Abstract
Purpose
Articulatory pattern variability describes the movement consistency across several repetitions of the same utterance. This study investigated speaking rate effects on articulatory pattern variability in talkers with mild ALS. Fast speech was used to differentiate disease-related and compensatory motor performance changes. Slow speech was used to evaluate therapeutic benefits.
Methods
Eight talkers with mild ALS and eleven controls participated in this study. Participants produced five repetitions of “Buy Bobby a puppy” at their typical rate, as fast as possible, and at a slow rate. Lower lip and jaw movements were captured using a motion capture system.
Results
Talkers with ALS tended to be more variable than controls during fast speech. During typical speech, however, talkers with ALS demonstrated significantly lower articulatory pattern variability than controls. Slow speech elicited elevated levels of articulatory pattern variability in both groups.
Conclusions
Increased variability during fast speech suggests that ALS negatively affects the ability to produce highly consistent articulatory movements; however, decreased variability during typical speech indicates a successful adaption to disease-related articulatory limitations. Findings of slow speech did not support the use of rate reductions to enhance this aspect of articulatory performance during the early stages of the ALS.
Introduction
Amyotrophic Lateral Sclerosis (ALS) is a progressive neurodegenerative disease that results in muscular weakness and loss of motor control. When the disease affects the head and neck musculature, talkers with ALS typically develop spastic-flaccid dysarthria (Darley, Aronson, & Brown, 1969). Although several aspects of speech motor performance have been investigated to better understand the neuromuscular mechanisms of speech decline [e.g., articulatory movement speed (Mefferd, Green, & Pattee, 2012; Yunusova et al., 2010; Yunusova et al., 2012), articulatory precision (Kuruvilla et al., 2012; Wang et al., 2011)], one overlooked but potentially important aspect of speech motor control is articulatory pattern variability.
Articulatory pattern variability is an aspect of speech motor performance that describes the consistency with which speech movements are repeated across multiple repetitions of the same utterance. This measure has been commonly used to determine if disruptions in the speech motor system affect speech motor control (Dromey, 2000; Kleinow, Smith, & Ramig, 2001; McHenry, 2003; Walsh & Smith, 2011). Although its impact on speech output is poorly understood, increased articulatory pattern variability may result in less predictable speech acoustic patterns and, therefore, may contribute to decrements in speech intelligibility in talkers with dysarthria (Mefferd & Green, 2010). In talkers with ALS, the study of articulatory pattern variability is particularly important for an enhanced understanding about the articulatory basis of the inevitable speech decline with disease progression, and for identifying clinical targets for therapeutic interventions that aim to improve speech function.
The spatiotemporal index (STI) is one metric that has been used to quantify articulatory pattern variability of the lip and jaw (Smith et al., 1995). The relatively low STI values of healthy mature talkers indicate consistent and predictable articulatory movements; in contrast, the relatively high STI values of immature talkers, talkers of old age, and talkers with dysarthria secondary to traumatic brain injury (TBI) or cerebral palsy (CP) indicate less consistent and less predictable speech movements (Chen et al., 2010; McHenry, 2003; Sadagopan & Smith, 2008; Wohlert & Smith, 1989).
These findings suggest that the degeneration of motoneurons in talkers with ALS will also result in increased variability of articulatory movements. Studies on talkers with Parkinson’s disease (PD), however, indicate that speech movements of talkers with mild to moderate PD are as consistent as those of healthy controls (Kleinow, Smith, & Ramig, 2001). One possible explanation for this finding is that, particularly during the early stages of a neurodegenerative disease, talkers may implement compensatory strategies to maintain articulatory performance. Speech movement variability of talkers with ALS may, therefore, also be unaffected during the earlier stages despite the presence of motoneuron degeneration.
A thorough testing of speech motor capacities should include a maximum performance task. Fast speech, for example, has been commonly used to detect speech deficits not evident during less demanding speech tasks (Duffy, 2005). Articulatory patterns have been shown to be significantly more variable in response to fast speech in talkers with dysarthria – regardless of the etiology – and also more variable than in healthy controls (Kleinow et al., 2001; McHenry, 2003). Thus, in ALS, fast speech tasks may be useful for assessing the impact of motoneuron degeneration on articulatory control, especially for talkers during the early stages of the disease who may be able to compensate during more typical speaking conditions.
The voluntary slowing of speech also elicited increased articulatory pattern variability in healthy talkers (Smith et al., 1995; Wohlert & Smith, 1998). This finding is notable because speaking rate reduction is a commonly prescribed speech treatment for persons with ALS; however, the effect of speaking rate reduction on articulatory pattern variability in this population has rarely been studied. Findings on the effect of slow speech in talkers with dysarthria due to neurologic conditions other than ALS have been mixed. For example, a decrease of articulatory pattern variability in response to slow speech has been reported for talkers with traumatic brain injury (TBI), particularly when the speech impairment was moderate to severe (McHenry, 2003). In contrast, an increase in articulatory pattern variability in response to slow speech was found in several studies of persons with PD (Dromey, 2000; Kleinow et al., 2001).
This investigation sought to determine how articulatory pattern variability is affected in talkers with mild ALS, and how speaking rate changes affect articulatory pattern variability. We hypothesized that, in persons with ALS, the variability of speech movement patterns will be greater during fast speech than during other speaking rates (i.e., slow and typical) because talkers with ALS may unable to compensate for the effects of motoneuron damage. The mixed findings in the literature for slow speech in persons with dysarthria make predictions about the articulatory performance in persons with ALS uncertain. For healthy talkers, however, we expected to replicate previous findings of elevated levels of speech movement variability in response to fast and slow speech and lowered levels of speech movement variability during typical speech (Kleinow et al., 2001; Smith et al., 1995).
Articulatory pattern variability has been commonly analyzed based on the compound movement of lower lip and jaw. In ALS, however, a differential effect of motoneuron degeneration on orofacial structures has been implicated; specifically, jaw muscles have been shown to be relatively more affected by the disease than lip muscles (DePaul et al., 1988; DePaul & Brooks, 1993; Langmore & Lehman, 1994). This differential effect has been observed in studies measuring muscular strength. It is currently unknown if articulatory performance, and specifically articulatory pattern variability is also differentially affected in talkers with ALS. Such knowledge would have important clinical implications for the assessment and treatment of talkers with ALS. Therefore, this study investigated the articulatory performance of the jaw and the lower lip independent of each other and as the compound movement of lower lip and jaw.
From previous findings on healthy talkers it is known that jaw movements are typically less variable than lower lip movements most likely due to differences in biomechanical properties of the two structures (Green et al., 2002). If a differential effect of motoneuron degeneration on orofacial structures were also observable in a measure of articulatory performance, then between group differences may be more pronounced for articulatory pattern variability of the jaw and less pronounced for the lower lip.
Methods
Participants
Eight talkers with mild ALS and eleven controls were included in this study. Participants passed a hearing screening at 0.5, 1, 2, and 4 kHz at 35 dB and had no self-reported history of neurological diseases other than ALS. Participants with ALS had been diagnosed by a certified neurologist. They also had the visual acuity required to read sentences and paragraphs printed on paper. English was their first and only language. Healthy controls were between 48 and 68 years of age (M = 58.09). Talkers with ALS were between 48 and 73 years of age (M = 61.25). Participants ALS1, ALS2, ALS4, and ALS8 reported taking pramipexole, a dopamine agonist that is currently being tested in several clinical trials. Further demographics and speech characteristics of talkers with ALS can be found in Table 1.
Table 1.
Demographic and Speech Characteristics of Talkers with ALS
| ID | Sex | Age | Initial Type of ALS | SIT | Speaking Rate | Articulatory Rate | Deviant Speech Characteristics |
|---|---|---|---|---|---|---|---|
| ALS1 | F | 74 | spinal | 100 | 162 | 164 | vocal fatigue |
| ALS2 | M | 52 | spinal | 99 | 142 | 142 | mild articulatory imprecision |
| ALS4 | M | 55 | spinal | 98 | 191 | 191 | mild articulatory imprecision |
| ALS7 | F | 48 | bulbar | 100 | 124 | 124 | mild articulatory imprecision |
| ALS8 | F | 64 | spinal | 100 | 173 | 173 | vocal hoarseness |
| ALS9 | M | 61 | spinal | 96 | 127 | 135 | mild articulatory imprecision |
| ALS10 | M | 77 | spinal | 98 | 135 | 158 | short breath groups |
| ALS11 | F | 60 | spinal | 100 | 180 | 180 | vocal fatigue |
|
| |||||||
|
ALS (SD) |
61.25 (8.91) |
98.75 (1.39) |
154.41 (25.80) |
172.13 (29.28) |
|||
|
| |||||||
|
Controls (SD) |
58.10 (2.7) |
100 |
190.70 (11.40) |
199.51 (9.66) |
|||
The Sentence Intelligibility Test (SIT) (Yorkston, Beukelman, Hakel, & Dorsey, 2007) was completed to determine speech intelligibility, speaking rate, and articulatory rate of all participants. SIT scores are also displayed in Table 1. The speaking rate calculation included pause times, whereas articulatory rates disregarded pauses longer than 200 ms.
Data Collection
All participants repeated the sentence “Buy Bobby a puppy” five times at their typical rate (“typical”), as fast as possible (“fast”), and approximately half of their articulatory rate (“slow”). Movements of the lower lip and jaw were captured using a 3-dimensional optical motion capture system (Motion Analysis, Ltd.). Four small (~ 2 mm) reflective markers were placed on each participant’s mouth (midline of the upper lip, midline of the lower lip, right and left corner of the mouth). Three small reflective markers were placed on the jaw (midline of the jaw, approx. 2 inches to the right and left of the midline). Reference markers were placed on the tip of the nose, the nose bridge, above the right and left eyebrow, and mounted in each corner of a rigid plate on the forehead. Movements were sampled at 120 Hz. A miniature microphone was placed on the rigid plate on the forehead to record the audio signal. The audio signal was sampled at 44.1 kHz. A digital video was also recorded as a reference during the analysis of movement data.
Data Analysis
The 3D Euclidian distance between the right jaw marker and right top head marker represented the JR signal, the 3D Euclidian distance between the center lower lip marker and the right top head marker represented the LLC+J signal, and the 3D Euclidian distance signal between the center lower lip marker and right jaw marker represented the iLLC signal (Figure 1).
Figure 1.
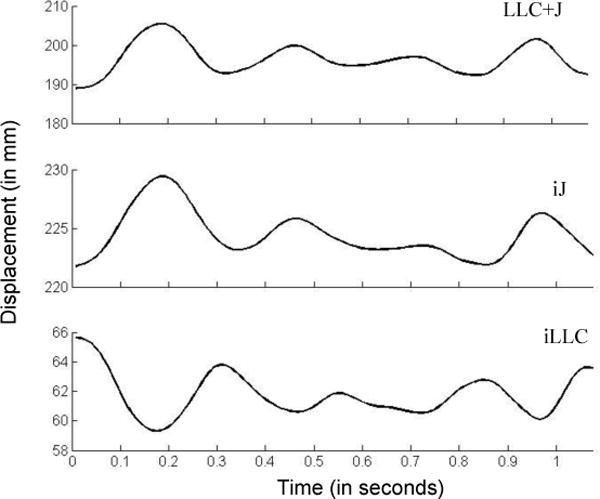
The 3D Euclidean distance signals of the target utterance used to calculate STI measures. LLC+J = compound movement of the lower lip and jaw, iJ = independent jaw movements, iLLC = independent lower lip movements
All three signals were smoothed using an 18 Hz low pass filter. Sentence repetitions were parsed based on the LLC+J signal. The distance minimum of “buy” and the distance minimum at the end of the syllable “pup” in “puppy” were used as landmarks to determine the beginning and end of each sentence. All five sentence repetitions of each rate were used for the data analyses. To examine the participants’ performance of articulatory rate modifications, movement durations of the target utterance were measured using the LLC+J signal.
To determine articulatory pattern variability during speech production, the STI was calculated for the jaw (iJ), the lower lip movements independent of the jaw (iLLC), and the lower lip-jaw compound movement (LLC+J). The STI was derived following the procedures by Smith et al. (1995). For each participant, five movement signals at each rate were demeaned and then divided by their standard deviations to spatially normalize the signals. Then, the signals were time normalized to 1000 points using a spline interpolation function. The STI was determined by calculating the sum of the standard deviations across 50 windows with 20 data points. For each participant, one STI value was derived for each movement signal in each articulatory rate.
Statistical Analyses
An outlier analysis was performed on each dependent measure. Consistent with conventional outlier definitions articulated by (Moore & McCabe, 1993), a data point was identified as an outlier when it was 1.5 × the interquartile range (IQR) smaller than the first quartile (lower bound), or 1.5 × IQR larger than the third quartile of its group’s distribution (upper bound). In the sentence duration measure, one data point was identified as an outlier in each group. Across all STI measures, six data points were identified as outliers in the control group. Outliers were winsorized by moving their values to the upper or lower bound in order to reduce the skewness of each group’s distribution.
To examine articulatory rate changes, movement durations of the LLC+J during sentence productions were submitted to a repeated measure ANOVA for each experimental group. To determine the effect of articulatory rate on movement variability (iJ, iLLC, LLC+J), repeated measure ANOVAs were completed separately for each group and each measure. All post hoc analyses were completed using the LSD approach with a critical alpha level at p = .05.
Methodological Considerations
Calculations of the STI values were computed on five sentence repetitions to minimize fatigue in the talkers with ALS. Prior work has demonstrated that five repetitions provide a sufficient sample for computing articulatory pattern variability (Walsh, Smith, & Weber-Fox, 2006). Due to tracking errors, the STI calculations were based on only three or four sentence repetitions in two out of 99 total calculations in the control group and in six out of 70 total calculations in the ALS group. Further, in one participant with ALS, STI values are missing for slow speech due to tracking errors of the jaw marker during that speech task.
Results
Task Performance
Figure 2 displays the group means for movement durations across all speaking rates. Repeated measure ANOVAs of talkers with ALS and healthy controls revealed significant speaking rate effects on movement durations for talkers with ALS [F(1.07, 7.45) = 14.149, p < .001] and healthy controls [F(1.09, 10.86) = 81.839, p = .006]. Post-hoc analyses showed that moment durations were significantly different between all speaking rates in talkers with ALS (p < .05) and healthy controls (p < .01).
Figure 2.
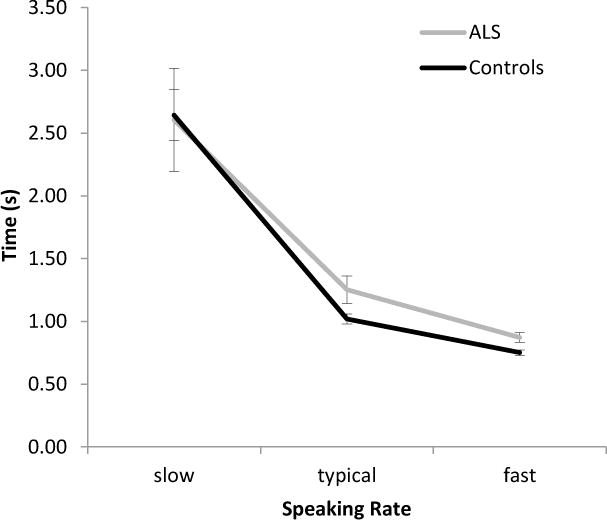
Mean movement durations (SE) measured based on the compound movements of the lower lip and jaw.
Between-group comparisons yielded significant differences in movement durations for typical and fast speech. Specifically, talkers with ALS had significantly longer movement durations than did healthy talkers during typical speech [t(17) = 2.254, p = .038] and fast speech [t(17) = 2.967, p = .009]. On average, healthy talkers shortened their movement durations by 26% to achieve fast speech, whereas talkers with ALS shortened their movement durations by 33%. To achieve slow speech, healthy talkers increased their movement durations by 147% whereas talkers with ALS increased their movement durations by 108%.
Speech Movement Variability
Figure 3 displays the group means for iJ, iLLC, and LLC+JR STI values. Although no significant main effects were found for any STI measure in either of the two groups, post-hoc analyses were conducted in both groups based on visual inspection of the data. Table 2 presents the statistical information of all significant post-hoc results. Significant differences were found for comparisons between typical speech and slow or fast speech for talkers with ALS. A trend towards a statistical difference in movement variability of the lower lip and jaw compound measure was also detected between slow and fast speech in the healthy group.
Figure 3.
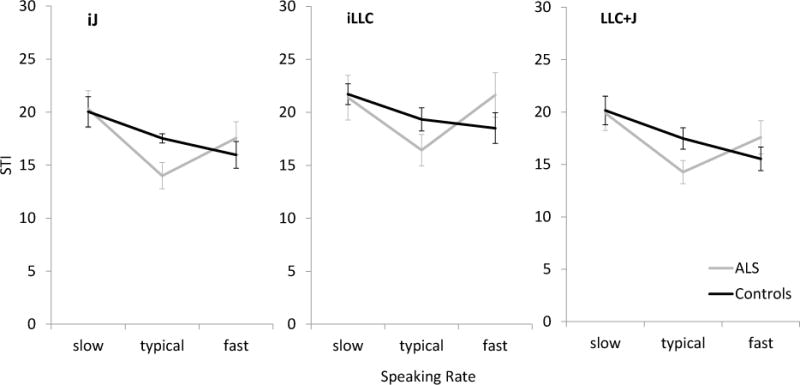
The mean STI values (SE) as a function of speaking rate to quantify speech movement variability across five repetitions of the target utterance. LLC+J = compound movement of the lower lip and jaw, iJ = independent jaw movements, iLLC = independent lower lip movements
Table 2.
Post-Hoc Summary for Rate Effects on Speech Movement Variability
| STI measure | Group | Comparison | df | p | Mean Difference |
|---|---|---|---|---|---|
| iJ | ALS | Slow vs. typical | 6 | .016 | 5.74 |
| Fast vs. typical | 7 | .039 | 3.56 | ||
| iLLC | ALS | Slow vs. typical | 6 | .026 | 4.39 |
| Fast vs. typical* | 7 | .070 | 5.07 | ||
| LLC+J | ALS | Slow vs. typical | 6 | .013 | 5.14 |
| Fast vs. typical | 7 | .045 | 3.32 | ||
| Controls | Slow vs. fast* | 10 | .059 | 4.61 |
statistical significance approaching
Between-group analyses of each STI measure revealed a significant difference for the iJ STI during typical speech. Specifically, talkers with ALS had significantly lower iJ STI values than did healthy controls during typical speech [t(8.7) = 2.679, p = .026, Mean difference = 3.53]. Further, significant differences between the ALS group and controls was approached for the LLC+J STI measure at typical speech with the ALS group tending to have lower LLC+J STI values than the controls [t(17) = 2.096, p = .051, Mean difference = 3.22].
Figure 4 displays the comparisons for the mean STI values of the independent jaw and lower lip movements within each group. In both groups, independent jaw movements were significantly less variable than independent lower lip movements during typical and fast speech (Table 3). In addition, in healthy talkers, independent movements of the jaw also tended to be less variable than independent movements of the lower lip during slow speech (p = .055).
Figure 4.
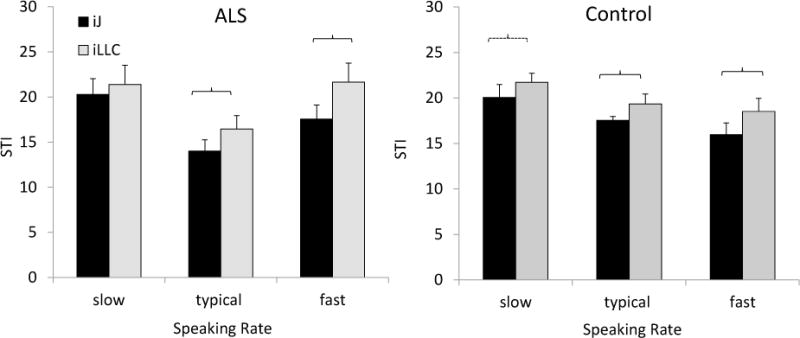
Comparisons between iJ and iLLC STI values within each group to determine the differential effect of motoneuron degeneration in talkers with mild ALS. iJ = independent jaw movements, iLLC = independent lower lip movements. Significant differences (p < .05) are marked by brackets.
Table 3.
Summary Statistics of Independent t-Tests (2-tailed) iJ STI vs. iLLC STI measures
| Group | Rate | t | p | Mean Difference |
|---|---|---|---|---|
| ALS* | Typical | 3.549 | .009 | 2.44 |
| Fast | 3.285 | .013 | 4.08 | |
| Controls** | Slow | 2.177 | .055 | 1.68 |
| Typical | 2.460 | .034 | 1.80 | |
| Fast | 3.436 | .006 | 2.53 |
df = 7
df=10
Discussion
The primary purpose of this study was to determine how articulatory pattern variability is affected by motoneuron degeneration in talkers with mild ALS. This study also sought to determine the effects of speaking rate manipulation on articulatory pattern variability. Fast speech served as a maximum performance task to examine articulatory performance under conditions that minimize options for articulatory compensation. Slow speech, in contrast to fast speech, was used to establish empirical evidence for a therapeutic approach commonly implemented to improve speech function in talkers with ALS.
The observation that jaw movement patterns were significantly less variable in talkers with ALS than in healthy talkers during typical speech was unexpected. The movements of the lower lip, and the lip- jaw complex also tended to be less variable in talkers with mild ALS than in healthy controls. One could argue that the disease-related neuromuscular changes constrain articulator movements resulting in decreased levels of movement pattern variability. The potential stabilizing effects of increased muscle stiffness have been documented in studies on limb movements (Burdet et al., 2001; Lametti, Houde, & Ostry, 2007). This explanation, however, is unlikely because speech movements during the slow and fast speech tasks were at least as variable as or even more variable than those of healthy talkers (see Figure 3).
Rather than a symptom of the disease, we suggest that the consistent jaw movements in talkers with ALS are the result of a compensatory strategy, which these talkers implement to maximize speech intelligibility. The potential compensatory function of a slowed speaking rate in talkers with mild ALS, for example, has been debated previously (e.g., Weismer, Laures, Jeng, Kent, & Kent, 2000). Although talkers with mild ALS enrolled in this study had a significantly slower speaking rate than their healthy controls, the observed increase in articulatory pattern variability during slow speech does not support the notion that talkers with ALS slow their habitual speaking rate to improve their ability to be more consistent in their speech movements.
However, the slowed habitual speech of talkers with mild ALS may be an epiphenomenon of a different compensatory strategy: clear speech. The implementation of a clear speech strategy to compensate for an impaired speech motor system has been suggested previously for talkers with Parkinson’s disease (Goberman & Elmer, 2005). Clear speech is associated with a reduced speaking rate (Picheny, Durlach, & Braida, 1986). Unfortunately, the effect of clear speech on articulatory pattern variability has not been studied in healthy talkers or in talkers with dysarthria. Future studies should examine the effects of clear speech on articulatory pattern variability to better understand the underlying reasons for the relatively low articulatory pattern variability in talkers with mild ALS during typical speech.
Fast Speech
We hypothesized that the fast speech condition would induce greater movement variability in talkers with mild ALS because this task afforded fewer options for compensation. Our findings supported this hypothesis. In talkers with mild ALS, we observed a relative increase in speech movement variability in all three measures (iJ, iLLC, LLC+J) during fast speech. In contrast, healthy controls tended to exhibit less variable articulatory movements during fast speech than during habitual speech. We speculate that this difference between groups was observed because talkers with mild ALS may operate at their articulatory capacity limit during fast speech whereas healthy talkers economize their articulatory efforts (Perkell, Zandipour, Matthies, & Lane, 2002). The findings of a previous study on talkers with mild ALS suggest that these talkers produce fast speech with larger lip and jaw displacements than do healthy talkers, which in turn requires them to move with greater articulatory speeds than healthy talkers and pushes them further towards their articulatory performance limit than healthy talkers (Mefferd, Green, & Pattee, 2012).
The observation that fast speech did not increase articulatory pattern variability in healthy talkers is not consistent with previous findings (Kleinow et al., 2001; McHenry, 2003; Smith et al., 1995; Wohlert & Smith, 1998). The discrepant findings for fast speech effects between the previous studies and this one may be explained by differences in the age of the participants. Although fast speech increases speech movement variability in young adults, its effect on the speech movements of aging individuals (65 years and older) is less pronounced (Kleinow et al.; McHenry; Smith et al.; Wohlert & Smith). In our study, the age-matched controls were on average younger than the aging individuals (Wohlert & Smith) or age-matched controls of talkers with PD (Kleinow et al.), but older than the young adults in previous studies (Kleinow et al.; McHenry, Smith et al.).
The observation that fast speech increases movement variability in talkers with mild ALS concurs with prior work on talkers with mild PD and mild TBI (Kleinow et al., 2001; McHenry, 2003). Interestingly, the LLC+J STI values for fast speech of our talkers with ALS were similar to those reported for talkers with mild PD (Kleinow et al.); however, they are lower than those of talkers with mild TBI (McHenry). The neuromuscular basis for these differences between talkers with various etiologies in response to fast speech is currently unknown and should be investigated in future studies.
Slow Speech
This study also sought to determine if further rate reduction would benefit articulatory control in talkers with mild ALS. The findings of this study do not support a stabilizing effect for speaking rate reductions during the early stages of the disease. Across all three measures (iJ, iLLC, LLC+J), speech movement variability increased during slow speech relative to habitual speech in talkers with mild ALS. This finding does not preclude the possibility, however, that rate reduction strategies may improve speech during the later stages of the disease. In talkers with TBI, for example, the benefit of slow speech was much greater for talkers with moderate-to-severe dysarthria than for talkers with mild dysarthria (McHenry, 2003). In healthy talkers, our findings of a trend towards increased movement variability in response to slow speech are concurrent with previous findings (Kleinow et al., 2001, Smith et al., 1995; Wohlert & Smith, 1989).
Differential Effects of ALS on Articulators
A secondary purpose of this study was to determine if lip and jaw performance are affected differentially by motoneuron disease. Based on previous observations that movements of the jaw are less variable than those of the lower lip (Green et al., 2002) and previous findings of relatively more affected jaw than lip muscles in talkers with ALS (DePaul et al., 1988; DePaul & Brooks, 1993; Langmore & Lehman, 1994), we expected to observe greater differences between talkers with ALS and healthy controls in jaw movement pattern variability than lower lip movement pattern variability, particularly during fast speech. Although no significant between-group differences were observed for iJ or iLLC STI measures during fast speech, between-group differences tended to be greater for the iLLC STI measure than the iJ STI measure. This observation suggests that a disease-related decline in the ability to produce consistent speech movements may be more evident in the lower lip than the jaw. Previous strength studies on talkers with ALS, however, have found the opposite pattern: more prominent jaw weakness than lower lip weakness in persons with ALS (DePaul et al., 1988; Langmore & Lehman, 1994). Future studies should examine speech performance of individual articulators in a larger sample of talkers with ALS with similar disease severity to determine if there is a differential decline in articulatory performance in ALS and if it is disassociated from differential declines in muscle force.
Conclusions
In sum, the findings of this study suggested that talkers with mild ALS may implement a compensatory strategy that preserves the consistency of speech movement patterns, particularly those of the jaw. Disease-related decrements in articulatory movement variability are more apparent during fast speech than during typical speech. Movement patterns of the lower lip tend to be more variable due to the disease than those of the jaw; however, further research is necessary to confirm this observation. Rate reductions do not appear to stabilize lip and jaw movements during the early stages of the disease.
Acknowledgments
This work was supported in part by research grant numbers R01 DC009890 and R01 DC06463 from the National Institute on Deafness and Other Communication Disorders (NIDCD-NIH) awarded to the second author, by the Nebraska Speech-Language-Hearing Endowment Fund Dissertation Award bestowed to the first author, and the Barkley Trust. We would like to thank Cynthia Didion, Cara Ullman, Lindsey Fairchild, Paige Leising, and Jenna Mroczek for their assistance with data collection and management.
Footnotes
Part of this work has been presented at the Motor Speech Conference in Savannah, GA in March 2010.
References
- Ball LJ, Beukelman DR, Pattee GL. Timing of speech deterioration in people with amyotrophic lateral sclerosis. Journal of Medical Speech-Language Pathology. 2002;10(4):231–235. [Google Scholar]
- Beukelman D, Fager S, Nordness A. Communication Support for People with ALS. Neurological Research International; 2011. Article ID 714693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdet E, Osu R, Franklin DW, Milner TE, Kawato M. The central nervous system stabilizes unstable dynamics by learning optimal impedance. Nature. 2001;414:446–449. doi: 10.1038/35106566. [DOI] [PubMed] [Google Scholar]
- Chen CL, Chen HC, Hong WH, Yang FP, Yang FP, Yang LY, Wu CY. Oromotor variability in children with mild spastic cerebral palsy: a kinematic study of speech motor control. Journal of Neuroengineering and Rehabilitation. 2010;27:7–54. doi: 10.1186/1743-0003-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Clusters of deviant speech dimensions in the dysarthrias. Journal of Speech and Hearing Research. 1969;12(3):462–492. doi: 10.1044/jshr.1203.462. [DOI] [PubMed] [Google Scholar]
- DePaul R, Abbs JH, Caligiuri MP, Gracco VL, Brooks BR. Differential involvement of hypoglossal, trigeminal and facial motoneurons in ALS. Neurology. 1988;38(2):281–283. doi: 10.1212/wnl.38.2.281. [DOI] [PubMed] [Google Scholar]
- Dromey C. Articulatory kinematics in patients with Parkinson Disease using different speech treatment approaches. Journal of Medical Speech-Language Pathology. 2000;8:155–161. [Google Scholar]
- Duffy JR. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management. 2nd. St. Louis, MO: Elsevier Mosby; 2005. [Google Scholar]
- Goberman A, Elmer LW. Acoustic analysis of clear versus conversational speech in individuals with Parkinson’s disease. Journal of Communication Disorders. 2005;38(3):215–230. doi: 10.1016/j.jcomdis.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Green JR, Moore CA, Reilly KJ. The sequential development of jaw and lip control for speech. Journal of Speech, Language, and Hearing Research. 2002;45:66–79. doi: 10.1044/1092-4388(2002/005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JR, Wilson EM, Wang Y, Moore CA. Estimating mandibular motion based on chin surface targets during speech. Journal of Speech, Language, and Hearing Research. 2007;50:928–939. doi: 10.1044/1092-4388(2007/066). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinow J, Smith A, Ramig LO. Speech motor stability in IPD: Effect of Rate and Loudness Manipulations. Journal of Speech, Language, and Hearing Research. 2001;44:1041–1051. doi: 10.1044/1092-4388(2001/082). [DOI] [PubMed] [Google Scholar]
- Kuruvila M, Green JR, Yunusova Y, Handford K. Spatiotemporal coupling of the tongue in amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research. 2012 doi: 10.1044/1092-4388(2012/11-0259). epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lametti DR, Houde G, Ostry DJ. Control of movement variability and the regulations of limb impedance. Journal of Neurophysiology. 2007;98:3516–3524. doi: 10.1152/jn.00970.2007. [DOI] [PubMed] [Google Scholar]
- Langmore SE, Lehman ME. Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. Journal of Speech and Hearing Research. 1994;37:28–37. doi: 10.1044/jshr.3701.28. [DOI] [PubMed] [Google Scholar]
- McHenry MA. The effect of pacing strategies on the variability of speech movement sequences in dysarthria. Journal of Speech, Language, and Hearing Research. 2003;46:702–710. doi: 10.1044/1092-4388(2003/055). [DOI] [PubMed] [Google Scholar]
- Mefferd AS, Green JR, Pattee G. A novel fixed-target task to determine articulatory speed constraints in persons with amyotropic lateral sclerosis. Journal of Communication Disorders. 2012;45(1):35–45. doi: 10.1016/j.jcomdis.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DS, McCabe GP. Introduction to the practice of statistics. 2nd. New York, NY: W. H. Freeman and Company; 1993. [Google Scholar]
- Picheny MA, Durlach NI, Braida LD. Speaking clearly for the hard of hearing. II: Acoustic characteristics of clear and conversational speech. Journal of Speech and Hearing Research. 1986;29(4):434–446. doi: 10.1044/jshr.2904.434. [DOI] [PubMed] [Google Scholar]
- Sadagopan N, Smith A. Developmental changes in the effects of utterance length and complexity on speech movement variability. Journal of Speech Language and Hearing Research. 2008;51(5):1138–1151. doi: 10.1044/1092-4388(2008/06-0222). [DOI] [PubMed] [Google Scholar]
- Smith A, Goffman L, Zelaznik HN, Ying G, McGillem C. Spatiotemporal stability and patterning of speech movement sequences. Experimental Brain Research. 1995;104:493–501. doi: 10.1007/BF00231983. [DOI] [PubMed] [Google Scholar]
- Yorkston K, Beukelman DR, Hakel M, Dorsey M. Speech Intelligibility Test for Windows [Computer software] Lincoln, NE: Institute for Rehabilitation Science and Engineering at Madonna Rehabilitation Hospital; 2007. [Google Scholar]
- Yunusova Y, Green JR, Lindstrom M, Ball L, Pattee G, Zinman L. Kinematics of disease progression in bulbar ALS. Journal of Communication Disorders. 2010;43(1):6–20. doi: 10.1016/j.jcomdis.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunusova Y, Green JR, Greenwood L, Wang J, Pattee G, Zinman L. Tongue movements and their acoustic consequences in amyotrophic lateral sclerosis. Folia Phoniatrica et Logopaedica. 2012;64(2):94–102. doi: 10.1159/000336890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B, Smith A. Linguistic complexity, speech production, and comprehension in Parkinson’s disease: behavioral and physiological indices. Journal of Speech, Language, and Hearing Research. 2011;4(3):787–802. doi: 10.1044/1092-4388(2010/09-0085). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B, Smith A, Weber-Fox C. Short-term plasticity in children’s speech motor system. Developmental Psychobiology. 2006;48(8):600–674. doi: 10.1002/dev.20185. [DOI] [PubMed] [Google Scholar]
- Wang J, Green JR, Samal A, Marx DB. Quantifying articulatory distinctiveness of vowels. InterSpeech; Florance, Italy: 2011. [Google Scholar]
- Weismer G, Laures JS, Jeng JY, Kent RD, Kent JF. Effects of speaking rate manipulations on acoustic and perceptual aspects of the dysarthria in Amyotrophic Lateral Sclerosis. Folia Phoniatrica et Logopaedica. 2000;52:201–219. doi: 10.1159/000021536. [DOI] [PubMed] [Google Scholar]


