Abstract
Social learning refers to individuals learning from others, including information gained through indirect social influences, such as the results of others’ actions and changes in the physical environment. One method to determine the relative influence of these varieties of information is the ‘ghost display’, in which no model is involved, but subjects can watch the results that a model would produce. Previous research has shown mixed success by chimpanzees (Pan troglodytes) learning from ghost displays, with some studies suggesting learning only in relatively simple tasks. To explore whether the failure of chimpanzees to learn from a ghost display may be due to neophobia when tested singly or a requirement for more detailed information for complex tasks, we presented ghost displays of a tool-use task to chimpanzees in their home social groups. Previous tests have revealed that chimpanzees are unable to easily solve this tool-use task asocially, or learn from ghost displays when tested singly, but can learn after observing conspecifics in a group setting. In the present study, despite being tested in a group situation, chimpanzees still showed no success in solving the task via trial-and-error learning, in a baseline condition, nor in learning the task from the ghost display. Simply being in the presence of their group mates and being shown the affordances of the task was not sufficient to encourage learning. Following this, in an escalating series of tests, we examined the chimpanzees’ ability to learn from a demonstration by models with agency: (1) a human; (2) video footage of a chimpanzee; (3) a live chimpanzee model. In the first two of these ‘social’ conditions, subjects showed limited success. By the end of the final open diffusion phase, which was run to determine whether this new behavior would be transmitted among the group after seeing a successful chimpanzee use the task, 83% of chimpanzees were now successful. This confirmed a marked overall effect of observing animate conspecific modeling, in contrast to the ghost condition.
This article is part of a Special Issue entitled: insert SI title.
Keywords: Social learning, Chimpanzee, Tool-use, Ghost display, Video display, Social facilitation
1. Introduction
To ask “how ‘social’ is social learning?” may seem paradoxical. By its very definition, social learning refers to individuals learning from others, necessitating some kind of social context. Indeed, for both humans (Over and Carpenter, 2012) and chimpanzees (Hopper et al., 2011) individuals may copy others for purely social reasons. However, much of what is referred to as ‘social learning’ is more specifically ‘observational learning’; learning from the direct observation of others. In contrast, the more global term of social learning can also refer to information gained through indirect social influences, including the results of others’ actions that cause changes in the physical environment. As Zentall (2011, 2012) notes, there are numerous examples of social learning that are likely mediated by such ‘non-social’ social learning mechanisms.
Consider chimpanzees (Pan troglodytes) in the wild cracking nuts with tools, a behavior that a variety of evidence suggests is transmitted socially (Inoue-Nakamura and Matsuzawa, 1997; Biro et al., 2003; Marshall-Pescini and Whiten, 2008; Luncz and Boesch, 2014). The performance of this behavior by a skilled individual could facilitate the learning of a naïve chimpanzee in two main ways. In one, direct observation of the proficient chimpanzee would allow imitative learning of the required actions (Whiten and Ham, 1992; Whiten et al., 2004). Alternatively, encountering the raw materials and/or by-products of the activity, such as an assemblage of hammers, anvils and cracked nuts, might facilitate learning of hammering through, for example, efforts to produce unshelled nuts or learning about the crucial properties of the materials (Byrne, 1998). Such ‘non-observational’ social learning (i.e. not dependent on observing the actions of another), has been described as a form of ‘emulation’ (Tomasello, 1999); reaching the same goal through independent means (Wood, 1989).
Beyond a simple dichotomy of imitation versus emulation, a number of social learning mechanisms have been identified (Whiten et al., 2004; Zentall, 2012; Moore, 2013), and it has been proposed that the social learning mechanism that individuals use may be mediated by the complexity of the task (Acerbi et al., 2010; Hopper et al., 2010). Much previous research has been concerned with distinguishing different social learning mechanisms used by a number of species, and their respective requirement for models with agency, including humans (Huang and Charman, 2005; Flynn and Whiten, 2013), apes (Call et al., 2005; Tennie et al., 2006), monkeys (Bugnyar and Huber, 1997; Subiaul et al., 2004; Hopper et al., 2013), dogs (Miller et al., 2009; Lakatos et al., 2014), rats (Heyes et al., 1994; Zohar and Terkel, 1991), birds (Akins et al., 2002; Auersperg et al., 2014), and reptiles (Kis et al., in press). To distinguish emulative from imitative learning, two key controls that have been used are ‘end-state’ and ‘ghost display’ conditions (reviewed in Hopper, 2010), often in conjunction with ‘two-action’ or ‘bi-directional’ tasks (Akins and Zentall, 1996; Zentall, 1996; Klein and Zentall, 2003). In an end-state condition naïve observers are shown the completed form of a task, and sometimes the initial state too, but no information is provided about the methods used to reach that end-state. In contrast, in a ghost display, championed by Zentall and colleagues, the observer sees only the required movements (or mechanical affordances) of objects, occurring without a live model. If learning arises from either of these two kinds of display, it is inferred that the observer was able to learn the task through emulation.
To date, studies with a number of species have indicated that subjects learn more quickly, and copy more accurately, after seeing a live (conspecific) model demonstrate the required actions, than they do when simply learning about the affordances of a task, for example via a ghost display (Hopper, 2010 provides a review). Indeed, Zentall (2012) noted that “the effect of demonstrator reinforcement may be to act as a catalyst to bring out imitative learning in an observer” (p. 121) and an early study by Zentall and Levine (1972) clearly demonstrated this effect. In that study, rats were presented with a lever that could be pressed in order to obtain a liquid reward. Naïve rats were tested in one of four conditions: (1) a rat in an adjacent cage pressing its lever and drinking the liquid, (2) a rat in the adjacent cage drinking the liquid when its lever moved automatically (it was yoked to the demonstrator's actions in condition 1; a ghost display), (3) a rat in the adjacent cage that neither pressed the bar nor drank liquid but simply provided social support, or (4) an empty cage. The rats learnt most quickly after seeing the full demonstration (condition 1) compared to the rats’ responses in the other three conditions. Furthermore, there was no difference between the responses of the rats that saw the ghost display or the empty cage, although rats in both these conditions were significantly more successful than those that were provided with a live companion who did not provide any demonstration (condition 3; the authors concluded that the responses of the rats in this condition were inhibited due to social facilitation, sensu Zajonc, 1965).
Considering Zentall and Levine's (1972) study, although the rats that saw the ghost display ultimately learnt how to press the lever to obtain the liquid reward, it took them longer than those that saw a live model demonstrate the required actions, despite the only difference across conditions being the model's failure to interact with the lever (see also Zentall and Hogan, 1976). It could be argued that certain tasks, such as tool-use tasks, are simply too complex to be learnt via emulative means, and individuals require a social demonstration that provides more information (Hopper et al., 2010; Whiten et al., 2009). However, in addition to considering the quantitative difference between the information provided in a ghost display compared to that given by a live model (e.g., number of cues provided), it has also been suggested that live models provide a qualitative advantage because a live model has agency and is goal-directed (Cannon and Woodward, 2012; Kano and Call, 2014).
It has been proposed that the success of children at learning from ghost displays, which can exceed that of chimpanzees (e.g., Hopper et al., 2010; Caldwell et al., 2012), is due to children's ability to attribute agency to any sequence of actions that appear as if the actions are goal-oriented; the ‘Agency Attribution Hypothesis’ (Subiaul et al., 2007). Children readily attribute agency to inanimate objects that act in a purposeful way (Subiaul et al., 2011) or that interact with animate beings (Gerson and Woodward, 2012), and even young infants can identify actions that are driven by agency (Saxe et al., 2007). However, despite this, attribution of agency does not always increase children's ability to replicate the actions seen (Subiaul et al., 2011) and there is perhaps a potential interplay between task complexity and the need for a model with agency for imitation to occur.
Our previous research has shown that chimpanzees are capable of learning how to operate tool-use tasks from observing live conspecific models (Whiten et al., 2005), but that a ghost display is often not sufficient to allow learning (Hopper et al., 2007), despite aiding learning of simpler tasks (e.g., bidirectional tasks, Hopper et al., 2008). To further assess chimpanzees’ requirement for a live model in order to learn tool use, we presented chimpanzees with either nonsocial displays – providing information purely about the mechanical properties of a task – or demonstrations of a task solution given by a live agent. Additionally, and extending upon our previous research, which has tested chimpanzees’ ability to learn from conspecifics (Whiten et al., 2005; Hopper et al., 2007), we wished to address whether chimpanzees might also learn tool-use from watching a human model (Hayes and Hayes, 1952; Nagell et al., 1993; Horner and Whiten, 2005) or from a video of a chimpanzee (Price et al., 2009; Hopper et al., 2012), both of which represent forms of models with agency.
It is well established that for social primate species, the presence of group mates can encourage exploration leading to increased success in operating (simple) novel tasks (Harlow and Yudin, 1933; Dindo et al., 2009). Consistent with this, chimpanzees appear better able to learn in tests of emulation when given social support (i.e. when conspecifics are present: Hopper et al., 2008; Tennie et al., 2010a) than when tested alone (Hopper et al., 2007, although the differing responses of chimpanzees in these studies could also relate to the differing complexity of the tasks involved). Indeed, Zentall (2006) noted that “an isolated animal in a novel environment may be fearful, and fear in an enclosed environment may reduce exploratory behavior. If the presence of a conspecific reduces fear and increases exploratory behavior, it may lead to a higher probability (by chance) that the target behavior will be performed . . .Thus, experiments concerned with imitation must include a control for the possibility that the presence of another animal might result in an increase (or decrease) in motivation that could lead to facilitate performance of the target behavior” (p. 338). Therefore, to test the importance of social support, we presented chimpanzees in a social group setting with ghost displays of a complex tool-use task that chimpanzees (1) rarely solve asocially (Whiten et al., 2005), and (2) fail to learn from ghost displays when tested singly (Hopper et al., 2007).
In this study, four social groups of chimpanzees were presented with a series of conditions escalating in their predicted power to elicit social learning, to ‘titrate’ the amount of information chimpanzees required in order to learn a tool-use task. The chimpanzees first saw a ghost display, then, if they did not acquire the technique from viewing the ghost display, they were shown a human demonstration. If there was still no learning, the chimpanzees were next shown video footage of an unfamiliar conspecific successfully completing the tool-use task. Finally, if they were still unsuccessful, they were allowed to observe a trained member of their group operate the tool-use task. From our earlier research, we know chimpanzees should learn from this social referent, if not the more ‘degraded’ models earlier in the series. This protocol created a more nuanced examination of chimpanzee social learning requirements than simply comparing their ability to learn from a live conspecific to their ability to learn from a ghost display. Globally, we predicted that, in order to successfully operate a complex tool-use task, chimpanzees would need to first observe an animate model (i.e. a live conspecific, video of a conspecific, or a human) demonstrate the required actions, and a live conspecific model would likely be the most effective model.
2. Materials and methods
2.1. Subjects and housing
The subjects for this study were 19 captive chimpanzees, housed at the Michale E. Keeling Center for Comparative Medicine and Research, UT MD Anderson Cancer Center, Bastrop, Texas, USA. The chimpanzees (eight females and 11 males; mean age: 26.7 years, range: 10–43 years) were socially housed in four groups. In group 1, there were four females and two males (average age = 25 years) and in group 2 there were four females and one male (average age = 34 years). Groups 3 and 4 were bachelor groups consisting of five (average age = 24 years) and three males (average age = 23 years) respectively. As the chimpanzees were tested in a group setting, no individual was isolated from its group for these experimental sessions and all participation on the part of the chimpanzees was voluntary. Over a year before the commencement of this study, eight of the 19 chimpanzees had individually been shown ghost displays of the apparatus used in the present study (the ‘Pan-pipes’, see below for details of the apparatus). None of these eight animals had had success in using the ‘Lift’ action, although one 17 year-old male, in group 1, successfully performed a single Poke action 29 min into his hour-long response period after seeing a ghost display of the Lift method (see Hopper et al., 2007 for details).
Each group was housed in a Primadome® (10.4 m diameter) connected to four spacious indoor dens. Across all testing conditions, the chimpanzees had access to both their highly-enriched outdoor enclosure and all four inside dens. The majority of test conditions were conducted in the outside enclosure, but one condition (the ‘video display’) was presented to the chimpanzees in their inside dens to reduce glare on the screen (see below for details). The chimpanzees were housed in facilities that are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) International, and in accordance with current United States Department of Agriculture (USDA), Department of Health and Human Services, and National Institutes of Health regulations and standards. This experiment was approved by The University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee (IACUC approval number: 07-92-03887). The chimpanzees had free access to water and primate chow and received three meals of fruits and vegetables per day, in addition to any food rewards obtained during testing.
2.2. Apparatus
The apparatus used in this study was the two-action ‘Pan-pipes’ tool-use task, which has been used previously in social learning studies with both chimpanzees (Whiten et al., 2005; Hopper et al., 2007) and children (Hopper et al., 2010; Fig. 1). The Pan-pipes consists of two sloping pipes, one on top of the other. The over-all dimensions of the apparatus are 33 cm long, 30 cm high, and 7 cm wide. From the back panel of the Pan-pipes, a food reward (a grape) could be dropped into the upper tube by an experimenter, where it would be trapped by a blockage that could only be moved through the use of a rod tool, which in turn would release the reward from a hole at the front of the Pan-pipes (Fig. 2). In all conditions, the Pan-pipes apparatus was presented outside the chimpanzees’ enclosure, such that its nearest point to their cage mesh was 20 cm away; thus the chimpanzees could not directly manipulate the Pan-pipes with their fingers but had to use the provided tool (a polycarbonate rod 45 cm long and 1.8 cm in diameter) to move the blockage and release the grape. A chute, connected to the base of the Pan-pipes, slotted through the cage mesh, allowed any food rewards that the chimpanzees obtained to fall into their enclosure.
Fig. 1.
An illustration of a chimpanzee operating the Pan-pipes using the Lift technique to obtain a reward. Artist: Alan Male.
Fig. 2.
A schematic of the Pan-pipes showing how they operated. (a) A side-view of the Pan-pipes tool-use apparatus with the food reward held in place by the blockage. The polycarbonate rod tool can be used to release the food reward in one of two ways: ‘Lift’ or ‘Poke.’ (b) To Lift, chimpanzees had to raise the blockage 5 cm by inserting the rod tool under the T-bar. (c) To Poke, chimpanzees had to push back the blockage by inserting the rod tool through a flap at the front of the Pan-pipes and sliding the blockage away from them to the back of the 30 cm long tube. It was this flap that was blocked in the current study so that the Poke method was impossible and the chimpanzees could only obtain the food reward by using the Lift method.
There are two methods–‘Lift’ and ‘Poke’ – that can be used to obtain the food reward from the Pan-pipes. In previous studies using the Pan-pipes, both Lift and Poke methods for retrieving the rewards, shown in Fig. 2, were possible. However, as a previous study had revealed that it was possible for at least one chimpanzee to individually learn the Poke method (Hopper et al., 2007), we were interested in the chimpanzees’ ability to learn the Lift technique, which no chimpanzee has been reported to individually discover (Whiten et al., 2005; Hopper et al., 2007). Therefore, in this study, the Poke method was prevented by screwing shut the flap at the front of the Pan-pipes into which the chimpanzees could insert the tool and push the blockage backwards to release the grape (Fig. 2c). Thus, the only way to retrieve the grapes from the Pan-pipes was through the use of the Lift method (Fig. 2b). For the Lift method, the chimpanzee had to place the rod tool under the T-bar attached to the top of the blockage and lift up the blockage by 5 cm. By using the tool to raise the T-bar, and the blockage as well, the food reward was released and rolled out of the front of the Pan-pipes along the chute to the chimpanzee. Following Hopper et al. (2007), the rod tool was attached at one end, by a 200 cm long wire cable, to the cart on which the Pan-pipes stood to ensure that a chimpanzee could not remove the tool from the vicinity of the Pan-pipes prohibiting other chimpanzees from performing the task.
2.3. Procedure: overview
This study involved a series of five experimental conditions run sequentially (Fig. 3). We first provide an overview of these conditions, before describing the methods for each in detail. Following previous studies of chimpanzee social learning (e.g., Hopper et al., 2007), the chimpanzees were first tested in a no-information baseline condition to determine whether any could spontaneously discover the Lift technique via individual learning during an hour-long free-interaction period with the apparatus. If no chimpanzee in a group successfully discovered Lift, then that group entered the experimental conditions, the first of which was a ghost display condition. In this condition, the group of chimpanzees was presented with 225 ghost displays of the Lift action in their group setting. After being shown the ghost displays, the chimpanzees were immediately given one hour to interact freely with the Pan-pipes to determine whether they had learnt Lift from the ghost display. If no chimpanzee in a group was successful, after these two asocial conditions, the group was then tested in the social conditions. The first was a ‘human model’ condition in which a familiar human demonstrated Lift 225 times before giving the chimpanzees access to the Pan-pipes. If no chimpanzee in the group was successful, they were next shown a presentation of a video of a female chimpanzee successfully using the Lift method 225 times. Finally, if no chimpanzees in the group had shown any success, then a group member was trained to perform Lift to determine if their group mates could learn from observing them. As for the ghost display condition, for each of the social conditions, after the demonstration, the group of chimpanzees was given one hour to freely interact with the Pan-pipes in order to determine whether they had learnt from the experimental manipulation. Thus, for the two asocial and three social conditions, the ‘response period’ for each was one hour. Each experimental condition was run on different days over a period of one month.
Fig. 3.
The experimental protocol. In each of the five possible experimental conditions, the group of chimpanzees first experienced the experimental manipulation (e.g., a ghost display) and were then given an hour-long free access period with the Pan-pipes. During this time, if any chimpanzee in the group successfully performed Lift (“YES”), the experimenter re-baited the Pan-pies and allowed the chimpanzees to continue interacting with the Pan-pipes. At this point, the chimpanzees entered an Open Diffusion phase which consisted of 20 one-hour, free-access sessions (run on different days). If, after experiencing the experimental manipulation, no chimpanzee in the group performed Lift during their one-hour, free-access period (“NO”), then (on a different day) that group was tested in the subsequent experimental condition as defined by this decision-tree.
2.4. Procedure: no-info baseline
Initially, each group was presented with the Pan-pipes placed against the caging of their outside enclosure as described in section 2.2 Apparatus. The group of chimpanzees observed the experimenter bait the Pan-pipes with a grape reward and push the tool through their mesh into their enclosure, but were not provided with any demonstration or encouragement to interact with the Pan-pipes. With the Pan-pipes pushed up against their cage mesh, each group was given one hour in which to interact freely with the Pan-pipes and could, if they solved the puzzle, retrieve the grape from within the Pan-pipes. If any chimpanzee did successfully obtain the grape from the Pan-pipes, the experimenter re-baited the apparatus with a grape.
2.5. Procedure: ghost display
The Pan-pipes apparatus was placed in front of the chimpanzees’ caging, in view, but out of reach of, the chimpanzees. A length of monofilament fishing line was tied to the top of the T-bar and the free end was looped through a pulley above the Pan-pipes (Fig. 4; cf. Hopper et al., 2007). With this set-up, the experimenter could raise and lower the T-bar remotely by discretely pulling on the free end of the fishing line threaded through the pulley. A total of 225 such ‘ghost displays’ of the Lift method were provided to each group. This number of demonstrations represented the average number of times that chimpanzees in our previous study observed the Lift action in a test of social learning from a conspecific (Hopper et al., 2007). For each ‘Lift’, a grape was loaded into the Pan-pipes in full view of the chimpanzees and when the T-bar was raised, the grape fell into a bucket placed in front of the Pan-pipes at the base of the food chute. For every ‘demonstration’, the experimenter first ensured that all group members were oriented toward the Pan-pipes, and attending to the display, by calling to the chimpanzees if needed. Additionally, to emulate the occasional scrounging observed in conditions in which chimpanzees see a conspecific demonstration, and following the methods reported by Hopper et al. (2007), for every 20th Lift demonstration, the food chute was inserted through the cage mesh so that the grape was delivered into the chimpanzees’ enclosure. This not only gave the chimpanzees’ personal experience with receiving food reward after a Lift action, but it also increased their motivation to attend to the demonstration. After all 225 displays had been given, the Pan-pipes was pushed against the caging, and baited with a grape in full view of the chimpanzees, who were then able to interact with the Pan-pipes for one hour. If any chimpanzee solved the puzzle and retrieved the grape from within the Pan-pipes, the experimenter re-baited the apparatus.
Fig. 4.
The ghost display condition. Photo shows the Pan-pipes placed near the chimpanzees’ enclosure and the pulley to raise and lower the T-bar for each ghost display of the Lift method.
2.6. Procedure: human model
The Pan-pipes was presented to the chimpanzees in their outside enclosure in a similar manner to that described for the ghost display (i.e. in view, but out of reach). However, instead of 225 ghost displays, the same number of demonstrations was provided by a familiar human (LMH), with whom the chimpanzees had a positive relationship. The experimenter sat to one side of the Pan-pipes on the outside of the cage, and used the tool to raise and lower the T-bar. As with the ghost displays, the experimenter only gave demonstrations when all group members were attending to the apparatus and also inserted the food chute through the cage mesh for every 20th demonstration so that the grape would roll into the chimpanzees’ enclosure periodically. After all the demonstrations had been given, the experimenter pushed the Pan-pipes up against the cage mesh and baited the apparatus with a grape, again in full view of the group. At this point, the group was given one hour to freely interact with the Pan-pipes and if any chimpanzee successfully obtained the grape from the Pan-pipes, the experimenter re-baited the Pan-pipes.
2.7. Procedure: video display of a chimpanzee agent
A video display of an unfamiliar chimpanzee was the fourth condition and previous experiments performed at this same facility that have indicated chimpanzees are capable of learning novel tasks from such information (i.e. video footage showing an unfamiliar chimpanzee, Perlman et al., 2010; Price et al., 2009; Hopper et al., 2012). For this condition, a Zenith colour television, with a 45 cm screen, was placed in front of the chimpanzees’ indoor caging on a trolley with the base of the screen 80 cm above ground level. The Pan-pipes task was placed alongside the television, out of reach, but in view, of the chimpanzees. The video-footage showed an unfamiliar female chimpanzee operating the Pan-pipes successfully using the Lift method 225 times.
A female chimpanzee (MY, aged 40 years) was used as the model for the video footage. (MY was also the model trained to perform Lift for our previous study of chimpanzee social learning, Hopper et al., 2007, and was housed in a different group from the observers in the present study.) MY was filmed using the Pan-pipes in her inside enclosure with a Sony MiniDV digital handycam (CDR-TRV27). Footage was captured from two angles: above and from the side (Fig. 5a shows a still from this footage). Due to the constraints of the indoor caging, no footage could be taken from behind the chimpanzee, as would be seen by observing cage mates (c.f. Hopper et al., 2012). The handycam was connected directly to the colour television to allow for the playback of the footage, which lasted for 15 min (Fig. 5b shows a female in group 1 observing the video display). After the presentation of the video, the television was moved away and the Pan-pipes was pushed against the caging and re-baited in full view of the group, which was then able to freely interact with it for one hour. If any chimpanzee was successful, the experimenter re-baited the apparatus as needed throughout the hour.
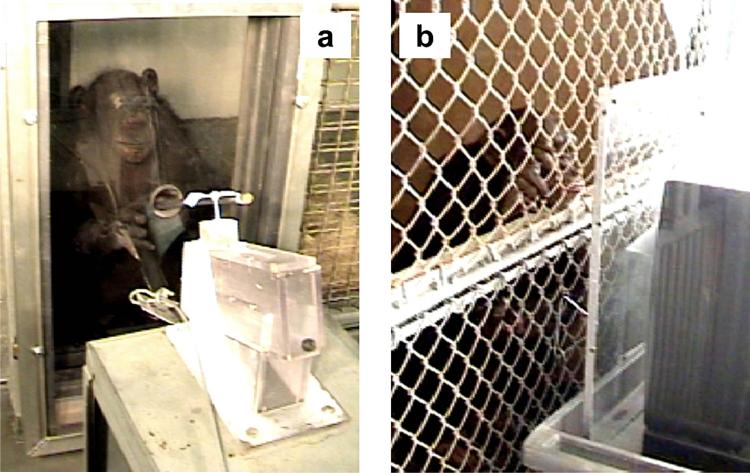
Fig. 5.
Video display of a chimpanzee agent. (a) A still from the video footage with female MY operating the Pan-pipes using the Lift technique and (b) 10 year-old female BR in group 1 watching the video demonstration before successfully using Lift to operate the Pan-pipes.
2.8. Procedure: live chimpanzee model
A mid-ranking or dominant chimpanzee from the group was selected to act as a conspecific model. Out of sight from their group, in one of their inside sleeping dens, which are separated from each other by concrete walls, this chimpanzee was trained by the experimenter, using positive reinforcement and shaping, to perform Lift (c.f. Whiten et al., 2005; Hopper et al., 2007). Training sessions were commenced by calling the chosen model chimpanzee into a sleeping den and separating them briefly from their social group. Each training session lasted no longer than 20 min and the individual was immediately reintroduced to their social group at the end of the training session. To ensure that participation was voluntary, training sessions were only run on days when the model chimpanzee would voluntarily separate from their group. Once the model chimpanzee was proficient in using Lift, defined as 30 consecutive successful operations, the experimenter presented the Pan-pipes to the group in their outside enclosure and called over the model to ‘demonstrate’ the Lift technique (c.f. Hopper et al., 2007). The model was then able to use the Lift technique to obtain grapes in view of their group mates. Following Hopper et al. (2007), to provide conspecific demonstrations by the model for 20 min, on the rare occasions that other chimpanzees in their group attempted to use the Pan-pipes, the experimenter pulled the apparatus out of reach. This ensured that all chimpanzees observed the chimpanzee model demonstrations of Lift before attempting to use the Pan-pipes themselves. After this first 20-min period, as with the other experimental conditions, the chimpanzees then entered a one-hour free access period in which any group member could interact with the Pan-pipes. Each time a chimpanzee successfully used the Lift technique to obtain a grape, the experimenter re-baited the apparatus.
2.9. Procedure: open diffusion phase
If one or more chimpanzees successfully learnt the Lift technique in the baseline or any of the experimental conditions, then the whole group continued into an ‘open diffusion’ phase (sensu Whiten et al., 2005), to discover if the technique spread to others within the group. For example, if a chimpanzee learnt from watching the human model, their group would continue with the open diffusion phase and would not be shown the video demonstration (Fig. 3). However, if no chimpanzee in a group had successfully learnt the Lift method from the human model, then that group would proceed to the video demonstration condition. In the open diffusion phase, as with each of the hour-long response periods, the group was given free access to the apparatus such that they could observe any successful individuals interacting with the Pan-pipes, as well as operate it themselves with the rod tool. This open diffusion phase lasted for a total of 20 h in order to provide all group members with an opportunity to interact with the device in case a single group member monopolized it in a single session. All open diffusion phase sessions were run in the chimpanzees’ outdoor enclosure and no more than one one-hour session was run with a group per day.
2.10. Coding
All test sessions were recorded on a Sony MiniDV digital handycam (DCR-HC35E). A running commentary was provided by the experimenter and the chimpanzees’ actions were coded from this videotape. One-zero sampling (Altmann, 1974) was used to code whether or not each chimpanzee was successful or not in each of the hour-long response periods and hour-long open diffusion phase sessions. A chimpanzee was coded as ‘successful’ if they could retrieve a grape from the Pan-pipes using the Lift method. As only one technique was possible to obtain a grape, the Lift method, there was no subjectivity as to which method had been used.
3. Results
3.1. Learning in the ‘nonsocial’ conditions
None of the chimpanzees from any of the four groups learnt how to operate the Pan-pipes in either the no-info condition or the ghost display condition (Table 1).
Table 1.
The number of chimpanzees that successfully used Lift in each of the experimental conditions and the total number (and percentage) of group members that used Lift one or more times during the open diffusion phase and experimental response periods combined.
| Group | Condition |
Total number of group successful | ||||
|---|---|---|---|---|---|---|
| No-info baseline | Ghost display | Human model | Video display (chimpanzee) | Chimpanzee model and open diffusion | ||
| 1 (N = 6) | 0 | 0 | 0 | 1(2?)a | 5 | 6 (100%) |
| 2 (N = 5) | 0 | 0 | 0 | 1(2?)a | 3 | 4 (80%) |
| 3 (N = 5) | 0 | 0 | 1 | – | 2 | 3 (60%) |
| 4 (N = 3) | 0 | 0 | 0 | 0 | 1 | 1 (50%)b |
In both groups 1 and 2, two females used Lift to obtain grapes from the Pan-pipes in the video display hour-long response period. However, given that the second successful female in each group also saw the first successful female in their group interact with the Pan-pipes, in addition to the video display, we cannot be sure whether these second females learnt from watching the video or their successful group mate, hence the question mark against ‘2’.
In group 4, one male was trained to use Lift so he could act as a social model for his group mates. Therefore, the potential number of chimpanzees in this group that could have learnt via social means was two.
3.2. Learning in the ‘social’ conditions
Once chimpanzees saw models with agency in conditions 3, 4 or 5, whether influenced directly by the presented model or by other successful group mates, the majority of chimpanzees showed success in using the Lift method. At the completion of the open diffusion phase sessions, 15 of 18 chimpanzees had successfully used the Lift technique at least once. Thus, the chimpanzees were significantly more successful after seeing an animate model than they were in the earlier no info and ghost conditions, in which there was zero success (two-tailed Fisher's Exact test, 0/19 vs. 15/18, P = 0.019: one chimpanzee acted as model in group 4, reducing the sample of potential social learners was reduced to 18, Table 1). At least one chimpanzee showed success as each model was introduced in the escalating series. This pattern, shown in Table 1, suggests that for different chimpanzees, the characteristics of a social model that engender social learning vary.
One male chimpanzee (KM, 16 years old) in group 3 acquired Lift after seeing the human model, so this group then entered the open diffusion phase (Table 1). Since this was the only group to learn in this condition, presentation of the human model in itself cannot be seen to have a significant effect on chimpanzee learning. Following this, two females in two groups (BR, 10 years old, from group 1 and VA, 36 years old, from group 2) learnt how to operate the Pan-pipes in the video display condition (Table 1). In these same groups, a further female in each group (MN, 21 years old, from group 1 and BA, 37 years old, from group 2) also avidly watched the television screen during the demonstration and went on to perform Lift during the one-hour response period. If, from this, we infer that these four chimpanzees learned from the video, the effect is significant (two-tailed Fisher's Exact test: 0/14 vs. 4/14, P = 0.042). However, caution is necessary in this conclusion because we cannot be certain the second chimpanzee to learn in each group was not influenced by the first. Both groups 1 and 2 then went on to the 20-h open diffusion phase, at the end of which all six chimpanzees from group 1 and four of the five chimpanzees in group 2 could successfully operate the Pan-pipes having seen demonstrations of Lift by conspecifics, either on the video or live in their group. Only one group (group 4) remained that had not performed any Lift behavior at the completion of the first four conditions. In this group, a male (SN, 21 years old) was trained to perform Lift and act as a model for his group members in their open diffusion phase. At the end of the open diffusion phase, there was one additional learner among the two remaining chimpanzees in his group (Table 1).
4. Discussion
In this study, the majority of chimpanzees required a live conspecific model in order to learn how to successfully execute ‘Lift’ with the Pan-pipes tool-use task. In the baseline condition, which provided no information about the task, chimpanzees were unable to solve the Pan-pipes via trial-and-error learning, and nor were they able to learn from a ghost display, despite (1) witnessing the tool and apparatus movements required to release a grape and (2) being tested in their social groups. Following these asocial conditions, we exposed the chimpanzees to an escalating series of social demonstrations, to identify what the chimpanzees’ ‘tipping point’ was for learning this complex tool-use method. Although one male was successful after observing a human model and at least two females showed success after seeing video footage of an unfamiliar adult female chimpanzee, the majority of the chimpanzees were only successful after having the opportunity to watch their group mates use Lift and obtain grapes from the Pan-pipes.
Our previous research had revealed that chimpanzees appear unable to discover the Lift method via trial-and-error when tested singly (Whiten et al., 2005), and nor were they able to learn it from observing ghost displays, also when tested singly (Hopper et al., 2007). However, the ‘escalation’ design of the present study, avoiding counterbalancing of the order of the human, video and live chimpanzee phases, raises the question of whether the chimpanzees’ increased success in later, social, conditions was due to the nature of the information provided (i.e. models with agency) or might instead be a result of the chimpanzees’ accumulated exposure to the task and increased demonstrations of its affordances that they saw. We conclude the latter is unlikely for two reasons. First, in each phase, the chimpanzees witnessed a massive number of displays, over 200, that should have provided ample information if they were to learn from this. Second, and extending this point, our previous research showed that chimpanzees were no more successful at solving the task after a ghost display, even if they were given an additional hour in which to explore and interact with the device (Hopper et al., 2007). Indeed, most chimpanzees made the majority of their interactions with the Pan-pipes within the first half of their response period, showing that simply providing increased time for exploration is not sufficient for learning the Lift technique. Thus, while acknowledging that the ‘escalation’ experimental design used here is logically and inherently vulnerable to order effects, we propose that the above considerations leave little doubt but that the social conditions had a power to elicit social learning, absent in the non-social conditions (see also, Hopper et al., 2012).
From their study with rats, which we outlined in the Introduction, Zentall and Levine (1972) concluded that “learning increased as a function of number of cues provided” (p. 1220). The present study expands this conclusion and suggests that it is likely that both the number and type of cues are important for learning. In all conditions, the chimpanzees were shown a large number of demonstrated actions, yet the subjects only showed successful learning when those acts were paired with an animate model. However, it was only during the 20, hour-long open diffusion sessions, when the chimpanzees had the opportunity to observe conspecific group mates operate the Pan-pipes, and had an extended period of time for both observation and practice, that the majority of test subjects showed success at using Lift. Ultimately, 83% of the chimpanzees used Lift one or more times. Comparably, in our previous study of chimpanzee social learning of the Pan-pipes (Hopper et al., 2007), 67% of the 18 chimpanzees, housed in two social groups, learnt Lift from observing live conspecific models. These chimpanzees were given a comparable open diffusion phase (25 h long) and they also required an extended period of time to acquire the Lift technique; the chimpanzees took an average of 7 h 58 min to first perform the Lift action (range: 39 min–16 h 11 min) and eight of the chimpanzees never performed Lift (five exclusively used the alternative Poke technique, and three were never successful with either method) highlighting how complex this behavior is for chimpanzees to master.
The present study also highlights that chimpanzees’ previous failure to learn from ghost displays when tested singly is unlikely to be purely due to being tested without social support. In the ghost display condition, despite being given numerous ‘demonstrations’ of the required actions (225), being given an extensive period of time to freely interact with the Pan-pipes afterwards (1 h), and being in the presence of their group mates, the chimpanzees still failed to show any success. This failure is not just restricted to chimpanzees. Even children, who were shown ghost displays of the Lift technique, and who were tested in a setting with conspecific social support (the experimenter was present), were significantly less likely to perform Lift than those children who saw a full demonstration by a conspecific adult model (Hopper et al., 2010; Caldwell et al., 2012). Thus, for such complex tasks, more than social support is required; both chimpanzees and children, it appears, require a demonstration by a model with agency. Although in some instances social support may be sufficient to aid exploration and learning (e.g., Dindo et al., 2009; Hopper et al., 2013), depending on the motivational changes induced, the mere presence of conspecifics is not always sufficient for learning (Zajonc, 1965; Zentall and Levine, 1972; Zentall and Hogan, 1976). Additionally, local enhancement, created by the presence of a conspecific in a particular location, may encourage exploration, but does not necessarily provide a naïve observer with enough information to learn a complex new skill (in this case, how to use a tool to operate the Pan-pipes using the Lift method). Indeed, many examples of learning via local enhancement pertain to individuals acquiring a novel variant of an already-known behavior, rather than a completely new action (e.g., ‘novel foods’ are learnt to be eaten, but the behavioral skill ‘eating’ is already known, see e.g., van de Waal et al., 2013; Finestone et al., 2014).
It is also important to acknowledge that ghost display experiments test for emulation conceived of in one particular, and perhaps extreme, way. This was well illustrated by Tomasello (1999), who noted that “if a mother chimpanzee rolls a log and eats the insects underneath, her child will very likely follow suit . . .. [but] the youngster would have learned the same thing if the wind, rather than the mother, had caused the log to roll over and expose the ants” (p. 29). Ghost experiments essentially test for effects like that of the wind, envisioned here. However, other forms of emulation might operate in situations where an animate agent is present, but the observer only recreates the outcomes of their actions in a different way (sensu Wood, 1989). A compelling example of this form of emulation was shown in a recent experiment in which a human model poured water from a bottle into a container to make a peanut inside float to the top, after which observer chimpanzees, who were not given a bottle to hold water, collected water in their mouths and spat it in the container to make the peanut float (Tennie et al., 2010a). Whether the chimpanzees’ success via emulating was due to the more causally-transparent nature of their task, the demonstration by an animate model (the human experimenter) rather than a ghost display, or because the subjects were tested with a social partner, cannot be determined.
Our present results, in conjunction with those reported previously for chimpanzees (Hopper et al., 2007, 2008; Tennie et al., 2010a) suggest the likelihood of an interplay between task complexity and the importance of an animate model; as complexity increases, so does the need for a model with agency (Hopper et al., 2010; Whiten et al., 2009). Indeed, this does not seem to be a requirement restricted to chimpanzees. A recent study with Goffin cockatoos (Cacatua goffini, Auersperg et al. 2014), also reported that the birds only learnt a novel tool-use behavior after observing a live conspecific, compared to seeing a ghost display of the required actions, while previous studies have shown learning (but not matching) from ghost displays of simpler actions by birds (e.g., Columbia livia, Klein and Zentall, 2003). Future research should aim to explicitly tease apart the interplay between a species’ requirement for a model with agency and the complexity of the behavior to-be-learnt. Direct comparisons of the social learning processes elicited when subjects are faced with different levels of task complexity (e.g., Tennie et al., 2010b; Russell et al., 2011; Massen et al., 2013), or other variables, such as individual traits (e.g., Massen et al., 2013; Lacreuse et al., 2014), or the dynamics of the social environment (e.g., Cronin et al., 2014), would also be valuable.
Acknowledgements
We thank Mike Brown, Guest Editor for the Comparative Cognition Society Special Issue of Behavioural Processes, for inviting us to contribute to this special issue honouring Tom Zentall and his work. We also thank the constructive feedback we received from two anonymous reviewers. We are extremely grateful to Jaine Perlman and Erica Thiele for their expert assistance with this study and to the colony manager and care staff for providing the highest standard of care to the chimpanzees. We also thank Andy Burnley for constructing the Pan-pipes tool-use task. This research was supported by a BBSRC postgraduate studentship (BBS/S/K/2004/11255) and, at the time of writing, LMH was supported by the Leo S. Guthman Fund. AW was supported by a Leverhulme Trust Major Research Fellowship. Support for the chimpanzee colony came from NIH/NCRR U42-RR015090.
Footnotes
This article is part of a Special Issue entitled: Tribute to Tom Zentall.
References
- Acerbi A, Tennie C, Nunn CL. Modeling imitation and emulation in constrained search spaces. Learning & Behavior. 2010;39(2):104–114. doi: 10.3758/s13420-010-0009-z. [DOI] [PubMed] [Google Scholar]
- Akins CK, Klein ED, Zentall TR. Imitative learning in Japanese quail (Coturnix japonica) using the bidirectional control procedure. Animal Learning and Behavior. 2002;30(3):275–281. doi: 10.3758/bf03192836. [DOI] [PubMed] [Google Scholar]
- Akins CK, Zentall TR. Imitative learning in male Japanese quail (Coturnix japonica) using the two-action method. Journal of Comparative Psychology. 1996;110(3):316–320. doi: 10.1037/0735-7036.110.3.316. [DOI] [PubMed] [Google Scholar]
- Altmann SA. Observational study of behavior: sampling methods. Behaviour. 1974;49(3):227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Auersperg AMI, von Bayern AMI, Weber S, Szabadvari A, Bugnyar T, Kacelnik A. Social transmission of tool use and tool manufacture in Goffin cockatoos (Cacatua goffini). Proceedings of the Royal Society B: Biological Sciences. 2014;281(1793):20140972. doi: 10.1098/rspb.2014.0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T. Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Animal Cognition. 2003;6(4):213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Bugnyar T, Huber L. Push or pull: an experimental study on imitation in marmosets. Animal Behaviour. 1997;54:817–831. doi: 10.1006/anbe.1996.0497. [DOI] [PubMed] [Google Scholar]
- Byrne RW. A comment on Boesch, C and Tomasello, M Chimpanzee and human culture. Current Anthropology. 1998;39(5):604–605. [Google Scholar]
- Caldwell CA, Schillinger K, Evans CL, Hopper LM. End state copying by humans (Homo sapiens): Implications for a comparative perspective on cumulative culture. Journal of Comparative Psychology. 2012;126(2):161–169. doi: 10.1037/a0026828. [DOI] [PubMed] [Google Scholar]
- Call J, Carpenter M, Tomasello M. Copying results and copying actions in the process of social learning: chimpanzees (Pan troglodytes) and human children (Homo sapiens). Animal Cognition. 2005;8(3):151–163. doi: 10.1007/s10071-004-0237-8. [DOI] [PubMed] [Google Scholar]
- Cannon EN, Woodward AL. Infants generate goal-based action predictions. Developmental Science. 2012;15(2):292–298. doi: 10.1111/j.1467-7687.2011.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin KA, Pieper BA, van Leeuwen EJC, Mundry R, Haun DBM. Problem solving in the presence of others: how rank and relationship quality impact resource acquisition in chimpanzees (Pan troglodytes). PLoS ONE. 2014;9(4):e93204. doi: 10.1371/journal.pone.0093204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindo M, Whiten A, de Waal FBM. Social facilitation of exploratory foraging behavior in capuchin monkeys (Cebus apella). American Journal of Primatology. 2009;71(5):419–426. doi: 10.1002/ajp.20669. [DOI] [PubMed] [Google Scholar]
- Finestone E, Bonnie KE, Hopper LM, Vreeman VM, Lonsdorf EV, Ross SR. The interplay between individual, social, and environmental influences on chimpanzee food choices. Behavioural Processes. 2014;105:71–78. doi: 10.1016/j.beproc.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Flynn EG, Whiten A. Dissecting children's observational learning of complex actions through selective video displays. Journal of Experimental Child Psychology. 2013;116(2):247–263. doi: 10.1016/j.jecp.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Gerson SA, Woodward AL. A claw is like my hand: comparison supports goal analysis in infants. Cognition. 2012;122(2):181–192. doi: 10.1016/j.cognition.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes K, Hayes C. Imitation in a home-raised chimpanzee. Journal of Comparative Physiological Psychology. 1952;45:450–459. doi: 10.1037/h0053609. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Yudin H. Social behavior of primates. I. Social facilitation of feeding in the monkey and its relation to attitudes of ascendance and submission. Journal of Comparative Psychology. 1933;16(2):171–185. [Google Scholar]
- Heyes CM, Jaldow E, Nokes T, Dawson GR. Imitation in rats (Rattus norvegicus): the role of demonstrator action. Behavioural Processes. 1994;32(2):173–182. doi: 10.1016/0376-6357(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Hopper LM. ‘Ghost’ experiments and the dissection of social learning in humans and animals. Biological Reviews. 2010;85(4):685–701. doi: 10.1111/j.1469-185X.2010.00120.x. [DOI] [PubMed] [Google Scholar]
- Hopper LM, Flynn EG, Wood LAN, Whiten A. Observational learning of tool use in children: investigating cultural spread through diffusion chains and learning mechanisms through ghost displays. Journal of Experimental Child Psychology. 2010;106(1):82–97. doi: 10.1016/j.jecp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Hopper LM, Holmes AN, Williams LE, Brosnan SF. Dissecting the mechanisms of squirrel monkey (Saimiri boliviensis) social learning. PeerJ. 2013;1:e13. doi: 10.7717/peerj.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Lambeth SP, Schapiro SJ. An evaluation of the efficacy of video displays for use with chimpanzees (Pan troglodytes). American Journal of Primatology. 2012;74(5):442–449. doi: 10.1002/ajp.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Lambeth SP, Schapiro SJ, Whiten A. Observational learning in chimpanzees and children studied through ‘ghost’ conditions. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1636):835–840. doi: 10.1098/rspb.2007.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Schapiro SJ, Lambeth SP, Brosnan SF. Chimpanzees’ socially maintained food preferences indicate both conservatism and conformity. Animal Behaviour. 2011;81(6):1195–1202. doi: 10.1016/j.anbehav.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Spiteri A, Lambeth SP, Schapiro SJ, Horner V, Whiten A. Experimental studies of traditions and underlying transmission processes in chimpanzees. Animal Behaviour. 2007;73(6):1021–1032. [Google Scholar]
- Horner V, Whiten A. Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens). Animal Cognition. 2005;8(3):164–181. doi: 10.1007/s10071-004-0239-6. [DOI] [PubMed] [Google Scholar]
- Huang C-T, Charman T. Gradation of emulation learning in infants’ imitation of actions on objects. Journal of Experimental Child Psychology. 2005;92(3):276–302. doi: 10.1016/j.jecp.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Inoue-Nakamura N, Matsuzawa T. Development of stone tool use by wild chimpanzees (Pan troglodytes). Journal of Comparative Psychology. 1997;111(2):159–173. doi: 10.1037/0735-7036.111.2.159. [DOI] [PubMed] [Google Scholar]
- Kano F, Call J. Great apes generate goal-based action predictions: an eye-tracking study. Psychological Science. 2014;25(9):1691–1698. doi: 10.1177/0956797614536402. [DOI] [PubMed] [Google Scholar]
- Kis A, Huber L, Wilkinson A. Social learning by imitation in a reptile (Pogona vitticeps). Animal Cognition. doi: 10.1007/s10071-014-0803-7. in press, http://dx.doi.org/10.1007/s10071-014. [DOI] [PubMed]
- Klein ED, Zentall TR. Imitation and affordance learning by pigeons (Columba livia). Journal of Comparative Psychology. 2003;117(4):414–419. doi: 10.1037/0735-7036.117.4.414. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Russell JL, Hopkins WD, Herndon JG. Cognitive and motor aging in female chimpanzees. Neurobiology of Aging. 2014;35(3):623–632. doi: 10.1016/j.neurobiolaging.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos G, Janiak M, Malek L, Muszynski R, Konok V, Tchon K, Miklósi Á. Sensing sociality in dogs: what may make an interactive robot social? Animal Cognition. 2014;17(2):387–397. doi: 10.1007/s10071-013-0670-7. [DOI] [PubMed] [Google Scholar]
- Luncz LV, Boesch C. Tradition over trend: neighboring chimpanzee communities maintain differences in cultural behavior despite frequent immigration of adult females. American Journal of Primatology. 2014;76(7):649–657. doi: 10.1002/ajp.22259. [DOI] [PubMed] [Google Scholar]
- Marshall-Pescini S, Whiten A. Social learning of nut-cracking behaviour in East African sanctuary-living chimpanzees (Pan troglodytes schweinfurthii). Journal of Comparative Psychology. 2008;122(2):186–194. doi: 10.1037/0735-7036.122.2.186. [DOI] [PubMed] [Google Scholar]
- Massen JJM, Antonides A, Arnold A-MK, Bionda T, Koski SE. A behavioral view on chimpanzee personality: exploration tendency, persistence, boldness, and tool-orientation measured with group experiments. American Journal of Primatology. 2013;75(9):947–958. doi: 10.1002/ajp.22159. [DOI] [PubMed] [Google Scholar]
- Miller HC, Rayburn-Reeves R, Zentall TR. Imitation and emulation by dogs using a bidirectional control procedure. Behavioural Processes. 2009;80(2):109–114. doi: 10.1016/j.beproc.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. Social learning and teaching in chimpanzees. Biology & Philosophy. 2013;28(6):879–901. [Google Scholar]
- Nagell K, Olguin RS, Tomasello M. Processes of social learning in the tool use of chimpanzees (Pan troglodytes) and human children (Homo sapiens). Journal of Comparative Psychology. 1993;107(2):174–186. doi: 10.1037/0735-7036.107.2.174. [DOI] [PubMed] [Google Scholar]
- Over H, Carpenter M. Putting the social into social learning: explaining both selectivity and fidelity in children's copying behavior. Journal of Comparative Psychology. 2012;126(2):182–192. doi: 10.1037/a0024555. [DOI] [PubMed] [Google Scholar]
- Perlman JE, Horner V, Bloomsmith MA, Lambeth SP, Schapiro SJ. Positive reinforcement training, social learning, and chimpanzee welfare. In: Lonsdorf EV, Ross SR, Matsuzawa T, Goodall J, editors. The Mind of The Chimpanzee: Ecological and Experimental Perspectives. University of Chicago Press; Chicago: 2010. pp. 320–331. [Google Scholar]
- Price EE, Lambeth SP, Schapiro SJ, Whiten A. A potent effect of observational learning on chimpanzee tool construction. Proceedings of the Royal Society B: Biological Sciences. 2009;276(1671):3377–3383. doi: 10.1098/rspb.2009.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JL, Lyn H, Schaeffer JA, Hopkins WD. The role of socio-communicative rearing environments in the development of social and physical cognition in apes. Developmental Science. 2011;14(6):1459–1470. doi: 10.1111/j.1467-7687.2011.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Tzelnic T, Carey S. Knowing who dunnit: infants identify the causal agent in an unseen causal interaction. Developmental Psychology. 2007;43(1):149–158. doi: 10.1037/0012-1649.43.1.149. [DOI] [PubMed] [Google Scholar]
- Subiaul F, Cantlon JF, Holloway RL, Terrace HS. Cognitive imitation in rhesus macaques. Science. 2004;305(5682):407–410. doi: 10.1126/science.1099136. [DOI] [PubMed] [Google Scholar]
- Subiaul F, Romansky K, Cantlon JF, Klein T, Terrace H. Cognitive imitation in 2-year-old children (Homo sapiens): a comparison with rhesus monkeys (Macaca mulatta). Animal Cognition. 2007;10(4):369–375. doi: 10.1007/s10071-006-0070-3. [DOI] [PubMed] [Google Scholar]
- Subiaul F, Vonk J, Rutherford MD. The ghosts in the computer: the role of agency and animacy attributions in ghost controls. PLoS ONE. 2011;6(11):e26429. doi: 10.1371/journal.pone.0026429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennie C, Call J, Tomasello M. Push or pull: imitation vs. emulation in great apes and human children. Ethology. 2006;112(12):1159–1169. [Google Scholar]
- Tennie C, Call J, Tomasello M. Evidence for emulation in chimpanzees in social settings using the floating peanut task. PLoS ONE. 2010a;5(5):e10544. doi: 10.1371/journal.pone.0010544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennie C, Greve K, Gretscher H, Call J. Two-year-old children copy more reliably and more often than nonhuman great apes in multiple observational learning tasks. Primates. 2010b;51(4):337–351. doi: 10.1007/s10329-010-0208-4. [DOI] [PubMed] [Google Scholar]
- Tomasello M. The Cultural Origins of Human Cognition. Harvard University Press; Cambridge, MA.: 1999. [Google Scholar]
- van de Waal E, Borgeaud C, Whiten A. Potent social learning and conformity shape a wild primate's foraging decisions. Science. 2013;340(6131):483–485. doi: 10.1126/science.1232769. [DOI] [PubMed] [Google Scholar]
- Whiten A, Ham R. On the nature and evolution of imitation in the animal kingdom: reappraisal of a century of research. Advances in the Study of Behavior. 1992;21:239–283. [Google Scholar]
- Whiten A, Horner V, Litchfield CA, Marshall-Pescini S. How do apes ape? Learning and Behavior. 2004;32(1):36–52. doi: 10.3758/bf03196005. [DOI] [PubMed] [Google Scholar]
- Whiten A, Horner V, de Waal FBM. Conformity to cultural norms of tool use in chimpanzees. Nature. 2005;437:737–740. doi: 10.1038/nature04047. [DOI] [PubMed] [Google Scholar]
- Whiten A, McGuigan N, Marshall-Pescini S, Hopper LM. Emulation, imitation, over-imitation and the scope of culture for child and chimpanzee. Philosophical transactions of the Royal Society of London: B. 2009;364(1528):2417–2428. doi: 10.1098/rstb.2009.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. Social interaction as tutoring. In: Bornstein MH, Bruner JS, editors. Interaction in Human Development. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1989. pp. 59–80. [Google Scholar]
- Zajonc RB. Social facilitation. Science. 1965;149(3681):269–274. doi: 10.1126/science.149.3681.269. [DOI] [PubMed] [Google Scholar]
- Zentall T. An analysis of imitative learning in animals. In: Heyes CM, Galef BG Jr., editors. Social Learning and Traditions in Animals. Erlbaum; Hillsdale, NJ: 1996. pp. 211–243. [Google Scholar]
- Zentall TR. Imitation: definitions, evidence, and mechanisms. Animal Cognition. 2006;9(4):335–353. doi: 10.1007/s10071-006-0039-2. [DOI] [PubMed] [Google Scholar]
- Zentall T. Social learning mechanisms: implications for a cognitive theory of imitation. Interaction Studies. 2011;12(2):233–261. [Google Scholar]
- Zentall T. Perspectives on observational learning in animals. Journal of Comparative Psychology. 2012;126(2):114–128. doi: 10.1037/a0025381. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Hogan DE. Imitation and social facilitation in the pigeon. Animal Learning & Behavior. 1976;4(4):4270430. [Google Scholar]
- Zentall TR, Levine JM. Observational learning and social facilitation in the rat. Science. 1972;178(4066):1220–1221. doi: 10.1126/science.178.4066.1220. [DOI] [PubMed] [Google Scholar]
- Zohar O, Terkel J. Acquisition of pine cone stripping behaviour in black rats (Rattus rattus). International Journal of Comparative Psychology. 1991;5(1):1–6. [Google Scholar]