Abstract
Current cell sheet-based blood vessels lack biomimetic structure and require excessively long culture times that may compromise smooth muscle cell phenotype. We modified a commercially available product for uniaxial cell sheet conditioning with thermoresponsive copolymers. Thus, culture of detachable conditioned cell sheets is shortened while retaining structural integrity and contractility.
Injury to cardiac tissue from ischemia often results in loss of contractile activity and ultimately reduces tissue function.1 Some therapeutic strategies have tried directly injecting cells or delivering cell-seeded scaffolds at the injury site; however, these methods are plagued with cell retention problems and teratoma formation.2,3 Although some regenerative therapies have been tested in clinical studies,4 currently no viable clinical options are commercially available to replace damaged myocardium and restore proper function.
Tissue engineering offers promise of true regeneration; however, a major obstacle is creating thick tissues (>2 mm) that remain viable to prevent necrosis from insufficient oxygen and nutrient diffusion.5 Cardiovascular cells also experience dynamic stresses constantly, and physiological feedback signals are fundamental to development and remodeling.6 Thus, truly regenerative cardiovascular tissue also requires mechanical and contractile properties that match the native tissue, followed by integration, and electrical and mechanical coupling to the host tissue.
Because polymeric scaffolding biomaterials may elicit undesired inflammation reactions,7–9 attention has turned to cell sheet technology to engineer scaffold-less three-dimensional tissues.10,11 Current commercial technology (UpCell, Thermo Scientific Nunc, Waltham, MA) allows wholly intact cell sheets to detach spontaneously and reversibly without enzymatic treatments using thermoresponsive polymer poly(N-isopropylacrylamide) (P(NIPAAm)). Cell sheets can attach at 37 °C; following a conformation change at temperatures below its lower critical solution temperature (LCST) at 32 °C, P(NIPAAm) becomes a loose, hydrated network, causing extracellular matrix (ECM) to detach intact with the cell sheet.12 Thus, 2-dimensional cell sheets can be detached and stacked to form 3-dimensional scaffold-less tissue. However, as P(NIPAAm) in the commercially available cell sheet technology is electron-beam grafted onto inelastic tissue culture polystyrene (TCPS), cells cultured on these surfaces cannot be subjected to dynamic stresses, such as cyclic strain. Mechanical stress is well-known to be a regulator of ECM gene expression,13–15 and changing the ECM profile may increase the robustness of a fragile cell sheet by changing its tissue mechanical properties. Thus, P(NIPAAm) grafted on a flexible substrate would be ideal to mechanically condition cell sheets that can subsequently detach without damage.
In this communication, we demonstrate that we can graft P(NIPAAm) copolymer on the elastic membranes of commercially available UniFlex plates (Flexcell, Hillsborough, NC), which enable users to apply cyclic uniaxial strain to cells. Subsequently, we can allow cell sheets to be mechanically conditioned on the Flexcell platform before detachment. Conditioned cell sheets can be dislodged at room temperature within 10 min without requiring any assistance. We also demonstrate that after transfer to another surface, the conditioned cell sheet remains viable and retains cellular alignment induced from stretching.
Previously, one of the authors modified the elastic surfaces of BioFlex plates bonded with a low density of amines (BioFlex, Flexcell) with a copolymer of P(NIPAAm) and acrylic acid (AAc) using N,N′-dicyclohexylcarbodiimide (DCC) (Scheme 1, left).16 P(NIPAAm-co-AAc) retained an LCST below cell culture temperature (37 °C) at 34 °C. Single cells could be detached from the silicone surface both before and after mechanical conditioning with a reduction in temperature. BioFlex plates have a single membrane that allow cells to be strained equiaxially; however, many native tissues require uniaxial strains, such as blood vessels. Thus, a cell culture platform that allows uniaxial strain to be applied to cell sheets that are subsequently detached would be a valuable tool to tissue engineers. The manufacturer of the BioFlex plates also produces UniFlex plates with amines bonded to the elastic surface as in BioFlex plates, but UniFlex plates also feature a strain field that is a 1 mm thick double membrane with an area of 15.25 mm × 24.18 mm or 3.68 cm2, with uniaxial strain orientation along the long axis (Fig. 1). As a result of the double membrane, water-insoluble reaction byproduct dicyclohexyl urea (DCU) was trapped between the membranes using the methods from the previous study (Fig. S1, ESI†).16 Whereas BioFlex plates modified with P(NIPAAm-co-AAc) can be cleared of DCU and other excess reagents with 3–4 cycles of the washing process (rinse in excess tert-butanol at 30 °C for 20–40 min, followed by a rinse in excess room temperature water), removing DCU from UniFlex plates was inconsistent and required numerous wash cycles over the course of 4–7 days at 3 h at a time. The repeated swelling consequently compromised the integrity of the membrane on occasion (Fig. S2, ESI†) and rendered the plate ineffective for mechanically conditioning cell sheets. Additionally, cell growth was slower if enough DCU was not removed from between the membranes (data not shown).
Scheme 1.
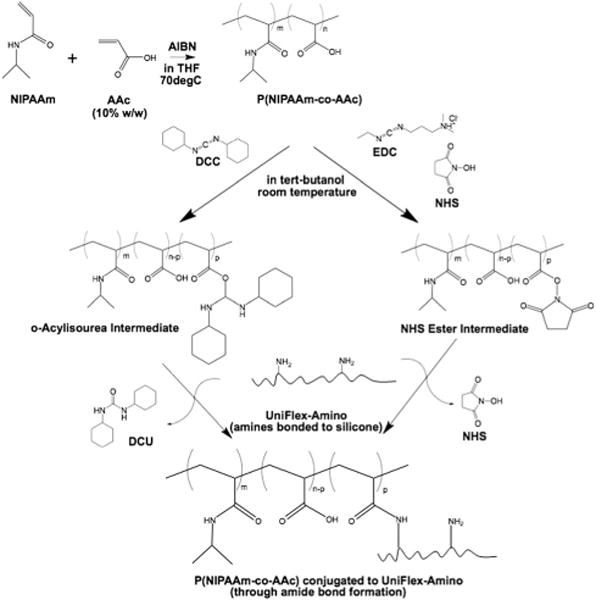
Conjugation of temperature-responsive P(NIPAAm) copolymer through amide bond formation to UniFlex-Amino cell culture plate using DCC (left) or EDC and NHS (right).
Fig. 1.
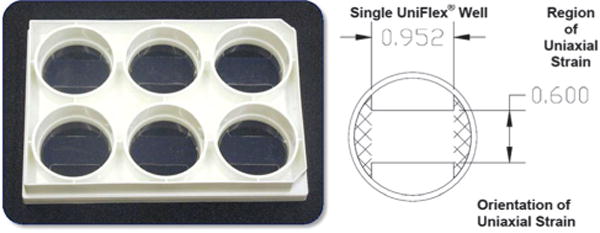
Dimensions of uniaxial strain field in a UniFlex plate.
Attempts to use water as the solvent to conjugate P(NIPAAm-co-AAc) alone with the less toxic 1-ethyl-3-[3-dimethylamino-propyl]carbodiimide hydrochloride (EDC) or in conjunction with N-hydroxysuccinimide (NHS) to increase stability of the intermediate products were unsuccessful (data not shown). Given the low density of target amines on the silicone surface that required the membrane to be swollen to access any primary amines,16 we surmised we could perform the reaction in organic solvent (Scheme 1, right).
Following complete dissolution of P(NIPAAm-co-AAc) (10 mg ml−1) in tert-butanol, 400 μg ml−1 EDC and 600 μg ml−1 NHS were added to the solution and allowed to react for 15 min at room temperature. At the end of the reaction time, EDC was quenched with 0.23 μl ml−1 β-mercaptoethanol. Simultaneously during the solution reaction, we swelled the silicone substrates in the UniFlex plate to equilibrium with tert-butanol (1 ml per well). The activated copolymer solution was then added (1 ml per well), along with additional tert-butanol (1 ml per well). Plates were agitated and allowed to react for 2 h at room temperature. The reaction was quenched with 50 mM Tris (pH 6.8). Excess reagents were removed from the wells before washing in phosphate buffered saline (PBS) with agitation overnight at room temperature. This method simplifies each step and significantly reduces the time required to conjugate P(NIPAAm-co-AAc) to UniFlex-Amino plates from 4–7 days to 12 h. Prior to characterizing the substrate surfaces, UniFlex-Amino plates modified with P(NIPAAm-co-AAc) were rinsed in deionized water at room temperature to remove any remaining salts.
To determine if the reaction was successful, horizontal attenuated total reflectance (HATR, Pike Technologies, Madison, WI) for Fourier transform infrared (FTIR) spectroscopy (Nicolet 4700, Thermo Scientific) was used to detect any surface modifications. The ratio of the peaks characteristic for P(NIPAAm) (i.e., 1650 cm−1 for carbonyls, 3300 cm−1 for amides) to the peaks characteristic for silicone (i.e., 1000–1100 cm−1 for methyl antisymmetric stretching) was approximately 2.5 times greater for P(NIPAAm-co-AAc)-modified UniFlex plates in comparison to nonmodified UniFlex plates (Fig. 2), indicating successful conjugation.
Fig. 2.
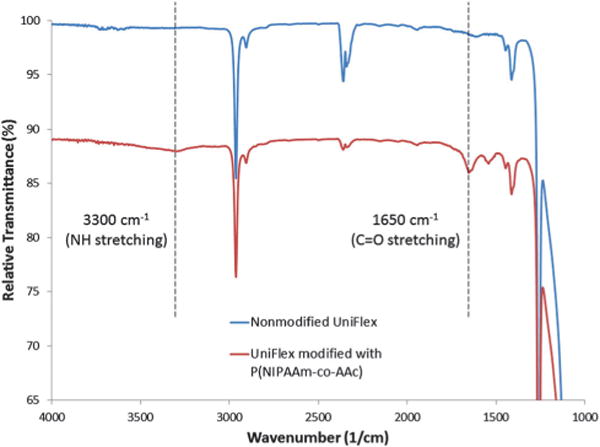
Attenuated total reflectance FTIR of nonmodified and P(NIPAAm-co-AAc)-grafted UniFlex substrates.
To show thermal reversibility in comparison to nonmodified silicone membrane surfaces, a contact angle goniometer and the pendant drop method were used to measure the surface tension of water on modified silicone membrane surfaces after washing at 37 °C and room temperature. No significant changes in contact angle (109.18 ± 0.6 degrees at 37 °C vs. 110.37 ± 1.2 degrees at room temperature, N = 4, P = 0.12) was observed for nonmodified silicone membranes (Fig. 3). In the previous study, no significant change in water contact angle was observed in TCPS surfaces as well.16 On the other hand, modified silicone surfaces conjugated with P(NIPAAm-co-AAc) using EDC and NHS exhibited a significant change in water contact angle (104.83 ± 1.6 degrees at 37 °C vs. 100.44 ± 0.82 degrees at room temperature, N = 8, P = 7.24 × 10−6) in response to a change in temperature of the substrate surface.
Fig. 3.
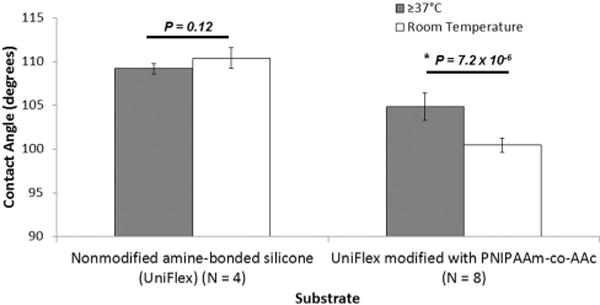
Contact angle of thermally reversible culture substrates above and below the LCST of P(NIPAAm-co-AAc).
Modified UniFlex silicone surfaces were tested for cell attachment and detachment with a change in temperature in comparison to nonmodified surfaces. Prior to cell culture, we treated the surface with ultraviolet light for 15–20 min to avoid contamination. Bovine vascular smooth muscle cells (BVSMCs), which are present in the tunica media of vasculature and generate contraction in the vessel for pulsatile blood flow, were grown into confluent cell sheets on nonmodified and modified substrates. In the previous study,16 UpCell surfaces were used as a positive control for cell sheets detached from thermo-responsive P(NIPAAm) for comparison to cell sheets that did not detach. Detached single cells (Fig. S3, ESI†) and cell sheets (Fig. 4) from this study showed a rounded morphology as displayed by single cells and cell sheets detached from UpCell dishes after temperature transition without requiring enzymatic treatments or other harmful physical means. Whereas substrates modified using methods from the previous study required gentle shear flow from a pipette to aid cell sheet detachment, we demonstrated in this study that cell sheets were capable of detaching completely spontaneously after a decrease in temperature. In addition, cell sheets cultured on substrates modified using the previous methods required anywhere from 20 min to 1 h for complete sheet detachment (Fig. 4, center column); in this study, we were able to demonstrate that cell sheets could consistently be detached 10–20 min following temperature decrease using the newer methods (Fig. 4, right column). Such a significant improvement in detachment time will allow for greater ease of handling and manipulating cell sheets without compromising cell viability.
Fig. 4.
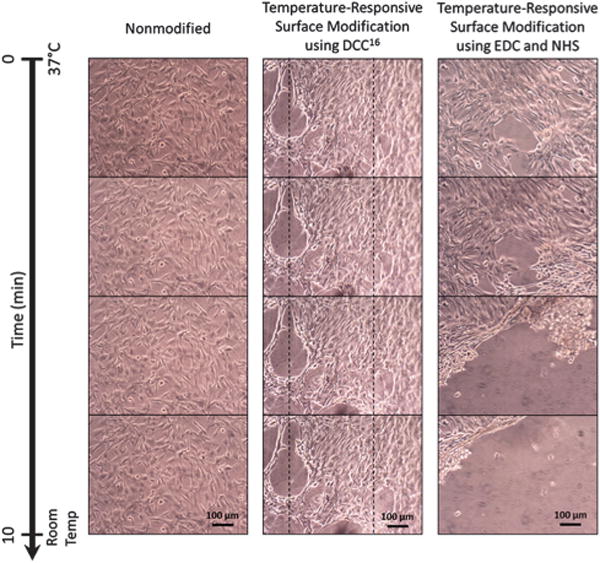
Cell sheets of BVSMCs detached over the course of 10 min from nonmodified UniFlex-Amino plates (left) and UniFlex-Amino plates modified with temperature-responsive copolymer P(NIPAAm-co-AAc) using DCC (center) or EDC and NHS (right). Dashed lines in the center column give a point of reference for cell sheet contraction during detachment.
Following mechanical conditioning (10% elongation strain, 1 Hz for 24 h), cell sheets were transferred to TCPS using the gelatin gel method17,18 and stained with live/dead cell viability kit (Life Technologies, Grand Island, NY), where live cells are stained green and dead cells are stained red. Stained cell sheets indicated they were still viable 24 and 72 h after transfer (Fig. 5). Furthermore, transferred cell sheets were quantified for cellular alignment as previously described.19 Briefly, 2D fast Fourier transform was used on the transferred cell sheet images (Fig. 5) to transform the spatial information into the frequency domain. ImageJ plug-in “Oval Profile” was used to determine radial sums, sampling every 6°, making the expected frequency distribution for a perfectly isotropic image 0.0333. To calculate percentage of aligned cells, distribution values were summed within 30° of the axis perpendicular to the direction of principal strain, a phenomenon known as strain-avoidance.15,20–22 A perfectly isotropic cell sheet would be approximately 36% of cells oriented within 30° of this axis; any values above 36% indicate increased alignment. At 24 h after cell sheet transfer, approximately 38% of cells were aligned within the calculated distribution (Fig. 6). At 72 h, aligned cells increased to approximately 41% of cells, which indicates cells from transferred sheets may influence alignment of proliferating cells. Thus, transferred cell sheets showed that alignment could be retained from induced mechanical conditioning, a key quality that will aid the tissue engineering of biomimetic tissues.
Fig. 5.
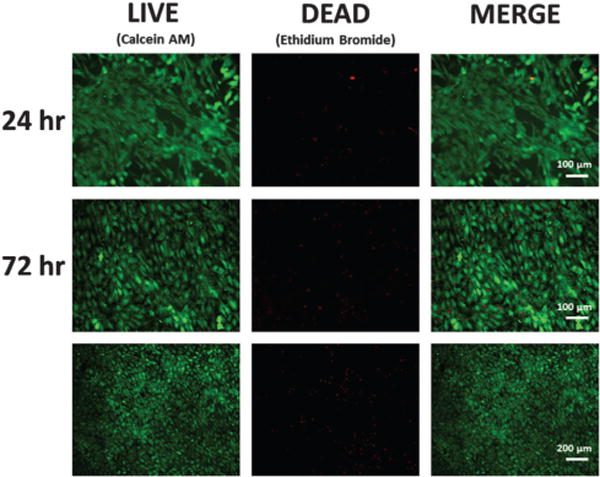
Live/dead viability stain of conditioned cell sheets 24 h and 72 h after transfer. (Note change in scale in last row to show larger area).
Fig. 6.
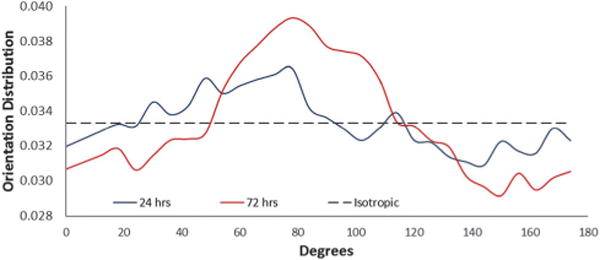
Transferred cell sheets were able to retain alignment after 24 h, which increased by 72 h after transfer.
Conclusions
We demonstrated that temperature-responsive NIPAAm synthesized into a copolymer with AAc can be conjugated to commercially available UniFlex-Amino plates using carbodiimide chemistry that eliminates insoluble waste products, thus reducing time necessary for grafting and washing from 4–7 days to 12 h, from the previously published method. In addition, we also showed that cell sheets can be detached wholly intact following a decrease in temperature, and the time to sheet detachment has been reduced to 10–20 min. Conditioned cell sheets that have been transferred to other culture surfaces were also able to remain viable and retain induced cellular alignment. Improving the method for producing UniFlex-Amino plates conjugated with temperature-responsive P(NIPAAm-co-AAc) can accelerate future studies that aim to examine the role mechanical conditioning plays in a single cell sheet and on subsequent tissue-level mechanical properties in tissue-engineered blood vessels and myocardium.
Supplementary Material
Acknowledgments
E. L. Lee was supported by Postdoctoral Fellowship Awards from the National Heart, Lung and Blood Institute under Award Number F32HL115999 and the National Institute of Allergy and Infectious Disease under Award Number T32AI089673. H. H. Bendre was supported by the Boston University Undergraduate Research Opportunities Program (UROP). This work was supported in part by The Hartwell Foundation and a Boston University College of Engineering Distinguished Professorship to J. Y. Wong.
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/ c5tb01171j
Notes and references
- 1.Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. J Biol Chem. 1998;273:11619–11624. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- 2.Damjanov I. Verh Dtsch Ges Pathol. 2004;88:39–44. [PubMed] [Google Scholar]
- 3.Lees JG, Lim SA, Croll T, Williams G, Lui S, Cooper-White J, McQuade LR, Mathiyalagan B, Tuch BE. Regener Med. 2007;2:289–300. doi: 10.2217/17460751.2.3.289. [DOI] [PubMed] [Google Scholar]
- 4.de Lazaro I, Yilmazer A, Kostarelos K. J Controlled Release. 2014;185C:37–44. doi: 10.1016/j.jconrel.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Griffith CK, Miller C, Sainson RC, Calvert JW, Jeon NL, Hughes CC, George SC. Tissue Eng. 2005;11:257–266. doi: 10.1089/ten.2005.11.257. [DOI] [PubMed] [Google Scholar]
- 6.Jones EA. Semin Cell Dev Biol. 2011;22:1028–1035. doi: 10.1016/j.semcdb.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Greisler HP. Ann Vasc Surg. 1990;4:98–103. doi: 10.1007/BF02042699. [DOI] [PubMed] [Google Scholar]
- 8.Bergamini TM, Bandyk DF, Govostis D, Kaebnick HW, Towne JB. J Vasc Surg. 1988;7:21–30. [PubMed] [Google Scholar]
- 9.Mertens RA, O’Hara PJ, Hertzer NR, Krajewski LP, Beven EG. J Vasc Surg. 1995;21:782–790. doi: 10.1016/s0741-5214(05)80009-6. discussion 790–781. [DOI] [PubMed] [Google Scholar]
- 10.Elloumi-Hannachi I, Yamato M, Okano T. J Intern Med. 2010;267:54–70. doi: 10.1111/j.1365-2796.2009.02185.x. [DOI] [PubMed] [Google Scholar]
- 11.von Recum HA, Kim SW, Kikuchi A, Okuhara M, Sakurai Y, Okano T. J Biomed Mater Res. 1998;40:631–639. doi: 10.1002/(sici)1097-4636(19980615)40:4<631::aid-jbm15>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Canavan HE, Cheng X, Graham DJ, Ratner BD, Castner DG. Langmuir. 2005;21:1949–1955. doi: 10.1021/la048546c. [DOI] [PubMed] [Google Scholar]
- 13.Chiquet M. Matrix Biol. 1999;18:417–426. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 14.Chiquet M, Matthisson M, Koch M, Tannheimer M, Chiquet-Ehrismann R. Biochem Cell Biol. 1996;74:737–744. doi: 10.1139/o96-080. [DOI] [PubMed] [Google Scholar]
- 15.Gupta V, Grande-Allen KJ. Cardiovasc Res. 2006;72:375–383. doi: 10.1016/j.cardiores.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Lee EL, von Recum HA. J Biomed Mater Res. 2010;93:411–418. doi: 10.1002/jbm.a.32754. [DOI] [PubMed] [Google Scholar]
- 17.Sasagawa T, Shimizu T, Sekiya S, Haraguchi Y, Yamato M, Sawa Y, Okano T. Biomaterials. 2010;31:1646–1654. doi: 10.1016/j.biomaterials.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 18.Tsuda Y, Shimizu T, Yamato M, Kikuchi A, Sasagawa T, Sekiya S, Kobayashi J, Chen G, Okano T. Biomaterials. 2007;28:4939–4946. doi: 10.1016/j.biomaterials.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Williams C, Brown XQ, Bartolak-Suki E, Ma H, Chilkoti A, Wong JY. Biomaterials. 2011;32:410–418. doi: 10.1016/j.biomaterials.2010.08.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaunas R, Nguyen P, Usami S, Chien S. Proc Natl Acad Sci U S A. 2005;102:15895–15900. doi: 10.1073/pnas.0506041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neidlinger-Wilke C, Grood ES, Wang JC, Brand RA, Claes L. J Orthop Res. 2001;19:286–293. doi: 10.1016/S0736-0266(00)00029-2. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Huang G, Zhang X, Wang L, Du Y, Lu TJ, Xu F. Biotechnol Adv. 2014;32:347–365. doi: 10.1016/j.biotechadv.2013.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


