Abstract
The spatiotemporal coordination of angiogenesis in synthetic materials is important for mimicking natural tissue morphogenesis. Here we report patterned hydrogel encapsulation of mesenchymal stem cells to direct endothelial tubulogenesis in co-culture. Tubulogenesis occurs preferentially over MSC patterns, suggesting this strategy may prove useful in guiding the design of heterotypic engineered tissues.
Angiogenesis is an integral part of wound healing1. Cytokine or growth factor therapy is a promising technique to promote angiogenesis; however there are several limitations including irregular vasculature, vasodilation and tumor angiogenesis2. Recently, stem cell therapy has emerged as a potential solution to the limitations associated with growth factor therapy, where dynamic secretion can improve angiogenesis3. Human mesenchymal stem cells (MSCs) are multipotent stem cells of mesoderm origin4 which are postulated to act as pericytes5 with therapeutic properties through secreted factors.
Several methods to modulate the MSC secretory profile have been reported3. Amongst these, extracellular matrix (ECM) properties are a potent factor in controlling MSC behavior. MSC differentiation is modulated by ECM stiffness6,7, composition8 and cell geometry7,9. In fact, mechanical properties of the ECM affect MSC cytokine secretion10 and subsequent angiogenic potential11. We have shown previously that stiffer matrix and protein composition act together to significantly alter the secretome and angiogenic potential of MSCs12.
For cell-based therapies, MSC delivery involves a more complex 3-D environment that would benefit from a design that recapitulates aspects of in vivo tissue13. It is well-established that signaling in 3-D matrices will influence cell behavior and secretory profiles differently than in 2-D assays14,15. Furthermore, MSC encapsulation within hydrogels has been shown to improve their viability during transplantation16. Taken together, this suggests that 3-D environments may be an important factor in MSC angiogenic potential.
Feedback between different cell types can also direct angiogenesis. In vivo, MSCs often secrete trophic factors in response to heterotypic cell-cell signaling17. Endothelial cells have been reported to alter gene expression profiles of MSCs18,19. Matrix properties also control network formation in 3-D co-culture systems20.
In this work, we demonstrate a chemical strategy to conjugate matrix proteins to poly(ethylene glycol) (PEG) hydrogels. We use these hydrogels as a platform to investigate the differences between 2-D and 3-D culture of MSCs on their angiogenic potential using a secondary in-vitro angiogenesis assay. Using the same material we can compare the influence of dimensionality when cells are either cultured on the surface or within the gel. Finally, we show how, using UV photopolymerization, we can ‘pattern’ vascularization in an MSC-endothelial cell co-culture system towards biomimetic architectures to study heterotypic signaling. The approach presented here may prove valuable for the design of 3-D biomaterials that are clinically viable for regenerative medicine.
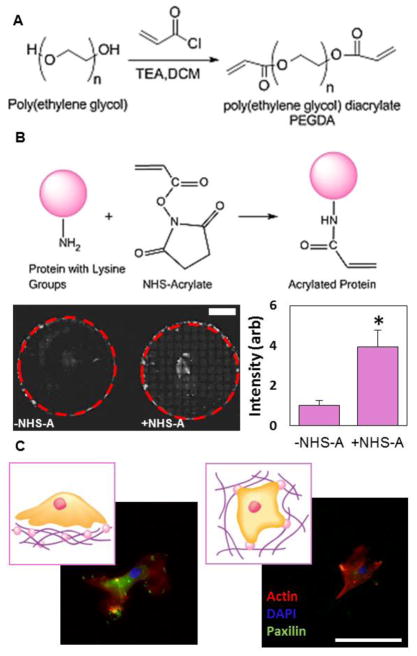
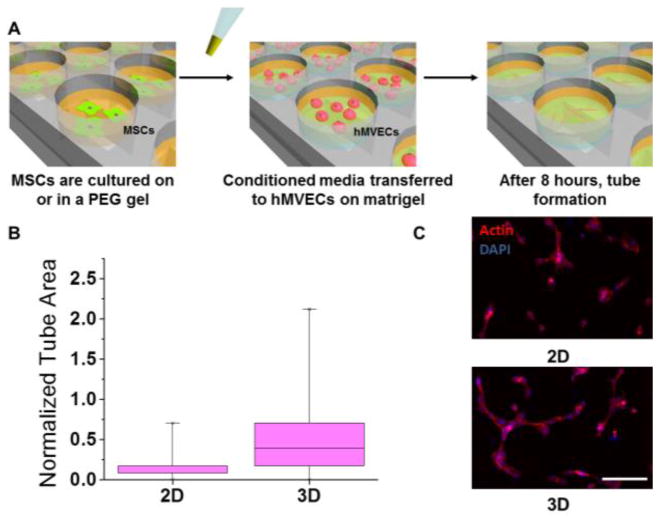
In order to compare MSCs cultured on the surface of 2-D gels to cells encapsulated inside a more clinically relevant 3-D hydrogel architecture, we used a poly(ethylene glycol) diacrylate (PEGDA) system. We modified the end groups of PEG as previously reported21 (Figure 1A) and confirmed modification using NMR (Figure S1). In order to incorporate protein into the 3-dimensional matrix, proteins were acrylated by reacting pendant amines with NHS-acrylate. We used a UV sensitive initiator to incorporate the matrix protein into the gels and confirmed higher protein incorporation in the NHS-acrylate condition using fluorescently labeled fibrinogen (Figure 1B). Based on our previous work12, we used fibronectin as the matrix protein and PEGDA hydrogels with an elasticity of around 40 kPa, as this condition had previously shown the highest angiogenic potential. PEGDA gels were made that were either flat with MSCs seeded on top (2-D) or they were mixed with MSCs before gelation so that the MSCs were encapsulated inside the gel (3-D). MSCs were cultured in both the 2-D and 3-D conditions for 2 days. Morphologically, MSCs look very different when cultured in 2-D vs 3-D (Figure 1C). On the flat 2-D surfaces, MSCs were spread out with a robust actin cytoskeleton, while inside the 3-D gels, the cells were more rounded up with a significantly smaller projected area. Paxillin staining shows focal adhesion formation on the surface of 2-D gels. After MSC culture, the conditioned media was used for an in-vitro tubulogenesis assay to investigate the differences in angiogenic potential12 (Figure 2A). After 2 days of culture, conditioned media containing cytokines secreted by MSCs was collected and then added to hMVECs seeded on a 3D matrigel matrix. After 8 hours, hMVECs angiogenic tube formation was quantitated and normalized to hMVEC tubulogenesis in complete growth factor supplemented media (EGM-2). Conditioned media collected from MSCs cultured in the 3-D environment showed approximately 2-fold increase in tubulogenesis compared to MSCs cultured in the 2-D system (Figure 2B). These differences can be discerned in the morphology of the hMVEC tubes (Figure 2C). Both conditions showed less than half the tube formation of hMVECs in complete medium, possibly due to the large number of growth factors included in that medium. It should be noted that hMVECs show very low tubulogenesis when cultured in serum free medium12.
Figure 1.
Protein conjugated PEG gels for MSC culture (A) Acryloyl chloride was used for end group modification of PEG into PEGDA (B) (top) NHS-acrylate (NHS-A) was used for the acrylation of proteins via pendant amine groups.(bottom) Higher incorporation of protein was confirmed using fluorescent protein. Scale bar is 5mm. * P<0.05 (C) MSC morphology when cultured on the surface of PEG gels (2-D) or encapsulated inside (3-D). Scale bar is 100μm
Figure 2.
In-vitro angiogenesis assay of MSC-conditioned media (A) Schematic showing the in-vitro angiogenesis assay using hMVECs (B) Box and whisker plot of quantitation of angiogenesis from conditioned media from MSCs cultured in 2-D or 3-D (C) Representative images of tube formation with conditioned media from 2-D and 3-D cultured MSCs. Scale bar is 200μm
In 3-D environments cells are in contact with the extracellular matrix on all sides, which will significantly influence the propagation of signals from the outside-in to regulate cell behavior. Cytokine secretions have been reported to increase up to 35-fold in 3-D vs 2-D environments15, so it is not surprising that the encapsulation of MSCs leads to higher angiogenesis. Although composition and mechanics are important factors, this result indicates that dimensionality, ligand presentation and other factors present in the switch from 2-D to 3-D culture13 have a major role in directing pro-angiogenic signaling from MSCs.
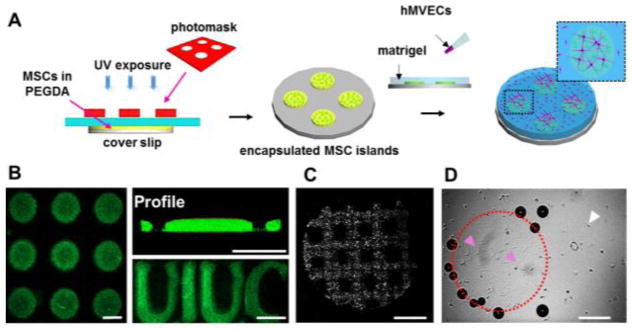
A direct readout of angiogenesis in one platform would be useful in the study of materials properties that direct pro-angiogenic signaling from MSCs. Towards this end, we designed and developed a co-culture system where both encapsulated MSCs and hMVECs can be cultured together (Figure 3A). By photopolymerizing gels under UV light through a photomask, we made ‘islands’ of MSCs encapsulated in PEGDA. To check the fidelity of these patterns we incorporated fluorescent beads in the PEGDA islands and imaged the islands using confocal microscopy (Figure 3B). Islands were of good dimensional accuracy and show good cross-sectional profiles and their shapes are not limited to circular but can be varied to adopt a range of geometries. Island height could be varied by changing gel crosslinking density but not by changing amount of gel solution prior to polymerization (Figure S2). After MSCs are encapsulated in these islands, cell patterning in the islands was verified using cell tracker (Figure 3C), and matrigel was added on top of the islands and then placed in the incubator to gel. hMVECs are then seeded on top of the matrigel and tube formation was monitored. After 8 hours, there is tube formation on the areas of the matrigel above the MSC islands with very little tubulogenesis elsewhere (Figure 3D). Due to the gelling of the matrigel, small bubbles are trapped at the base of the islands and can be seen around the edges. Tube formation may be enhanced on top of the islands due to closer affinity of these areas to MSCs or higher concentration of MSC-secreted cytokines.
Figure 3.
MSC-hMVEC co-culture (A) Schematic showing the procedure for co-culturing PEG encapsulated MSCs and hMVECs on matrigel (B) Confocal images of fluorescent beads embedded into PEGDA islands showing top view (left) and profile. PEGDA islands can also be formed into irregular letters. Scale bars are 500μm (C) Fluorescence image of MSCs captured in a PEG grid. Scale bar is 5mm (D) Brightfield images of hMVECs at the surface of matrigel after 8 hours of co-culture. Dotted outline indicates PEG island; pink arrows indicate tube formation; white arrow indicates rounded cells. Scale bar is 1mm.
Conclusions
We developed a chemical strategy to conjugate proteins within a 3-D poly(ethylene) glycol hydrogel towards tissue-mimetic architectures for exploration of heterotypic cell-cell signaling. We show the importance of dimensionality and ligand presentation on MSC angiogenic efficacy. We extend this to a heterotypic co-culture system where presence of MSCs greatly increases tube formation from surrounding hMVECs. Spatial control of angiogenesis signaling in vitro, with supporting MSC co-culture, may be a good model for studying vasculature-pericyte interactions. Furthermore, this system is modular allowing assessment of virtually any hydrogel and cell type of interest to aid the design of cell-based therapeutic biomaterials.
Supplementary Material
Acknowledgments
This work was supported by the National Heart Lung and Blood Institute of the National Institutes of Health, grant number HL121757.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Tonnesen MG, Feng X, Clark RA. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 2.Epstein SE, Kornowski R, Fuchs S, Dvorak HF. Circulation. 2001;104:115–119. doi: 10.1161/01.cir.104.1.115. [DOI] [PubMed] [Google Scholar]
- 3.Ranganath SH, Levy O, Inamdar MS, Karp JM. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science (80-) 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Abdeen AA, Zhang D, Kilian KA. Biomaterials. 2013;34:8140–8148. doi: 10.1016/j.biomaterials.2013.07.074. [DOI] [PubMed] [Google Scholar]
- 8.Rowlands AS, George PA, Cooper-White JJ. Am J Physiol Cell Physiol. 2008;295:1037–1044. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 9.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Proc Natl Acad Sci U S A. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seib FP, Prewitz M, Werner C, Bornhäuser M. Biochem Biophys Res Commun. 2009;389:663–667. doi: 10.1016/j.bbrc.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 11.Kasper G, Dankert N, Tuischer J, Hoeft M, Gaber T, Glaeser JD, Zander D, Tschirschmann M, Thompson M, Matziolis G, Duda GN. Stem Cells. 2007;25:903–910. doi: 10.1634/stemcells.2006-0432. [DOI] [PubMed] [Google Scholar]
- 12.Abdeen AA, Weiss JB, Lee J, Kilian KA. Tissue Eng part A. 2014;20:2737–45. doi: 10.1089/ten.tea.2013.0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker BM, Chen CS. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-feliciano J, Mooney DJ. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Proc Natl Acad Sci U S A. 2009;106:399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Tissue Eng Part A. 2012;18:806–815. doi: 10.1089/ten.tea.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy MB, Moncivais K, Caplan AI. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen TO, Blois AL, Xing Z, Xue Y, Sun Y, Finne-Wistrand A, Akslen La, Lorens JB, Leknes KN, Fristad I, Mustafa K. Stem Cell Res Ther. 2013;4:52. doi: 10.1186/scrt202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saleh Fa, Whyte M, Ashton P, Genever PG. 2011;20 doi: 10.1089/scd.2010.0168. [DOI] [PubMed] [Google Scholar]
- 20.Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Angiogenesis. 2012;15:253–264. doi: 10.1007/s10456-012-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aydin D, Louban I, Perschmann N, Blümmel J, Lohmüller T, Cavalcanti-Adam EA, Haas TL, Walczak H, Kessler H, Fiammengo R, Spatz JP. Langmuir. 2010;26:15472–15480. doi: 10.1021/la103065x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.