Abstract
Background
The acceptability of and retention on extended-release naltrexone (XR-NTX), an FDA-approved medication for the treatment of alcohol and opioid use disorders, among persons living with HIV disease (PLH) under criminal justice setting (CJS) supervision has not been evaluated to date.
Methods
Two double-blind placebo-controlled randomized trials of XR-NTX for inmates with HIV disease transitioning to the community with (1) alcohol use disorders (AUDs) or (2) opioid use disorders, are underway. Reasons for not accepting XR-NTX and an evaluation of differences in demographic features between those who were retained on study drug and those who did not return for their second injection post-release are discussed.
Results
70% of eligible persons consented to participate; almost 90% received their first injection; and almost 60% returned for their second injection after release. Variables found to be associated (p<0.10) with returning for the second injection included: not meeting criteria for hazardous drinking (p=0.035; OR 0.424 (CI 0.191–0.941)); being prescribed antiretroviral therapy (p=0.068; OR 2.170 (CI 0.943–4.992)); expressing experiencing serious depression 30 days prior to incarceration (p=0.068; OR 1.889 (CI 0.955–3.737)); not having a positive cocaine urine screen on the day of release (DOR) (p=0.011; OR 0.258 (CI 0.091–0.729)); and not meeting criteria for an AUD plus any substance use disorder (p=0.068; OR 0.521 (CI 0.259–1.048)). Only positive cocaine urine test on DOR was statistically significant after multivariate regression analyses (p=0.005; OR 0.207 (CI 0.068–0.623)).
Conclusion
CJS based XR-NTX programs are highly acceptable among PLH, however retention on XR-NTX after release is negatively impacted by relapse to cocaine use.
Keywords: criminal justice system, opioid use disorders, alcohol use disorders, Vivitrol, extended-release naltrexone, HIV
1. INTRODUCTION
Currently 1 in 31 persons in the United States (U.S.) is under criminal justice (CJ) system (CJS) supervision (Pew Center on the States, 2009). HIV prevalence rates are three times greater (Maruschak and Beavers, 2009; Spaulding et al., 2009) and drug and alcohol dependence is ten times greater among CJ-involved persons as compared to the general population. Ten million persons are released every year to the community with an estimated 16% who have HIV infection (Spaulding et al., 2009) and relapse to drug and alcohol use occurs in approximately 90% within one year after release regardless of time of incarceration (Kinlock et al., 2009, 2008) Relapse to drug and alcohol use contributes to poor retention in care, non-adherence to antiretroviral therapy (ART), and increased HIV and drug related morbidity and mortality (Binswanger et al., 2007; Lim et al., 2012; Springer et al., 2011b).
Current FDA-approved medication assisted therapies (MAT) for opioid dependence are methadone (MTH), buprenorphine (BPN), naltrexone (NTX), and extended-release naltrexone (XR-NTX); and for alcohol use disorders include NTX, XR-NTX, acamprosate and disulfiram (Altice et al., 2010; Springer et al., 2011a, 2011b). Most CJ-involved persons (80%) are not, however, offered routine MAT during incarceration or upon release despite strong evidence to support the effectiveness of MAT in decreasing relapse to opioid and alcohol use as well as improving adherence to ART, decreasing HIV transmission and potentially improving HIV viral load suppression (Springer, 2010a, Springer et al., 2011a, 2010b, 2012). It is possible that the stigma, diversion issues, poor adherence, and additional professional licensing required to prescribe an opioid agonist treatment (BPN or MTH) may be possible reasons they are not widely used within the U.S. CJS for opioid use disorders, but reasons for why NTX is not routinely used for CJS persons with alcohol use disorders (AUDs) is not clearly understood (Springer et al., 2011a). The World Health Organization (WHO) recommends that MAT predominantly in the form of MTH and BPN should be offered to those with opioid use disorders to improve morbidity as well as decrease transmission of HIV to the uninfected (World Health Organization et al., 2004); no specific recommendations have been made for AUDs globally, however. Unfortunately, it is difficult for released prisoners to take daily oral MAT regimens or even obtain prescriptions for these treatments either at time of release to the community or soon after release.
XR-NTX, the depot formulation of NTX that is administered intramuscularly every 28 days, provides an alternative treatment for both opioid and alcohol use disorders and is an opioid antagonist that does not require additional certifications or licenses to administer and store as the agonists do for opioid dependence (Gastfriend, 2011; Krupitsky and Blokhina, 2010). In 2006, XR-NTX was FDA-approved in the U.S. for the treatment of alcohol dependence, and in 2010 approved for treatment of opioid dependence. The monthly administration schedule of XR-NTX injections could potentially reduce adherence issues with daily oral medications (Kranzler et al., 2008; Minozzi et al., 2011; Swift et al., 2011) and eliminate concerns of diversion. Additionally, the protective properties of the long-acting formulation of NTX, due to the long half-life, may reduce the risk of relapse to opioids and alcohol as well as reduce risk of overdose (Hulse, 2005; Hulse and Tait, 2003) for prisoners upon release from the correctional system when administered prior to release. Despite these potential benefits, very few community settings or CJS settings offer XR-NTX.
In this observational study we describe the acceptability of initiation of XR-NTX in CJS settings and the retention on XR-NTX post-release into the community among people living with HIV disease (PLH) with alcohol and opioid use disorders as part of two NIH-funded double blind, placebo controlled trials of XR-NTX.
2. METHODS
2.1. Setting
In Connecticut approximately 16,551 persons (N=15,422 men, N=1,129 women as of July 2014) are incarcerated and are able to opt-in for HIV testing. 1.5% of men (N=234) and 2.4% of women (N=27) are HIV+ (personal communication, C. Gallagher CT Department of Correction [CTDOC], September 4, 2014; Connecticut Department of Correction, 2014). Approximately 60% of the population within the CTDOC that is living with HIV disease have an opioid use disorder, 80% have a cocaine use disorder, and 50% have an alcohol use disorder (Springer et al., 2010b, 2012) Voluntary substance use treatment counselors are available in some, but not all of the CT prisons and jails. MAT for alcohol use disorders is not routinely offered, while MTH has been offered for many years to pregnant women for detoxification upon incarceration. No form of opioid detoxification was offered for non-pregnant women or men until the year 2010, when BPN was offered to detoxify opioid dependent persons in the CT jails; and as of 2012 MTH was made available upon transition to the community for incarcerated women and men with opioid dependence in the one female CTDOC facility and within the New Haven city jail for men.
2.2. Approach
The eligibility, exclusion criteria, protocols, and planned analyses for primary outcomes of the two NIH-funded double-blind, placebo controlled trials of XR-NTX for PLH who are transitioning from prison or jail to the community with either (1) alcohol dependence or hazardous drinking (Project INSPIRE), or (2) opioid dependence (Project NEW HOPE), have been previously published (Di Paola et al., 2014b; Springer et al., 2014). Briefly, all HIV+ individuals incarcerated in CT with a history of drinking alcohol or opioid use (and opioid use in one jail in Springfield, MA) were eligible to participate in these two separate double-blind placebo-controlled randomized trials of XR-NTX to evaluate HIV-1 RNA viral load suppression and relapse to alcohol and opioid use outcomes, respectively, post-release to the community. This required written and verbal informed consent procedures during incarceration and again after release, education of the participants and clinicians regarding clinical uses and side effects of XR-NTX, as well as performing a physical assessment and clinical evaluation to ensure that all participants could safely receive the injections. As described in the previously published detailed protocols (Di Paola et al., 2014b; Springer et al., 2014), two-thirds of all participants are randomized to receive XR-NTX and one-third are to receive the placebo. All participants receive their first injection within 5–7 days prior to release from prison or jail to the community, some participants were released from a jail or prison facility to either a treatment or housing facility while under Department of Correction (DOC) care and were unable to receive their initial injection until their release into the community. The second injection is scheduled 25–35 days after the initial injection, within one-month postrelease to the community. In the event that a participant returns for their second injection after the scheduled date, he or she would miss that injection but the interview would be conducted and the next injection would be scheduled; an amendment to the studies was implemented in 2014 where participants who missed a study injection could receive the injection based on the closest due date.
2.3. Participants
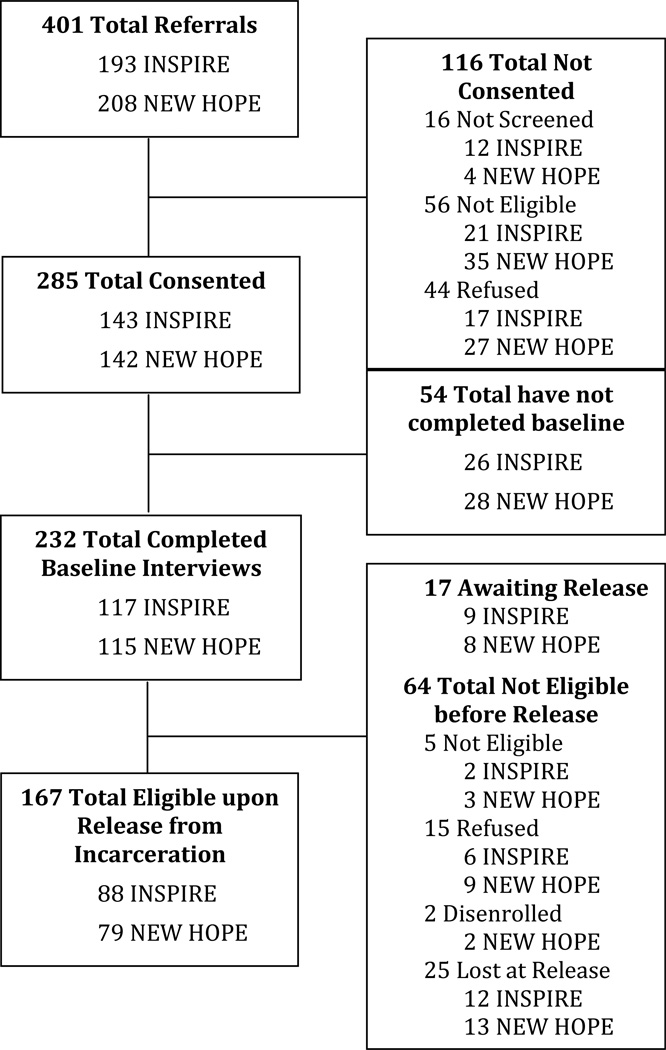
The sample of participants used in this analysis was combined from the studies described above. The enrollment consort diagram for the two studies is depicted in Figure 1. Of the 401 people who were referred to the two studies, 71.1% (285) consented to participate. Of those who did not consent to participate in one of the studies, 13.8% (N=16) did not complete the screening process, 28.3% (N=56) did not meet either study eligibility criteria, and 37.9% (N=44) refused to participate (reasons for refusal discussed in previous publications; Di Paola et al., 2014b; Springer et al., 2014). Of those who consented to participate in either of the studies, 81.4% (N=232) completed the baseline interview and 72.0% (N=167) were eligible for study injection upon release at the time of this analysis. These acceptability rates are similar to those found in community based treatment studies for injection drug users (Ahamad et al., 2015).
Figure 1.
Enrollment Flow
2.4. Ethical Responsibilities
Specific internal institutional review board (IRB) approval needed to be undertaken by the CTDOC as well as the Yale School of Medicine IRB for the CT sites (for a brief period approval was needed from Waterbury Hospital in CT to conduct the opioid study). Because these studies involve vulnerable populations, the Office of Human Research Protections at the Department of Health and Human Services granted additional protections and a Certificate of Confidentiality was obtained for both studies. Additional approvals were needed for the opioid study (Project NEW HOPE) at the MA site including the Baystate Medical Center IRB and the Hampden County Correctional Center. Both studies are registered at www.clinicaltrials.gov (NCT0124601, NCT0107731). A data and safety monitoring board for the opioid dependent study was also requested and set up as well.
2.5. Variable Definitions
Included in this analysis were consented participants who received their initial injection of the study drug. For this evaluation, the dependent variable was a dichotomous variable based upon whether or not the participant received the second injection in the one-month post–release time period.
Data used for this analysis included baseline interviews for behaviors prior to incarceration; day of release interviews for behavior during incarceration, and immediately after release; and CJS medical record review for additional data during incarceration. Interview data included at baseline: demographic data, housing status, the Alcohol Use Disorder Identification Test (AUDIT; Saunders et al., 1993) for alcohol use disorder, Addiction Severity Index (ASI; McLellan et al., 1992) for drug use history, and the Mini International Neuropsychiatric Interview (M.I.N.I.; Sheehan et al., 1997, 1998) for DSM-IV psychiatric and alcohol and substance use disorder diagnosis criteria; day of release interview data included urine toxicology results and housing status; and DOC medical record review included: HIV-1 RNA viral load, CD4 T lymphocyte count, and current HIV ART regimen.
Categorical demographic variables used in this analysis were recorded as part of the baseline interview, including: sex, highest level of education, and self-reported race/ethnicity. Calculated variables included age, from the date of birth to the date of the baseline interview; and months incarcerated was calculated from the dates of admission into the CTDOC and date of release; both where analyzed as continuous variables. Housing status was categorized into homeless (self-identifying as homeless: living on the street or in a shelter), unstably housed (in a transitional housing, in a drug treatment facility, living with a friend, or living in a group home), or stably housed (permanent single room occupancy, renting an apartment/home, or own their own home) for time periods prior to incarceration and post release.
Scores from the AUDIT conducted at baseline were dichotomously coded for hazardous drinking for women with a score of 4 or above and men with a score of 8 or above. Drug use behavior data as collected by the ASI included: lifetime drug use, drug use in the 30 days prior to incarceration and addition severity score; years of lifetime use and days use for the 30 days prior to incarceration of any alcohol use, alcohol use to intoxication, heroin, cocaine, methadone, opiates, barbiturates, and cannabis were analyzed as continuous variables. Addiction severity composite scores were calculated for alcohol and drug use; those with a score >0.17 for alcohol and >0.16 for drug use were coded as dichotomous variables.(Rikoon et al., 2006) Additional data in this analysis include the number of times the participant reported being detoxed for alcohol or drugs prior to incarceration, the sum of arrest charges in their lifetime, and psychiatric disorder symptoms.
Modules from the M.I.N.I. were coded dichotomously for those that met criteria for psychiatric disorders and for alcohol and substance use disorders.
Data collected for this analysis the correctional medical record review included: ART regimen, HIV-1 RNA viral load at time of release, and CD4 count at time of release. CD4 count was analyzed as a continuous variable; and HIV viral load data was coded into dichotomous variables of achieving viral suppression of <400 copies/mL and <50 copies/mL. Prescription of ART prior to release was also coded as a dichotomous variable for this analysis.
2.6. Statistical Analysis
To determine the association of the difference in retention between those that received their scheduled second injection of study drug and those that did not return for the second injection, bivariate logistic regressions were conducted using IBM SPSS statistical software version 19. Covariates that were reaching significance at p<0.10 were subsequently included in a multivariate logistical regression module, where odds ratios with 95% confidence intervals were reported; p<0.05 were deemed statistically significant for the multivariate analysis.
3. RESULTS
3.1. Baseline Demographics
As described in Table 1, the majority of the participants were male (95.6%), black non-Hispanic (47.5%) or Hispanic (41.1%), with a mean age of 44.7 years, met criteria for an alcohol use disorder (61.5%), opioid use disorder (50.0%) or cocaine use disorder (71.4%) via the MINI, and were incarcerated for an average of 11.3 months.
Table 1.
Demographic Characteristics
| Baseline Characteristic | N = 141 | Received 2nd injection N = 83 |
Did Not Receive 2nd Injection N=58 |
|---|---|---|---|
| Study | |||
| Inspire | 81 | 46 | 35 |
| New Hope | 60 | 37 | 23 |
| Gender | |||
| Female | 31 | 18 | 13 |
| Male | 109 | 65 | 44 |
| Transgender | 1 | 0 | 1 |
| Mean age | 44.7 (± 8.17) | 45.1 (± 7.47) | 44.3 (± 9.12) |
| Race | |||
| White non-Hispanic | 16 | 9 | 7 |
| Black non-Hispanic | 67 | 40 | 27 |
| Hispanic | 58 | 34 | 24 |
| Mean Months Incarcerated* | 11.8 (± 22.80) | 14.5 (± 28.38) | 7.9 (± 9.10) |
| Housing pre incarceration | |||
| Homeless | 50 | 28 | 22 |
| Unstable housing | 40 | 24 | 16 |
| Stable Housing | 51 | 31 | 20 |
| Study Site | |||
| New Haven | 61 | 33 | 28 |
| Hartford | 55 | 36 | 19 |
| Other | 25 | 14 | 11 |
| AUDIT Hazardous Drinking* | 97 | 52 | 45 |
| HIV Viral Load <400 copies/mL (N=136) | 89 | 55 | 34 |
| HIV Viral Load <50 copies/mL (N=136) | 45 | 30 | 15 |
| Mean CD4 Count (N=139) | 478.6 (± 273.6) | 498.2 (± 294.4) | 450.4 (± 240.4) |
| Prescribed ART at baseline | 105 | 67 | 38 |
| Addiction Severity Index | |||
| Experienced Serious Depression 30 days prior to incarceration | 78 | 51 | 27 |
| Alcohol Severity Composite Score >.17 | 93 | 51 | 42 |
| Drug Severity Composite Score >.16 | 100 | 55 | 45 |
| Positive urine toxicology at Day of Release interview, N=122 | |||
| Cocaine | 18 | 7 | 11 |
| Opiates | 4 | 2 | 2 |
| Marijuana | 6 | 4 | 2 |
| Breathalyzer >0.0 (N=117) | 4 | 3 | 1 |
| Mini International Psychiatric Interview N=136 | |||
| Current Major Depression | 47 | 29 | 18 |
| Bipolar Disorder | 5 | 3 | 2 |
| Post-Traumatic Stress Disorder | 19 | 10 | 9 |
| Generalized Anxiety Disorder | 15 | 9 | 6 |
| Antisocial Personality | 65 | 41 | 24 |
| Alcohol Use Disorder (N=135) | 83 | 46 | 37 |
| Narcotic Use Disorder (N=126) | 63 | 38 | 25 |
| Marijuana Use Disorder (N=117) | 23 | 15 | 8 |
| Cocaine Use Disorder (N=128) | 90 | 51 | 39 |
| Any Substance Use Disorder (N=135) | 108 | 63 | 45 |
| Any Alcohol AND a SUD (N=134) | 64 | 33 | 31 |
N=138
3.2. Reasons for not receiving the First Injection while incarcerated
As shown in Table 2, the initial study injection acceptability was 86.8% (N=145/167 participants who were eligible to receive an injection) for both studies combined with more accepting in the alcohol study (INSPIRE, 94.3%, N=83/88) compared to 78.5% of the participants enrolled in the opioid study (NEW HOPE, N=62/79). The predominant reason for not receiving the initial injection combined between the two studies was being ‘temporarily lost’ in which these participants were released prior to their expected DOC release date to the community and thus did not receive their first injection prior to release. The second most common reason for not receiving the initial injection was having a clinical need for prescription opioids (an exclusion criteria for XR-NTX as it is an opioid antagonist) and being released to DOC supervision and an inpatient drug treatment program where XR-NTX/placebo was not authorized. Another reason why some of the NEW HOPE participants did not accept receipt of an injection prior to release was a request for a form of opioid agonist treatment like BPN or MTH. Of note the majority of the opioid dependent participants in the NEW HOPE study had a history of prescription of methadone or buprenorphine treatment (79%) for their opioid addiction prior to their incarceration (data not shown).
Table 2.
Injection Acceptability
| COMBINED | Study INSPIRE |
NEW HOPE | |
|---|---|---|---|
| Initial Injection Acceptance | |||
| Received Injection | 145 of 167 (86.8%) | 83 of 88 (94.3%) | 62 of 79 (78.5%) |
| Reasons did not receive injection | |||
| Opioid dependent | 3 | 0 | 3 |
| Positive for opioids | 1 | 0 | 1 |
| Clinical need for opioids | 4 | 2 | 2 |
| Inpatient Drug Treatment | 4 | 2 | 2 |
| Moved out of area | 1 | 0 | 1 |
| Quit study | 1 | 0 | 1 |
| Refused injection | 1 | 1 | 0 |
| Temporarily Lost | 5 | 0 | 5 |
| Lost to follow-up | 2 | 0 | 2 |
| Second Injection Acceptance | |||
| Received Injection | 86 of 145 (59.3%) | 48 of 83 (57.8%)* | 38 of 62 (61.3%) |
| Reasons did not receive injection | |||
| Opioid dependent | 3 | 0 | 3 |
| Clinical need for opioids | 1 | 1 | 0 |
| Inpatient Hospital | 1 | 0 | 1 |
| Inpatient Drug Treatment | 5 | 2 | 3 |
| No recent labs on file | 1 | 1 | 0 |
| Reincarcerated | 7 | 5 | 2 |
| Not released after initial injection | 5 | 5 | 0 |
| Moved out of area | 1 | 1 | 0 |
| Quit study | 1 | 0 | 1 |
| Disenrolled, dementia | 1 | 1 | 0 |
| Refused injection | 3 | 3 | 0 |
| Temporarily Lost | 22 | 14 | 8 |
| Lost to follow-up | 7 | 1 | 6 |
plus 1 that did not receive the initial injection due to inpatient drug treatment
The proportion who received their second injection after release within 30 days to the community are depicted in Table 2 as well, where 59.3% of the eligible participants in the two combined studies received their second injection (N=86/145). The reasons for missing the injections were similarly matched between the studies. The predominant reason for not returning for their second injection was ‘temporarily lost’ where this represented persons who did not show for the second injection but later came for subsequent injections or interviews within the 6-month study injection period, and additional 6 month interview period. Participants who were considered ‘temporarily lost’ were later found and reengaged in the study while reincarcerated, admitted to the hospital or inpatient drug treatment facility, or found through community outreach after admitting to have been actively using drugs. These factors will be explored upon completion of the studies for retention in the studies during the full length of the incarceration.
3.3. Evaluation of Receipt of the Second Injection post-release
3.3.1. Bivariate Logistic Regression Analyses
Table 3 depicts selected results from the bivariate logistic regressions conducted. Covariates that were shown to be approaching statistical significance (p<0.10) for receiving the second injection were: (1) not meeting criteria for hazardous drinking via the AUDIT (p=0.035 OR 0.424 (CI 0.191–0.941)), (2) being prescribed ART prior to release (p=0.068 OR 2.170 (CI 0.934–4.992)), (3) expressed experiencing serious depression 30 days prior to incarceration via the ASI (p=0.068 OR 1.889 (CI 0.955–3.737)), (4) not having a positive cocaine urine toxicology screen on the day of release (p=0.011 OR 0.258 (CI 0.091–0.729)), and (5) meeting criteria for an alcohol use disorder plus any substance use disorder via the M.I.N.I. (p=0.068 OR 0.521 (CI 0.259–1.048)).
Table 3.
Bivariate Regression Results
| Baseline Characteristic | Bivariate Analysis | ||
|---|---|---|---|
| p value | Odds Ratio | 95% CI | |
| Study | |||
| Inspire | |||
| New Hope | 0.561 | 1.224 | 0.619–2.419 |
| Gender | |||
| Female | |||
| Male | 0.875 | 1.067 | 0.475–2.397 |
| Transgender | 1.000 | 0.000 | 0.000 |
| Mean age | 0.571 | 1.012 | 0.971–1.055 |
| Race | |||
| White non-Hispanic | |||
| Black non-Hispanic | 0.865 | 1.102 | 0.360–3.368 |
| Hispanic | 0.801 | 1.152 | 0.383–3.468 |
| Mean Months Incarcerated* | 0.087 | 1.029 | 0.996–1.063 |
| Housing pre incarceration | |||
| Homeless | 0.626 | 0.821 | 0.372–1.813 |
| Unstable housing | 0.939 | 0.968 | 0.415–2.256 |
| Stable Housing | |||
| Study Site | |||
| New Haven | |||
| Hartford | 0.215 | 1.608 | 0.759–3.403 |
| Other | 0.872 | 1.080 | 0.423–2.755 |
| AUDIT Hazardous Drinking* ** | 0.035 | 0.424 | 0.191–0.941 |
| HIV Viral Load <400 copies/mL | 0.622 | 1.198 | 0.584–2.460 |
| HIV Viral Load <50 copies/mL | 0.287 | 1.500 | 0.711–3.163 |
| CD4 Count | 0.311 | 1.001 | 0.999–1.002 |
| Prescribed ART at baseline** | 0.068 | 2.170 | 0.943–4.992 |
| Addiction Severity Index | |||
| Experienced Serious Depression 30 days prior inc** | 0.068 | 1.889 | 0.955–3.737 |
| Alcohol Severity Composite Score >.17 (ASI) | 0.122 | 0.486 | 0.194–1.214 |
| Drug Severity Composite Score >.16 (ASI) | 0.177 | 0.588 | 0.272–1.271 |
| Positive urine toxicology at Day of Release interview | |||
| Cocaine** | 0.011 | 0.258 | 0.091–0.729 |
| Opiates | 0.488 | 0.494 | 0.067–3.637 |
| Marijuana | 0.988 | 1.013 | 0.178–5.774 |
| Breathalyzer >0.0 | 0.696 | 1.581 | 0.159–15.710 |
| Mini International Psychiatric Interview N=136 | |||
| Current Major Depression | 0.620 | 1.200 | 0.583–2.473 |
| Bipolar Disorder | 0.941 | 1.071 | 0.173–6.628 |
| Post-Traumatic Stress Disorder | 0.265 | 0.538 | 0.181–1.601 |
| Generalized Anxiety Disorder | 0.878 | 0.911 | 0.275–3.011 |
| Antisocial Personality | 0.456 | 1.304 | 0.629–2.620 |
| Alcohol Use Disorder (N=135) | 0.253 | 0.658 | 0.320–1.348 |
| Narcotic Use Disorder (N=126) | 0.856 | 1.068 | 0.524–2.176 |
| Marijuana Use Disorder (n=117) | 0.755 | 1.164 | 0.449–3.020 |
| Cocaine Use Disorder (N=126) | 0.496 | 0.763 | 0.350–1.664 |
| Any Substance Use Disorder (N=135) | 0.662 | 0.824 | 0.345–1.965 |
| Any Alcohol AND SUD (N=134)** | 0.068 | 0.521 | 0.259–1.048 |
N=138;
significant p<0.10
Variables that were examined but did not approach statistical significance between the two groups and not depicted in Table 3 included: sexual orientation, educational level, years of lifetime use or days used in the past 30 days of any alcohol use, alcohol use to intoxication, heroin, cocaine, methadone, opiates, barbiturates, and cannabis, time reported being detoxed from alcohol or drugs, the sum of lifetime arrest charges, meeting criteria for stimulant use disorders, the number of substances he/she met criteria for substance use disorders, and having co-morbid hepatitis C infection. Additionally, not depicted in Table 3, having positive urine toxicology for benzodiazepines upon release from incarceration did not approach statistical significance between the two groups.
3.3.2. Multivariate Logistic Regression Analyses
The covariates that were found to be approaching statistical significance, as state in section 3.3.1, were then entered into multivariate logistic regression as shown in Table 4. Only having positive cocaine urine drug toxicology after release was statistically significantly associated with not returning for the second injection within the 30 days post- release (p=0.005; OR 0.207 (CI 0.068–0.623)).
Table 4.
Multivariate Regression Results
| Characteristic | Multivariate Analysis N=110 | ||
|---|---|---|---|
| p value | OR | 95% CI | |
| AUDIT Hazardous Drinking | 0.159 | 0.448 | 0.146–1.371 |
| Prescribed ART at baseline | 0.975 | 1.020 | 0.305–3.408 |
| Experienced Serious Depression 30 days | |||
| prior to incarceration | 0.415 | 1.443 | 0.598–3.483 |
| Comorbid Alcohol and Substance Use | |||
| Disorder per M.I.N.I. | 0.464 | 0.698 | 0.267–1.822 |
| Positive Cocaine Urine Toxicology at Day of Release* | 0.005 | 0.207 | 0.068–0.623 |
Significant p<0.05; Goodness of Fit, Nagelkerk R2 = 0.156
4. DISCUSSION
Although these studies are ongoing, they provide useful information regarding the implementation of XR-NTX among PLH within CJS settings prior to- and after release to the community. XR-NTX may be a viable option for CJ-involved persons to prevent relapse to opioid and alcohol use after release to the community and thus assist as a conduit to HIV and other medical care. Overall, there was high acceptability in both the opioid dependent population as well as the population with AUDs (over 80%) all with HIV infection and all within CJS settings even when enrolled in double blind, placebo-controlled randomized trials. Thus, the potential uptake of this treatment is high for this highly affected population.
Of special importance is the fact that the majority of the opioid dependent participants in the NEW HOPE study had a history of prescription of methadone or buprenorphine treatment (79%) for their opioid addiction prior to their incarceration. This is in stark contrast to the one published double blind, placebo-controlled randomized controlled trial of XR-NTX that led to the U.S. FDA approval of Vivitrol® (XR-NTX) for the treatment of opioid dependence (Krupitsky et al., 2011). This referenced trial was conducted in Russia and none of the participants had a history of prior opioid agonist treatments due to the unavailability of MTH and BPN in that country. It was determined early in the course of our study that one of the main reasons for refusal to participate in our study was the interest in opioid agonist treatments. The other issue was fear of needles, a well-known phenomenon among persons who inject drugs. Tolerability and side effects was another important issue in this study and participants were most interested in the potential for withdrawal if they were to be incarcerated again when the drug could no longer be given to them.
Similarly concerning is that not 100% of the eligible participants could receive the first injection prior to release to the community, and five of the participants were released early by the DOC, thus making them ‘temporarily lost’ with two additional participants completely lost for the study. This is important and speaks to the need of more careful discharge planning by CJS settings to ensure that inmates are adequately prepared prior to release to ensure retention in care for any program after release (Springer et al., 2011b).
Additional factors that affected retention included: (1) contact information given to research assistants by some participants while incarcerated was found to be invalid upon release, and in particular this transient group often changed addresses and phone numbers multiple times, thus affecting our ability to contact individuals to remind them of their subsequent follow up appointments; (2) recidivism back to prison / jail or living in a housing or treatment facility that would not allow phone calls made it difficult for our staff to contact participants and confirm appointments; and (3) although the research sites are on public transportation routes and we provided bus tokens, some participants stated they had difficulty with transportation to get to our offices.
Of note, although careful protocols were followed as previously published (Di Paola et al., 2014b; Springer et al., 2014) that included education of DOC staff and community providers as well as of the participants about side effects and safety of XR-NTX, monetary remuneration, cell phone reminders, optional individual and group substance use counseling, optional case management services, and very close follow-up with research assistants, only approximately 60% of those who received their first injection while incarcerated came back for their second injection after release to the community. There was a high rate of recidivism within the first 6 months post release of initiating the study. Although specific reasons for rearrests are not described in this analysis, it does speak to the high recidivism rate among previously incarcerated persons in CT as previously reported (Meyer et al., 2014a, 2014b; Springer et al., 2004); it is possible that the behavior leading to the rearrests was initiated upon release and may include illicit drug use as a cause for reduced retention in this study given the high prevalence of comorbid substance use disorders in this sample (Meyer et al., 2014a). Reducing the recidivism rate includes not only alcohol and drug treatment as addressed by the two parent randomized controlled trials in this paper, but also importantly includes psychiatric disorder evaluation and treatment, linkage to effective housing, as well as identification of other important psychosocial factors that have been found to be associated with recidivism and poor retention in care post-release (Di Paola et al., 2014a; Meyer et al., 2014a, 2014b; Springer et al., 2011b). Recidivism will be explored in the retention for the full intervention period.
Of the factors found to be associated with a decrease in receipt of XR-NTX post release, cocaine use at time of release was the only factor after multiple regression analyses that remained statistically significantly associated with poor likelihood for returning for the second injection within one month post-release. It is well known that cocaine use negatively impacts adherence to ART and retention in HIV care (Chitsaz et al., 2013; Krishnan et al., 2013; Sullivan et al., 2011), is associated with increased risk for psychiatric disorders and HIV risk behaviors (Roy et al., 2015), and interferes with retention in drug treatment care with other forms of opioid treatment such as MTH (Levine et al., 2015) and BPN (Sullivan et al., 2010). Thus, this finding in itself might not be surprising. It does, however, highlight the fact that drug relapse is common in persons returning from incarcerated settings despite incarceration for a mean time of almost 12 months as identified in these two combined studies of PLH with substance use disorder. More importantly, it is concerning that without yet having effective MAT for preventing cocaine relapse, all treatments for opioid and alcohol use disorders, not just XR-NTX, will be difficult to adhere to if more attention is not paid to the relapse potential for those with cocaine use disorders identified prior to incarceration. More MAT development for cocaine use disorders is urgently needed, and linkage with cognitive behavioral programs, the most effective treatment for cocaine use disorders, is urgently required to prevent relapse to cocaine for this population. If cocaine use impacts the ability of released CJ-involved persons to stay retained on XR-NTX for relapse prevention to opioid and alcohol use, then the likelihood of reducing morbidity and mortality from relapse to substance use as well as improving linkage to care for PLH will be poor without effective strategies to identify cocaine use disorders prior to release.
4.1. Limitations
The two studies are on-going; thus, final unblinding of the studies could not be done at this time. It is possible that those who received the placebo (one-third of participants) were less likely to return for the injection and this could be an important variable in these analyses. Despite this, there are important results from this analysis that underscore the high acceptability of XR-NTX among PLH in CJ-settings, as well as implications of the effect of relapse to cocaine use on retention on XR-NTX post-release for routine clinical care for opioid and alcohol use disorders. Additionally, a larger sample size may change the levels of significance for variables in the multivariate analysis for independent variables trending toward significance.
4.2 Conclusions
XR-NTX is highly acceptable for CJS-involved PLH with alcohol and opioid use disorders. Although almost 90% of study participants received their first injection prior to incarceration, less than 60% returned for their next injection within 28 days after release to the community. Retention in this observational study for receipt of XR-NTX are close to what has been found in other studies of XR-NTX (Comer et al., 2006; Lee et al., 2015), MMT (Proctor et al., 2015), and BPN (Soyka et al., 2008; Stein et al., 2005), followed for longer periods. Relapse to cocaine use was found to be the only statistically significant variable associated with not returning for their second injection after release after multivariate regression analyses were completed. Thus, interventions are required to identify not only alcohol and opioid use disorders for which we have effective MAT for treatment prior to release from CJS settings, but also to identify a history of cocaine use disorder for which we do not yet have effective MAT interventions. Persons with comorbid cocaine use disorders who are identified as candidates for starting MAT to prevent relapse to alcohol and opioid use at time of release, might be targeted for closer monitoring discharge planning interventions that may include cognitive behavioral interventions to prevent relapse to cocaine use at time of release, thus allowing them to continue to participate in their MAT interventions for alcohol and opioid use disorder and improve linkage to care in the community and, most importantly, reduce morbidity and mortality.
Highlights.
XR-NTX is highly accepted by HIV+ opioid and alcohol dependent CJ-involved persons
Relapse to cocaine use is associated with poor retention on XR-NTX after release
Identification of cocaine addiction is necessary to improve retention on XR-NTX
Acknowledgements
Role of Funding Source: Research reported in this publication was supported by the National Institutes of Health under Award Numbers NIAAA R01 AA018944 (INSPIRE, SAS and FLA); NIDA R01 DA030762 (NEW HOPE, SAS and FLA); NIDA K02 DA032322 (SAS); and NIDA K24 DA017072 (FLA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Both studies’ medication and placebo were provided in-kind through investigator-initiated grants from Alkermes, Inc. The authors would like to thank co-Investigators Thomas Lincoln, MD, Daniel Skiest, MD, Baystate Study Director Maureen Desabrais; the study participants, Connecticut Department of Corrections, Hampden County Correctional Center, all research staff and the funding sources as indicated above.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none
Contributors: All authors have read and approved the final manuscript submitted for submission. S.A.S. designed the studies and prepared the manuscript. F.L.A contributed to the design of the studies and contributed to the analytic approach. A.D. conducted the analyses. S.B. provided editing to the analyses and manuscript.
REFERENCES
- Ahamad K, Milloy MJ, Nguyen P, Uhlmann S, Johnson C, Korthuis TP, Kerr T, Wood E. Factors associated with willingness to take extended release naltrexone among injection drug users. Addict. Sci. Clin. Pract. 2015;10:12. doi: 10.1186/s13722-015-0034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, Koepsell TD. Release from prison--a high risk of death for former inmates. N. Engl. J. Med. 2007;356:157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitsaz E, Meyer JP, Krishnan A, Springer SA, Marcus R, Zaller N, Jordan AO, Lincoln T, Flanigan TP, Porterfield J, Altice FL. Contribution of substance use disorders on HIV treatment outcomes and antiretroviral medication adherence among HIV-infected persons entering jail. AIDS Behav. 2013;17(Suppl. 2):S118–S127. doi: 10.1007/s10461-013-0506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O'Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch. Gen. Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connecticut Department of Correction. 2014 http://www.ct.gov/doc/lib/doc/PDF/MonthlyStat/Stat201407.pdf.accessed on.
- Di Paola A, Altice FL, Powell ML, Trestman RL, Springer SA. A comparison of psychiatric diagnoses among HIV-infected prisoners receiving combination antiretroviral therapy and transitioning to the community. Health Justice. 2014a;2 doi: 10.1186/s40352-014-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola A, Lincoln T, Skiest DJ, Desabrais M, Altice FL, Springer SA. Design and methods of a double blind randomized placebo-controlled trial of extended-release naltrexone for HIV-infected, opioid dependent prisoners and jail detainees who are transitioning to the community. Contemp. Clin. Trials. 2014b;39:256–268. doi: 10.1016/j.cct.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastfriend DR. Intramuscular extended-release naltrexone: current evidence. Ann. N.Y. Acad. Sci. 2011;1216:144–166. doi: 10.1111/j.1749-6632.2010.05900.x. [DOI] [PubMed] [Google Scholar]
- Hulse GK. Reducing hospital presentations for opioid overdose in patients treated with sustained release naltrexone implants. Drug Alcohol Depend. 2005;79:351–357. doi: 10.1016/j.drugalcdep.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulse GK, Tait RJ. A pilot study to assess the impact of naltrexone implant on accidental opiate overdose in 'high-risk' adolescent heroin users. Addict. Biol. 2003;8:337–342. doi: 10.1080/13556210310001602257. [DOI] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O'Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J. Subst. Abuse Treat. 2009;37:277–285. doi: 10.1016/j.jsat.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, O'Grady KE. A study of methadone maintenance for male prisoners: 3-month postrelease outcomes. Crim. Justice Behav. 2008;35:34–47. doi: 10.1177/0093854807309111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Stephenson JJ, Montejano L, Wang S, Gastfriend DR. Persistence with oral naltrexone for alcohol treatment: implications for health-care utilization. Addiction. 2008;103:1801–1808. doi: 10.1111/j.1360-0443.2008.02345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Wickersham JA, Chitsaz E, Springer SA, Jordan AO, Zaller N, Altice FL. Post-release substance abuse outcomes among HIV-infected jail detainees: results from a multisite study. AIDS Behav. 2013;17(Suppl. 2):S171–S180. doi: 10.1007/s10461-012-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Blokhina EA. Long-acting depot formulations of naltrexone for heroin dependence: a review. Curr. Opin. Psychiatry. 2010;23:210–214. doi: 10.1097/YCO.0b013e3283386578. 210. [DOI] [PubMed] [Google Scholar]
- Lee JD, McDonald R, Grossman E, McNeely J, Laska E, Rotrosen J, Gourevitch MN. Opioid treatment at release from jail using extended-release naltrexone: a pilot proof-of-concept randomized effectiveness trial. Addiction. 2015;110:1008–1014. doi: 10.1111/add.12894. [DOI] [PubMed] [Google Scholar]
- Levine AR, Lundahl LH, Ledgerwood DM, Lisieski M, Rhodes GL, Greenwald MK. Gender-specific predictors of retention and opioid abstinence during methadone maintenance treatment. J. Subst. Abuse Treat. 2015;54:37–43. doi: 10.1016/j.jsat.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Lim S, Seligson AL, Parvez FM, Luther CW, Mavinkurve MP, Binswanger IA, Kerker BD. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from new york city jails, 2001–2005. Am. J. Epidemiol. 2012;175:519–526. doi: 10.1093/aje/kwr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruschak L, Beavers R. HIV in Prisons, 2007–08. In: Bureau of Justice Statistics, editor. Bureau of Justice Statistics Bulletin. Washington, D.C.: U.S. Department of Justice, Office of Justice Programs; 2009. [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J. Subst. Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Meyer JP, Cepeda J, Springer SA, Wu J, Trestman RL, Altice FL. HIV in people reincarcerated in Connecticut prisons and jails: an observational cohort study. Lancet HIV. 2014a;1:e77–e84. doi: 10.1016/S2352-3018(14)70022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JP, Cepeda J, Wu J, Trestman RL, Altice FL, Springer SA. Optimization of human immunodeficiency virus treatment during incarceration: viral suppression at the prison gate. JAMA Int. Med. 2014b;174:721–729. doi: 10.1001/jamainternmed.2014.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst. Rev. 2011:CD001333. doi: 10.1002/14651858.CD001333.pub2. [DOI] [PubMed] [Google Scholar]
- Pew Center on the States. One in 31: The Long Reach of American Corrections. The Pew Charitable Trusts; Washington, DC: 2009. [Google Scholar]
- Proctor SL, Copeland AL, Kopak AM, Hoffmann NG, Herschman PL, Polukhina N. Predictors of patient retention in methadone maintenance treatment. Psychol. Addict. Behav. 2015 doi: 10.1037/adb0000090. [DOI] [PubMed] [Google Scholar]
- Rikoon SH, Cacciola JS, Carise D, Alterman AI, McLellan AT. Predicting DSM-IV dependence diagnoses from Addiction Severity Index composite scores. J. Subst. Abuse Treat. 2006;31:17–24. doi: 10.1016/j.jsat.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Roy E, Jutras-Aswad D, Bertrand K, Dufour M, Perreault M, Laverdiere E, Bene-Tchaleu F, Bruneau J. Anxiety, mood disorders and injection risk behaviors among cocaine users: results from the COSMO study. Am. J. Addict. 2015;24:654–660. doi: 10.1111/ajad.12286. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Harnett-Sheehan K, Janavs J, Weiller E, Bonors L, Keskiner A, Schinka J, Knapp E, Sheehan M, Dunbar G. Reliability and validity of the MINI International Neuropsychiatric Interview (M.I.N.I.): according to the SCID-P. Eur. Psychiatry. 1997;12:232–241. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar G. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. International. J. Neuropsychopharmacol. 2008;11:641–653. doi: 10.1017/S146114570700836X. [DOI] [PubMed] [Google Scholar]
- Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PLoS One. 2009;4:e7558. doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S. Commentary on Larney (2010a): a call to action—opioid substitution therapy as a conduit to routine care and primary prevention of HIV transmission among opioid-dependent prisoners. Addiction. 2010;105:224–225. doi: 10.1111/j.1360-0443.2009.02893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Altice FL, Herme M, Di Paola A. Design and methods of a double blind randomized placebo-controlled trial of extended-release naltrexone for alcohol dependent and hazardous drinking prisoners with HIV who are transitioning to the community. Contemp. Clin. Trials. 2014;37:209–218. doi: 10.1016/j.cct.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Azar MM, Altice FL. HIV, alcohol dependence, and the criminal justice system: a review and call for evidence-based treatment for released prisoners. Am. J. Drug Alcohol Abuse. 2011a;37:12–21. doi: 10.3109/00952990.2010.540280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Chen S, Altice FL. Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment. J. Urban Health. 2010b;87:592–602. doi: 10.1007/s11524-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin. Infect. Dis. 2004;38:1754–1760. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- Springer SA, Qiu J, Saber-Tehrani AS, Altice FL. Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PLoS One. 2012;7:e38335. doi: 10.1371/journal.pone.0038335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Spaulding AC, Meyer JP, Altice FL. Public health implications for adequate transitional care for hiv-infected prisoners: five essential components. Clin. Infect. Dis. 2011b;53:469–479. doi: 10.1093/cid/cir446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Patricia C, Friedmann PD. Buprenorphine retention in primary care. J. Gen. Intern. Med. 2005;20:1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LE, Botsko M, Cunningham CO, O'Connor PG, Hersh D, Mitty J, Lum PJ, Schottenfeld RS, Fiellin DA, Collaborative B. The impact of cocaine use on outcomes in HIV-infected patients receiving buprenorphine/naloxone. J. Acquir. Immune Defic. Syndr. 2011;56(Suppl. 1):S54–S61. doi: 10.1097/QAI.0b013e3182097576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LE, Moore BA, O'Connor PG, Barry DT, Chawarski MC, Schottenfeld RS, Fiellin DA. The association between cocaine use and treatment outcomes in patients receiving office-based buprenorphine/naloxone for the treatment of opioid dependence. Am. J. Addict. 2010;19:53–58. doi: 10.1111/j.1521-0391.2009.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift R, Oslin DW, Alexander M, Forman R. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J. Stud. Alcohol Drugs. 2011;72:1012–1018. doi: 10.15288/jsad.2011.72.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, United Nations Office On Drugs and Crime, and the Joint United Nations Programme on HIV/AIDS. Geneva: WHO; 2004. Joint Position Paper on Substitution Maintenance Therapy in the Management of Opioid Dependence and HIV/AIDS Prevention. [Google Scholar]