion of cocaine-generated silent synapses mature into fully functional synapses by recruiting AMPARs during cocaine withdrawal (Lee and others 2013; Ma and others 2014). Thus, cocaine exposure may remodel NAc circuits by adding new excitatory input.
Our unpublished data reveal that exposure to morphine also generated silent synapses in the NAc (Graziane and Dong). However, morphine-induced generation of silent synapses is not accompanied by insertion of GluN2B NMDARs, and can be prevented by inhibition of AMPAR internalization. These results suggest that morphine exposure may remodel NAc circuits by weakening or eliminating certain preexisting excitatory inputs.
The Nucleus Accumbens and Drug Addiction
The forebrain region NAc is an essential component of the mesocorticolimbic dopamine system, receiving dopaminergic innervation from the ventral tegmental area and extensive glutamatergic projections from a number of limbic and paralimbic brain regions. Based on experimental results and theoretical analyses, Gordon Mogensen and colleagues proposed in 1980 that the NAc is an interface between motivation and action; it regulates the pri-oritization of emotional and motivational arousals for behavioral output. At any given time point, multiple emotional and motivational arousals often flood in simultaneously, competing for behavioral output. It is thought that these emotional and motivational arousals are critically regulated by glutamatergic inputs to the NAc. Glutamatergic transmissions to the NAc are one of the primary targets of drugs of abuse; they are extensively altered after exposure to cocaine or other drugs of abuse (Dong and Nestler 2014; Luscher and Malenka 2011; Wolf 1998, 2010). More important, many drug-induced alterations in NAc glutamatergic transmission are essential for the expression of addiction-associated behaviors (Wolf 1998, 2010). Thus, at the theoretical level, addiction-associated information can be stored as alterations in NAc glutamatergic transmissions from different projections, and expressed as strengthened prioritization of drug-associated emotional and motivational arousals.
Generation of Silent Synapses
A prominent cellular event in NAc glutamatergic transmission after cocaine exposure is generation of AMPAR-silent synapses. Repeated exposure to cocaine, either noncontingent (e.g., intraperitoneal injections) or contingent (self-administration), leads to generation of silent synapses in principal medium spiny neurons (MSNs) of the NAc (Fig. 2). These synapses are readily detectable by electrophysiological recordings by the end of the typical 5-day cocaine procedures (either intraperitoneal injection of cocaine or cocaine self-administration), and but “disappear” after withdrawal (Huang and others 2009; Lee and others 2013). The molecular mechanisms underlying silent synapse generation has been recently reviewed thoroughly (Dong and Nestler 2014), whereas the “disappearance” is likely mediated by a maturation process through which silent synapses are unsilenced and thus cannot be detectable (see below). Here we will focus on attempts to characterize the cell-type and pathway specificity of silent synapse generation within the NAc.
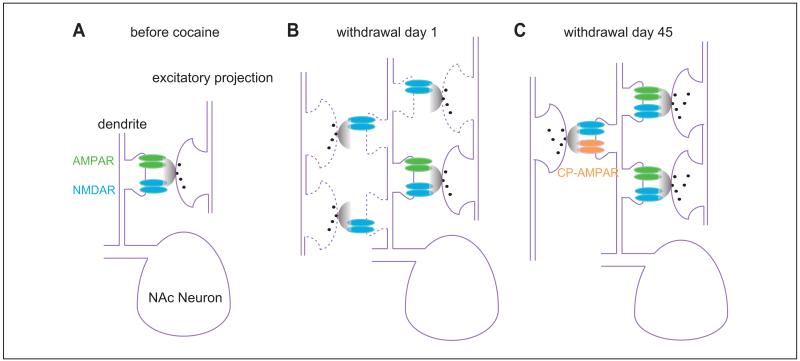
Figure 2.
Generation and maturation of silent synapses after cocaine exposure. (A) Diagram showing preexisting glutamatergic synapses in the nucleus accumbens before exposure to cocaine. At this time point, most glutamatergic synapses are typical synapses containing both AMPARs and NMDARs. (B) Diagram showing that 1 day after cocaine withdrawal, AMPAR-silent synapses are detected. They can be generated in either preexisting axons or new axons. (C) Diagram showing that after long-term withdrawal, some cocaine-generated silent synapses are pruned away, whereas others mature into fully functional synapses by recruiting new AMPARs. Within some efferents, newly inserted AMPARs are atypical, calcium-permeable AMPARs (CP-AMPARs). AMPAR = α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor; NMDAR = N-methyl-d-aspartate receptor.
The pathway specificity for silent synapse generation has been characterized in a small set of excitatory inputs to NAc MSNs. Both the basolateral amygdala (BLA) and medial prefrontal cortex (mPFC), the latter including the prelimbic (PrL) and infralimbic (IL) mPFC, are shown to exhibit similarly elevated silent synapse levels soon after cocaine self-administration, which return to the baseline levels at a similar time point after withdrawal (Lee and others 2013; Ma and others 2014). Notably, the basal levels of silent synapses (measured in naïve or saline-exposed animals) appear to be different between these NAc efferents. Specifically, in >2-month-old rats, a minimal level (~4%) of silent synapses is detected among BLA-to-NAc synapses, whereas ~8% of silent synapses is detected among synapses of the two mPFC projections (Lee and others 2013; Ma and others 2014). It may also be interesting to point out that the basal levels of silent synapses in a thalamic projection to the NAc are even higher (~20%; Neumann and Dong, unpublished data). Furthermore, the basal levels of synapses in the NAc are also higher in mice than in age-matched rats (Schlüter and Dong, unpublished data). Whereas the different levels of silent synapses may reflect differential plastic potentials of these projections (Kerchner and Nicoll 2008), the difference may also stem from their different pre- and postsynaptic properties (Box 2).
Box 2.
To estimate the % of silent synapses, the minimal stimulation assay, which was developed by Malinow and colleagues in 1995, was used. This assay is based on two theoretical assumptions: (1) the presynaptic release sites are independent and (2) release probability across all synapses, including silent synapses, are identical. Thus, the percentage of silent synapses among all recorded synapses was calculated using the equation: 1 – Ln(F−70)/Ln(F+50), in which F−70 was the failure rate at −70 mV and F+50 was the failure rate at +50 mV, as rationalized previously (Liao and others, 1995). However, these two theoretical assumptions may not hold in the NAc. A likely possibility is that the release probability is changed by cocaine administration or is different at silent synapses (Suska and others 2013). This will affect estimated values (%) of silent synapses. Specifically, let us define the total number of synapses as t, which is equal to the sum of the number of nonsilent synapses (n) and the number of silent synapses (s). Thus, t = n + s. Let us further define the release probability at nonsilent synapses as Pn, and the release prob-ability at silent synapses as Ps. At −70 mV, for a fixed number of synapses (t), the failure rate is exclusively determined by the release probability and the number of nonsilent synapses, Pn: F−70 = (1 – Pn)n. At +50 mV, silent synapses make contributions: F+50 = (1 – Pn)n(1 – Ps)s. These two equations can be transferred to
| (1) |
and
| (2) |
After rearrangement of Equations (1) and (2),
| (3) |
and
| (4) |
Let (1 - Ps) = (1 – Pn)a, then (4) will be
| (5) |
Thus, the number of silent synapses over the number of total synapses should be
| (6) |
Rearrange (6) to get
| (7) |
where (1 – Ps) = (1 – Pn)a.
Thus, if Ps = Pn, a = Ln(1 – Ps)/Ln(1 – Pn) = 1, and Equation (7) can be converted to the original equation s/t = 1 – Ln(F−70)/Ln(F+50).
If Ps > Pn, then a < 1, and thus the s/t assessed by (7) will be higher than that assessed by the original equation. Similarly, if Ps < Pn, then a > 1, and the s/t assessed by (7) will be lower than that assessed by the original equation.
Thus, if the release probability differs between silent and nonsilent synapses, the actual percentage silent synapses should be higher or lower than the estimated percentage using the equation s/t = 1 – Ln(F−70)/Ln(F+50).
Thus, different presynaptic properties (e.g., different Pr) may result in assessment errors. Nonetheless, as demonstration in the derivation process, this introduces quantitative errors, but should not qualitatively affect the results.
Nonetheless, it remains to be determined whether silent synapses are generated ubiquitously in all glutamatergic pathways onto NAc MSNs after cocaine exposure, and whether via similar molecular mechanisms. For example, it is still open for discovery whether all silent synapses are generated by insertion of NMDARs into newly generated synaptic loci or by removal of AMPARs at preexisting, mature synapses in all glutamatergic projections; and whether the former is always accompanied by insertions of GluN2B-containing NMDARs as demonstrated after repeated i.p. injections of cocaine (Huang and others 2009). The “rejuvenation hypothesis” suggests that after exposure to cocaine, some excitatory circuits within the NAc reenter the developmental stage for circuitry remodeling. Synapse formation and elimination are two key components in the assembly and refinement of neural circuits. During development, these two components often occur simultaneously to establish new synaptic contacts while at the same time remove others to arrange or rearrange neural circuits. Thus, in addition to the potential synaptogenesis process (Dong and Nestler 2014), synaptodegeneration also likely occurs in certain excitatory projections to the NAc. Thus, the increased spine density observed in the NAc after cocaine exposure (Robinson and Kolb 2004) may be a net effect of a substantial synaptogenesis in some projections and a modest synaptodegeneration in some other projections. Similar scenario may apply to exposure to opioids (e.g., morphine), after which the decreased spine density in the NAc may be a net effect of a modest synaptogenesis and a substantial synaptodegeneration in different projections.
Effort to further differentiate silent synapse generation in different subpopulations of NAc principal neurons has been limited. NAc MSNs can be largely divided into two main subgroups based on the types of dopamine receptors that are expressed, those that express D1 versus D2 receptors, likely with a third subgroup that expresses both D1 and D2 receptors. The two main subgroups exhibit different receptor and neural peptide expression profiles, and are anatomically and functionally distinct, even though these neurons are similar in morphology and electrophysiological properties. Nonetheless, there has been no direct measurement of silent synapse generation in D1-versus D2-expressing MSNs, although statistical analysis of the levels of silent synapse in mixed cell populations failed to detect a binomial distribution (Brown and others 2011). This is consistent with a prior morphological study showing that both D1- and D2-expressing MSNs have significantly increased dendritic spine density soon after repeated cocaine exposure, suggesting synaptogenesis in both subpopulations (Lee and others 2006). On the other hand, rather than segregating NAc MSNs into D1 and D2-expressing neurons, an elegant study by Koya and others (2012) used Fos-GFP (Fos–green fluorescence protein) reporter animals to examine silent synapse generation in NAc neurons that are strongly activated by cocaine versus those that are not, and showed that silent synapses are selectively induced in the most strongly activated neurons. The identity of the neurons is decidedly MSNs for their expression of DARPP-32—and most likely D1-expressing MSNs, based on previous observations that cocaine-induced Fos expression occurs predominantly in D1-expressing neurons (Bertran-Gonzalez and others 2008). This is also consistent with the observation that over-expression of delta FosB, a member of Fos family transcription factor that is critically implicated in reward and addiction, likely increases silent synapses only in NAc D1-but not D2-expressing MSNs (Grueter and others 2013; Koya and others 2012). Interestingly, silent synapses in the Fos-positive neurons were detected at high levels at least 6-11 days after cocaine exposure, which suggests distinctly greater longevity than what is observed in the mixed population of NAc neurons (Huang and others 2009; Koya and others 2012). One complication is that these synapses were recorded after an acute, challenge injection of cocaine (reexposure to cocaine) after cocaine withdrawal (Koya and others 2012); it could either be a response to the acute cocaine reexposure after withdrawal from cocaine administration (e.g., regeneration of silent synapses), or more interestingly, represent the unique cell ensemble that has developed during repeated cocaine exposure.
Maturation of Silent Synapses: To Be, or Not to Be?
Are most cocaine-induced silent synapses eliminated over time or do some of them mature and incorporate into relevant circuit to reshape future behaviors? Early morphological studies show sustained increase in dendritic spines of D1-expressing neurons but shorter-lasting increase in D2-expressing neurons over a 1-month withdrawal period (Lee and others 2006). If the increased spines detected after short-term cocaine withdrawal are silent synapses, they should be present on both D1- and D2-expressing neurons, but selectively decline in D2-expressing neurons after long-term withdrawal. It is important to note that thus far there is no direct evidence illustrating the relationship between “new” spines and silent synapses. Indeed, the time courses of spine generation and elimination are likely more dynamic than cocaine-induced generation and subsequent maturation of silent synapses. Nonetheless, what could be a feasible tag to track these drug-induced synapses? One important clue comes from biochemical measurements of the protein expression levels of glutamate receptor subunits in the NAc during long-term cocaine withdrawal. It has been demonstrated that cocaine-induced silent synapses in certain projections mature by incorporating calcium-permeable (CP)-AMPARs, either immediately after generation or in a delayed manner (Dong and Nestler 2014; Lee and others 2013; Loweth and others 2014; Ma and others 2014). Insertion of new CP-AMPARs can be detected by the increased rectifying current-voltage relationship or unique pharmacology. Indeed, application of Naspm, a selective antagonist for CP-AMPARs, revealed an elevated level of silent synapses within at least two NAc glutamatergic projections after long-term withdrawal, long after silent synapses have “disappeared” during early withdrawal, suggesting that at least a significant portion of drug-induced silent synapses mature by recruiting CP-AMPARs. Supporting this view, strong evidence from hippocampal studies show that maturation of silent synapses is primarily mediated by selectively insertion of GluA1-containing, GluA2-lacking AMPARs (Shi and others 2001). Nonetheless, the level of silent synapses revealed by Naspm after long-term withdrawal is not quite as high as that after early withdrawal, suggesting that some silent synapses have either matured by recruiting nonCP-AMPARs (Ma and others 2014) or have been eliminated by this time. During brain development, a large number of immature excitatory synapses, often silent in nature, are generated at the beginning, but only a portion of these synapses mature and eventually incorporate into neural circuits, while others are pruned away via metabolic turnover. If exposure to cocaine rejuvenates excitatory circuits in the NAc as hypothesized (Dong and Nestler 2014), the subsequent rematuration process would inevitably be highly regulated, involving synapse pruning and refinement. One possible regulation may occur in a cell type-specific manner. As discussed above, whereas both types of NAc MSNs exhibit cocaine-induced spinogenesis, it appears that only new spines on D1-expressing MSNs are maintained through the long-term withdrawal (Lee and others 2006). If new spines selectively mature on D1-expressing MSNs but are pruned away on D2-expressing MSNs after cocaine withdrawal, there must be at least two sets of highly selective regulatory signaling, one promoting maturation of silent synapses on D1-expressing MSNs, while the other promoting synapse pruning on D2-expressing MSNs. Another possible regulation may occur in a projection-specific manner. Among all excitatory projections, it is conceivable that some of them are more susceptible than others to cocaine-induced synaptic remodeling. Again, critical cellular mechanisms must exist to differentiate different projections and their maturation/pruning processes.
The molecular and cellular mechanisms underlying synapse generation and, particularly, those underlying refinement/elimination after exposure to drugs of abuse remain largely unexplored, but stand as an important future research direction.
The other appreciable feature of newly matured synapses is that they may be more susceptible to removal of AMPARs, for example, to stimuli that induce long-term depression (LTD). A modified protocol was recently shown to preferentially induce LTD in NAc glutamatergic transmissions in rats after long-term withdrawal from cocaine self-administration but not saline control rats. Importantly, in pathways where there is upregulation of CP-AMPARs, the induction of LTD is accompanied by a loss of Naspm sensitivity of these pathways, suggesting a preferential internalization of CP-AMPARs that are presumably recruited by drug-induced nascent synapses. In pathways where there is no upregulation of CP-AMPARs, LTD still can also be selectively induced in cocaine but not saline-treated animals, suggesting that some drug-induced silent synapses may have matured by recruiting the typical calcium-impermeable AMPARs.
Maturation or elimination of silent synapses and recruitment of different subtypes of AMPARs may be cell type and pathway specific. Research in this realm is still limited, but there has been evidence showing that following cocaine withdrawal, there is potentiation of AMPAR EPSCs within the ventral hippocampal (vHipp) and mPFC inputs onto NAc D1R-but not D2-expressing MSNs; and that only the mPFC but not vHipp projections exhibit enhanced CP-AMPARs (Pascoli and others 2014). Another study shows that whereas IL-to-NAc shell pathway recruits CP-AMPARs following long-term withdrawal, PrL-to-NAc core recruits nonCP-AMPARs during the same withdrawal period (Ma and others 2014). These results suggest that CP-AMPARs are not the only players in the game, and the role of nonCP-AMPARs in cocaine seeking and relapse remains less understood.
Behavioral Consequences: A Glimpse via “Incubation” of Cocaine Craving
What could be the functional consequences of cocaine-induced silent synapses? They perhaps contribute negligibly to basal transmission because of a lack of AMPARs, and yet they have the potential on maturation to be incorporated into the reward circuitry. Could they possibly contribute to the addiction-associated behaviors after long-term withdrawal? Although direct manipulation of silent synapses has not been reported, pharmacological approach targeting CP-AMPARs and in vivo optogenetics with selective LTD protocols reveals a corner of the iceberg.
In rodent models of drug relapse and craving, cue-induced drug craving progressively increases after withdrawal from cocaine or other abused drugs, which is termed “incubation of drug craving” (Grimm and others 2001; Pickens and others 2011). The development of incubation of cocaine craving correlates with the upregulation of CP-AMPARs in the NAc during long-term withdrawal; reduction of CP-AMPAR function in the NAc, either by selective receptor antagonist or by mGluR1-induced CP-AMPAR internalization, suppresses the expression of incubation of cocaine craving (Conrad and others 2008; Loweth and others 2014). Pathway-specific optogenetic manipulations also show that low frequency stimulation, which spares synapses in saline-treated animals, induces LTD of NAc excitatory synaptic transmission in cocaine-treated animals, and alters behavior in a pathway-dependent manner. It has been shown that following long-term withdrawal, LTD induction in the PrL-or BLA-to-NAc inputs reduces incubation, whereas LTD in the IL inputs enhances incubation (Lee and others 2013; Ma and others 2014) (Fig. 3). One note of caution here is that although these LTD induction protocols have proved specificity in brain slices, direct evidence that the affected synapses are the newly matured cocaine-induced silent synapses has been scarce and difficult to obtain.
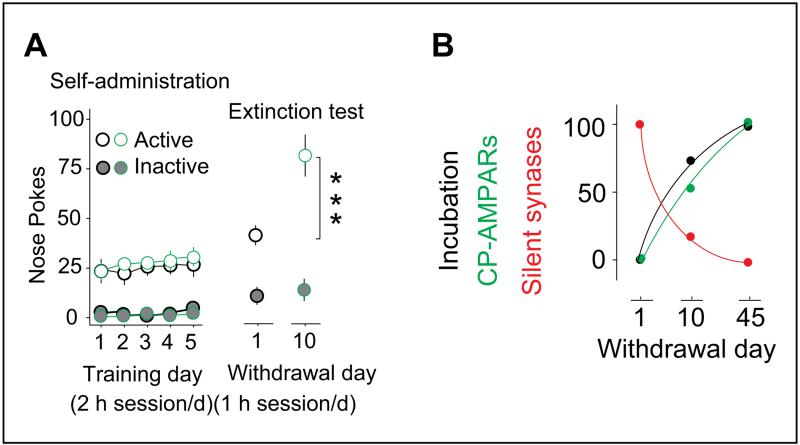
Figure 3.
Maturation of BLA-to-NAc silent synapses correlates with incubation of cocaine craving. (A) Summarized results show that rats are subject to a 5-day cocaine self-administration regimen acquire stable drug taking. After abstinence, cocaine-associated cues (without cocaine infusion) elicit cocaine seeking on withdrawal day 1 and day 10, and this cue-induced cocaine seeking becomes increasingly intensified over the period of withdrawal (right). (B) Diagram showing that over the 45-day withdrawal period, cue-induced cocaine seeking becomes progressively increased. This intensification process is correlated with a progressive increase in CP-AMPARs and a progressive decrease in silent synapses. Data are normalized by setting the withdrawal scores to 0% and withdrawal day 45 score to 100%. n values are presented as the number of neurons/number of rats sampled. ***P < .0001. This figure is modified from Lee and others (2013) with permission from Nature Neuroscience. BLA = basolateral amygdala; CP-AMPAR = calcium-permeable α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor; NAc = nucleus accumbens.
Revisiting the Rejuvenation Hypothesis
Cocaine-induced silent synapses share many molecular events and building blocks that construct nascent excitatory synapses during development, which has been extensively discussed recently (Dong and Nestler 2014). This leads to the hypothesis that addictive drugs “rejuvenate” neural substrates within the brain reward circuitry, using some highly efficient plasticity mechanisms—normally seen in development—to form addiction-related memories. Interestingly, similar to developmental synaptogenesis, whose pruning and stabilization are pathway- and time-dependent, cocaine-induced silent synapse is also pathway-specific and evolves over the course of long-term withdrawal. Nonetheless, whereas developmental synaptogenesis is clearly shaped by experience, it is not clear what kind of experience—or the lack of which—shapes the fate of drug-induced silent synapses: in the absence of cocaine exposure during long-term withdrawal, what could be the driving force for the synaptic delivery of new AMPARs? Even when they are temporarily internalized, what drives them to come back again? Is there a “critical period” like those during development for the maturation of drug-induced silent synapses? Can the maturation process during drug abstinence be modified by positive experience? These are but a few tantalizing examples of what remains to be researched and revealed.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research of the authors is supported by National Institutes of Health funds DA029565 (YHH), DA035805 (YHH), MH101147 (YHH), DA023206 (YD), DA030379 (YD), DA034856 (YD), and the Pennsulvania Department of Health Commonwealth Universal Research Enhancement (YHH).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28(22):5671–85. doi: 10.1523/JNEUROSCI.1039-08.2008. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31(22):8163–74. doi: 10.1523/JNEUROSCI.0016-11.2011. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–21. doi: 10.1038/nature06995. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed MC, Luscher C. Drug-evoked synaptic plasticity: beyond metaplasticity. Curr Opin Neurobiol. 2013;23(4):553–8. doi: 10.1016/j.conb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci. 2014;35(8):374–83. doi: 10.1016/j.tips.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381(6577):71–5. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci U S A. 2013;110(5):1923–8. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanse E, Seth H, Riebe I. AMPA-silent synapses in brain development and pathology. Nat Rev Neurosci. 2013;14(12):839–50. doi: 10.1038/nrn3642. [DOI] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G. In vivo cocaine experience generates silent synapses. Neuron. 2009;63(1):40–7. doi: 10.1016/j.neuron.2009.06.007. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Schluter OM, Dong Y. An unusual suspect in cocaine addiction. Neuron. 2013;80(4):835–6. doi: 10.1016/j.neuron.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9(11):813–25. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15(11):1556–62. doi: 10.1038/nn.3232. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond. Neuropharmacology. 2011;61(7):1060–9. doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16(11):1644–51. doi: 10.1038/nn.3533. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103(9):3399–404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci. 2014;17(1):73–80. doi: 10.1038/nn.3590. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69(4):650–63. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83(6):1453–67. doi: 10.1016/j.neuron.2014.08.023. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Luscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509(7501):459–64. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34(8):411–20. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105(3):331–43. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Suska A, Lee BR, Huang YH, Dong Y, Schluter OM. Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine. Proc Natl Acad Sci U S A. 2013;110(2):713–8. doi: 10.1073/pnas.1206287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54(6):679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33(9):391–8. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao MY, Wasling P, Hanse E, Gustafsson B. Creation of AMPA-silent synapses in the neonatal hippocampus. Nat Neurosci. 2004;7(3):236–43. doi: 10.1038/nn1196. [DOI] [PubMed] [Google Scholar]