Abstract
Recent advances in immunoncology have dramatically changed the treatment options available to cancer patients. However, the fundamental challenges with this therapeutic modality are not new and still persist with the current wave of immunoncology compounds. These challenges are centered on the activation and expansion, induction of intratumoral infiltration and persistence of highly activated, cytotoxic, tumor antigen specific CD8+ T cells. We have investigated the anti-tumor mechanism of action of pegylated recombinant interleukin-10, (PEG-rIL-10) both pre-clinically with murine (PEG-rMuIL-10) and now clinically (AM0010) with human pegylated interleukin-10. The preponderance of data suggest that IL-10’s engagement of its receptor on CD8+ T cells enhances their activation status leading to antigen specific expansion. Quantitation of CD8+ T cell tumor infiltration reveals that treatment of both humans and mice with pegylated rIL-10 results in 3–4 fold increases of intratumoral, cytotoxic, CD8+ T cells. In addition, mice cured of their tumors with PEG-rMuIL-10 exhibit long term immunological protection from tumor re-challenge and long term treatment of cancer patients with AM0010 results in the persistence of highly activated CD8+ T cells. Cumulatively, these data suggest the IL-10 represents an emerging therapeutic that specifically addresses the fundamental challenges of the current wave of immunoncology assets.
Keywords: Interleukin-10, Immunoncology, CD8+ T cells, Activated, Cytotoxic, Tumor Infiltrating, Persistence
Introduction
The idea of immunotherapy for oncology is not new, as the link between tumor regressions and infection was first noted by both Anton Chekhov[1] and William Coley[2]. Since then a slow march of progress has led to the current wave of immunoncology. Interleukin-2 (IL-2) was one of the first approved biological therapies[3]. Though IL-2 receptor engagement leads to broad spectrum immune activation that exhibits a narrow therapeutic window, this pioneering work was among the first to show that the immune system could be harnessed in a meaningful way to treat cancer. Monoclonal antibodies such as Herceptin[4] and Rituximab[5] were developed to target innate cytotoxic immune cell functions to the tumor via a process of antibody dependent cellular cytotoxicity. Other antibodies, such as Cetuximab, were developed to block specific growth factor receptor pathways[6]. The current immunoncology wave was initiated by the clinical results generated by Ipilimumab[7]. This was followed closely by the development of the anti-PD1 antibodies. The anti-PD1 receptor antibodies were the first to exhibit reproducible anti-tumor effects across multiple cancer indications[8, 9]. Additionally, the development of chimeric antigen receptor T cells (CARTs)[10] coupled with the development of technologies for ex vivo T cell expansion and adoptive transfer[11, 12] have firmly established immunoncology as a promising therapeutic class.
However, even with the rapid development of these technologies, challenges still persist. The key requirements necessary for generating effective anti-tumor immunity are becoming clearer as we elucidate the complex interactions between the immune system, the tumor, and the tumor microenvironment, as well as the consequences of therapeutically induced changes to the antigenic profile of the tumor. It has become more evident that CD8+ T cells are the most important lymphocytic cell population to stimulate in order to therapeutically induce initial control of tumor growth and long term anti-tumor immunity[13, 14]. The presence of intratumoral CD8+ T cells correlates with progression free survival across multiple solid tissue tumor indications[15–17]. Further analysis of these tumor-infiltrating CD8+ T cells indicates that their expression of cytotoxic enzymes further distinguishes a patient’s likelihood for long term survival[18].
CD8+ T cells
Intratumoral CD8+ T cells have become even more important as the current wave of immunoncology compounds, such as Nivolumab or Pembrolizumab, requires a pre-existing immune response represented by infiltrating CD8+ T cells and commensurate PD1/PDL1 expression[19]. Problematically, tumors are exquisitely adept at preventing T cells from infiltrating their microenvironment[20]. Approximately 35% of patients with immune sensitive tumors exhibit sufficient immune infiltration to receive benefit from first wave of immunotherapy compounds[21].
Immunoncology Challenges
There appear to be three fundamental requirements of CD8+ T cell biology needed for the generation of durable anti-tumor immunity. The first is the need to activate and expand tumor antigen specific cytotoxic CD8+ T cells[22, 23]. The second is to increase the density of these cells in the tumor to levels that will induce substantial tumor cell destruction[24, 25]. Finally, tumor eradication and long term immunity requires that these T cells persist in their highly activated cytotoxic state and avoid exhaustion[26–28].
Activation and Expansion
The first requirement, activating and expanding the number of tumor antigen specific CD8+ T cells, seems like an extraordinarily solvable challenge as vaccine technology has been used since the mid 1800’s to establish long term and potent antigen specific immunity[29]. While prophylactic vaccines are highly efficacious, there are unfortunately no broadly utilized therapeutic vaccines, especially in an oncology setting[30, 31]. However, immunoncology vaccines effectively “work” in that cancer patients treated with a variety of vaccine technologies undergo the peripheral expansion of antigen specific CD4+ and CD8+ T cells[32].
Intratumoral Infiltration
The second fundamental requirement is to drive the activated, tumor antigen specific T cells to infiltrate the tumor microenvironment in sufficient numbers to exert effective anti-tumor cytotoxic function. High dose IL-2 was the first therapeutic attempt at changing the intratumoral lymphoid infiltrate. The initial trial resulted in three striking findings: One was that the use of cytokines to activate the peripheral immune system is fraught with danger. Too much activation can lead to profound but potentially manageable toxicities[33]. Two, the immune system can be harnessed to exert long lasting immunity and tumor control, resulting in the most durable cures ever reported[34, 35]. Three, the high doses of IL-2 did not substantially increase the number of activated CD8+ T cells within the tumor, leading investigators to move very quickly to high dose IL-2 in combination with adoptive T cell therapy[36]. Subsequent use of other stimulatory cytokines has not resulted in the same degree of prolonged anti-tumor function. The lack of efficacy has been driven by narrow therapeutic windows due to systemic and uncontrollable immune related toxicities[37, 38]. Furthermore, while some studies show that antigen specific T cells infiltrate into tumors, these cells are ultimately unable to mount an effective anti-tumor immune response due to compensatory intratumoral inhibitory feedback pathways induced by the activated cells[39]. The subsequent challenge then appears to be one of persistence of the activated, tumor antigen specific cytotoxic CD8+ T cell population[40].
Persistence
The requirement of persistence is perhaps best elucidated by one of the first CART studies by Rosenberg and colleagues. In this study, 15 melanoma patients were treated with their own ex vivo expanded T cells transduced to express a T cell receptor specific for MART-1, gp100, NY-ESO-1 or p53 tumor antigen. After expansion, the cells were re-infused and their presence in the periphery was assessed over time. Two patients exhibited long term persistence of their transgene, however the remaining patients did not. This elucidates a fundamental self-limiting immunological mechanism inherent in all immunostimulatory pathways, termed activation induced cell death[41]. This is the process by which activated cells self-limit by generating the means of their own destruction. Cytotoxic cells become sensitive to the same factors that they secrete to induce apoptosis in target cells, undergoing apoptosis themselves. In this manner, the immune system and host do not become overwhelmed by the presence of T cell clones that no longer have a target to engage. In context of immunoncology, since all immunologically activated cytotoxic cells are the architects of their own destruction, the largest challenge is how to maintain the longer term persistence of an antigen specific, cytotoxic T cell repertoire. This challenge is most abundantly clear with the advent of CART technology. By circumventing the immunological machinery of antigen presentation, CD4+ T cell activation, expansion and cytokine secretion followed by CD8+ T cell activation, as well as expansion of cytotoxic effector functions, CART technology jumps straight to providing antigen targeted, activated and cytotoxic cells. By virtue of the numbers provided and the nature of the CART, these cells tether to the target expressing tumor cell population and initiate substantial tumor cell destruction. However, as with all activated cytotoxic T cell populations, these cells self-limit and undergo apoptosis, often prior to complete eradication of the tumor mass. The therapy then loses efficacy with subsequent treatments as the CART construct can become immunogenic.
Therefore, how can we solve these three primary immunoncology challenges? How do we facilitate activation and expansion of tumor antigen specific cytotoxic CD8+ T cells, intratumoral T cell infiltration, and enhance the persistence of these cells in a highly activated and cytotoxic state? Each of these challenges can be addressed by different ligands interacting with their cognate receptors. Chemokines gradients can cause T cell infiltration[42]. Cytokines such as IL-2, IL-7, IL-12, IL-15, IL-21, and IL-27 enhance CD8+ T cell cytotoxic function and cytokines such as IL-7 and IL-15 are reported to be survival factors for memory CD8+ T cells[43]. These data suggest that the complexity of the immune system is so significant that a multiplicity of factors would be required to appropriately engage all of the cells and receptors in order to activate and maintain a tumor antigen specific, cytotoxic T cell population.
IL-10 Enhances CD8+ T cell Function
It was therefore surprising when we investigated the potent anti-tumor effect elicited by treating mice with PEG-rMuIL-10 that the fundamental challenges of immunoncology are addressed by IL-10, by itself. As previously reported, PEG-IL-10/IL-10 engagement of its receptor enhances the cytotoxic function of CD8+ T cells on a per cell basis[44], increases the number of CD8+ T cells within the tumor and elicits the generation of potent and long lasting anti-tumor immunity[45, 46]. For these reasons we tested the effects of PEG-rHuIL-10 (AM0010), in cancer patients and have uncovered that the immunostimulatory biology resulting from IL-10 engaging its receptor, is conserved between mice and humans.
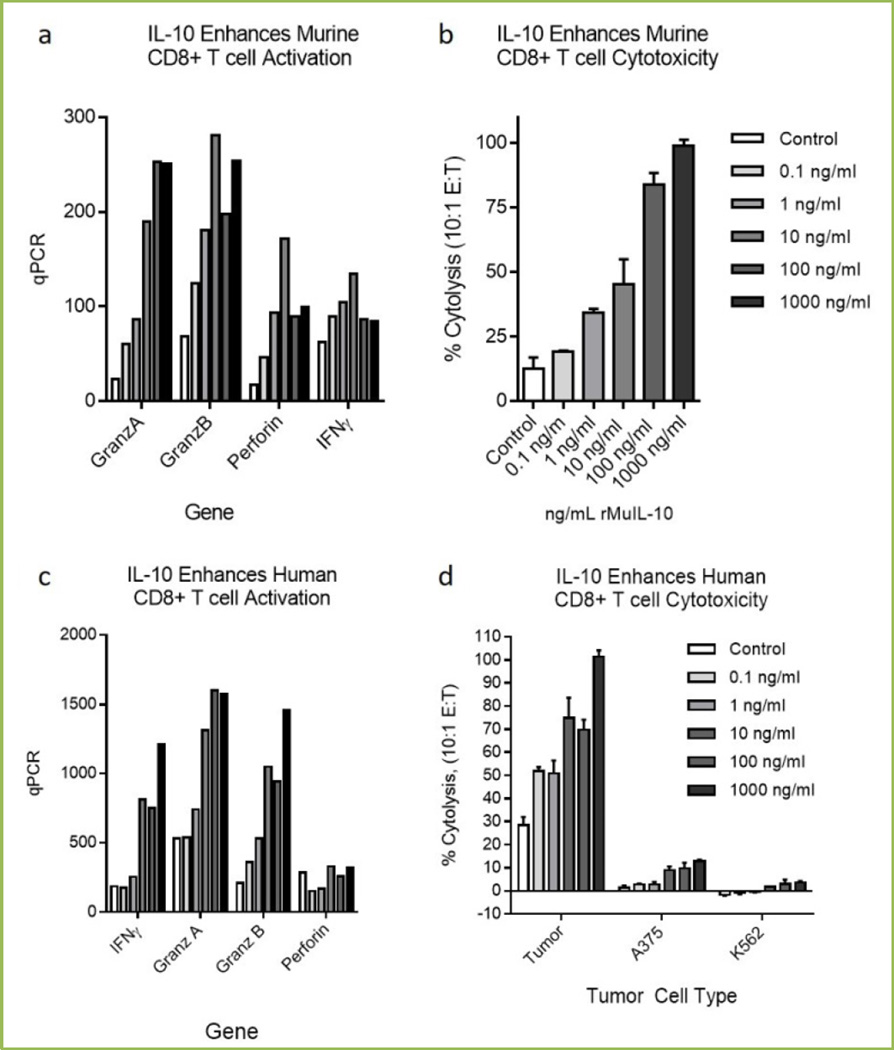
Figure 1 illustrates that IL-10 exposures enhances the cytotoxic profile and function of murine and human CD8+ T cells, (reproduced in part from[46]). Figure 1a shows that rMuIL-10 enhances murine OT1 CD8+ activation expression profile, (IFNγ, Granzyme A/B and Perforin) and cytotoxic function 1b, when exposed to SIINFEKL pulsed tumor cells. Figure 1c shows that IL-10 enhances human CD8+ TIL activation expression profile, (IFNγ, Granzyme A/B and Perforin) and cytotoxic function, 1d when exposed to autologous tumor cells vs. melanoma control tumor cells (A375) or NK cell targets, (K562).
Fig 1. IL-10 exposure directly stimulates murine and human CD8+ T cell cytotoxic function.
Murine OT1 T cells isolated by magnetic (Miltenyi) bead isolation were stimulated and exposed to species specific IL-10 for 3–5 days. Cells were assessed for cytotoxic mRNA regulation (1a) and for cytolytic function (1b). OT1 cells were exposed at 10:1 effector to target ratio and cytolysis determined by CytoTox96 (Promega) LDH release. Target cells were PDV6 squamous tumor cells pulsed with SIINFEKL for 24 hours. Cytolysis was assessed after 4 hours. Human CD8+ T cells were isolated by magnetic (Miltenyi) bead isolation (1c), stimulated and exposed to IL-10 for 3–5 days. Cells were assessed for cytotoxic mRNA regulation (1c) and for cytolytic function (1d). Tumor antigen specific CD8+ T cells were isolated from surgically resected human melanoma tumors and stimulated with irradiated autologous tumor cells in the presence of 100U/mL IL-2 (Centocor) and rested for 5 days with IL-10. Activated CD8+ T cells were exposed at 10:1 effector to target ratio and cytolysis of Cr51 labeled autologous tumor cells, A375 (ATCC) or K562 (ATCC) assessed after 4 hours.
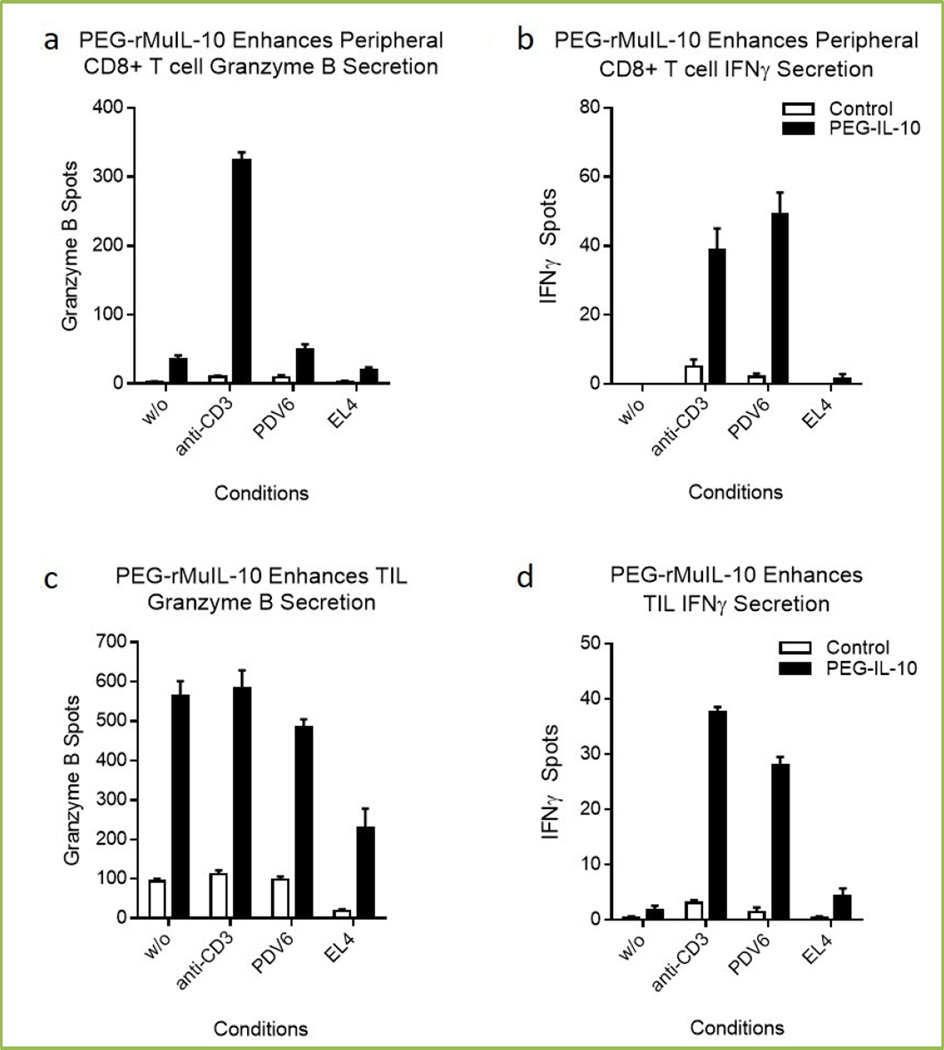
Figure 2 shows that continued exposure of mice to PEG-rMuIL-10 increase the number of peripheral CD8+ T cells (2a–2b) and tumor infiltrating lymphocytes (TIL) (2c–2d), which secrete Granzyme B and IFNγ. Similar to the in vitro results in Figure 1a–1b, these data suggest exposure to PEG-rMuIL-10 increases the functional activation state of both the peripheral and intratumoral CD8+ T cells. In direct comparison to the in vitro results reported by Chan et al., Granzyme B (2a) and IFNγ (2b) secretion are similarly controlled by T cell receptor engagement in the periphery. However in the more target rich environment in the tumor, Granzyme B (2c) secretion is less controlled by T cell receptor engagement than IFNγ secretion (2d). Consistent with these data of PEG-IL-10-induced immune activation, we’ve reported that intratumoral T cells of cancer patients exhibit increased expression of Granzyme B after treatment with AM0010[47]. These data suggest that PEG-IL-10/IL-10 ligation of its receptor and resultant immuno-stimulatory function is a conserved biology between mice and humans and provide a solution to the first challenge of immunoncology.
Fig 2. Effect of PEG-rMuIL-10 dosing on peripheral and intratumoral CD8+ T cell cytotoxicity.
These ELISPOTs (R&D Systems) were generated by magnetic (Miltenyi) bead isolation of 1000 CD8+ T cells from PBMC or mechanically disrupted and enzyme digested PDV6 (Schering Plough) squamous tumors. CD8+ T cells were exposed for 24 hrs without any secondary stimulus, (w/o), or 1 µg/mL soluble anti-CD3 (eBiosciences), 100 PDV6 squamous tumor or EL4 (ATCC) (as negative control) tumor cells. Spots were quantified with ImmunoSpot Software.
IL-10 Treatment Increases Intratumoral CD8+ Cell Density and Antigen Specificity
To address the second challenge, we assessed whether treatment of mice with PEG-rMuIL-10 increased the numbers of tumor infiltrating CD8+ T cells. As previously reported[46], treatment with PEG-rMuIL-10 causes a 3–4 fold increase of intratumoral CD8+ T cells. Similar results were generated when serial cancer patient biopsies were assessed for intratumoral CD8+ T cells pre and post AM0010 treatment[47]. We also assessed whether the number of antigen specific T cells within the tumor are changed by exposure of tumor bearing mice to PEG-rMuIL-10.
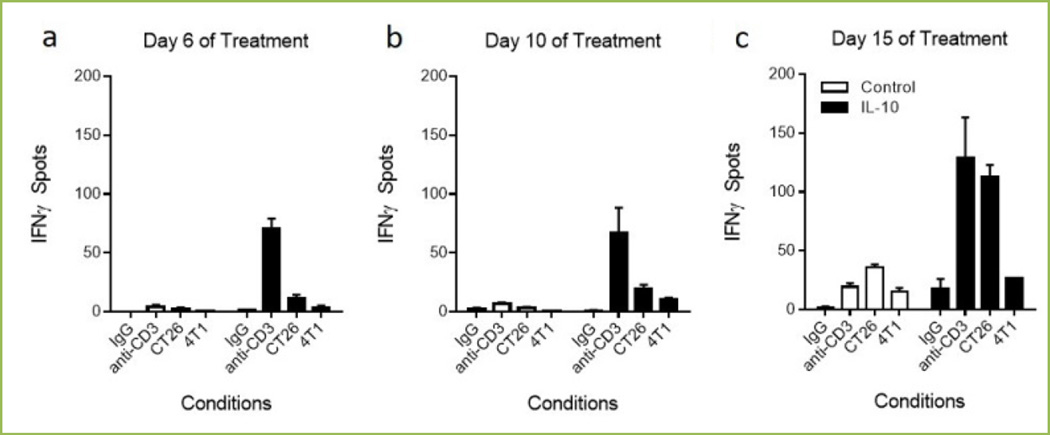
Figure 3a–c shows that over the time course of dosing from 6 (3a), 10 (3b) and 15 (3c) days, tumor antigen specific CD8+ T cells within the tumor increase. Figure 3a–c shows that continued exposure of CT26 tumor bearing mice to PEG-rMuIL-10 increase the number of tumor reactive (IFNγ ELISPOT spots) and therefore tumor antigen specific, (compare CT26 vs. 4T1 columns) CD8+ T cells within the TIL population over time. In context of the second challenge of immunoncology, these data suggest that continued dosing with either murine or human PEG-rIL-10 leads to the intratumoral increase of activated, highly cytotoxic and antigen specific CD8+ T cells.
Fig 3. Effect of PEG-rMuIL-10 dosing on intratumoral CD8+ T cell infiltration and function.
ELISPOTs (R&D Systems) were generated by magnetic (Miltenyi) bead isolation of 1000 CD8+ T cells from PBMC or mechanically disrupted and enzyme digested CT26 (ATCC) tumors. CD8+ T cells were exposed for 24 hrs to no secondary stimulus, (w/o), 1 µg/mL soluble anti-CD3 (eBiosciences), 100 CT26 squamous tumor or 4T1 (ATCC) (as negative control) tumor cells. Spots were quantified with ImmunoSpot Software.
IL-10 Facilitates CD8+ T cell Persistence
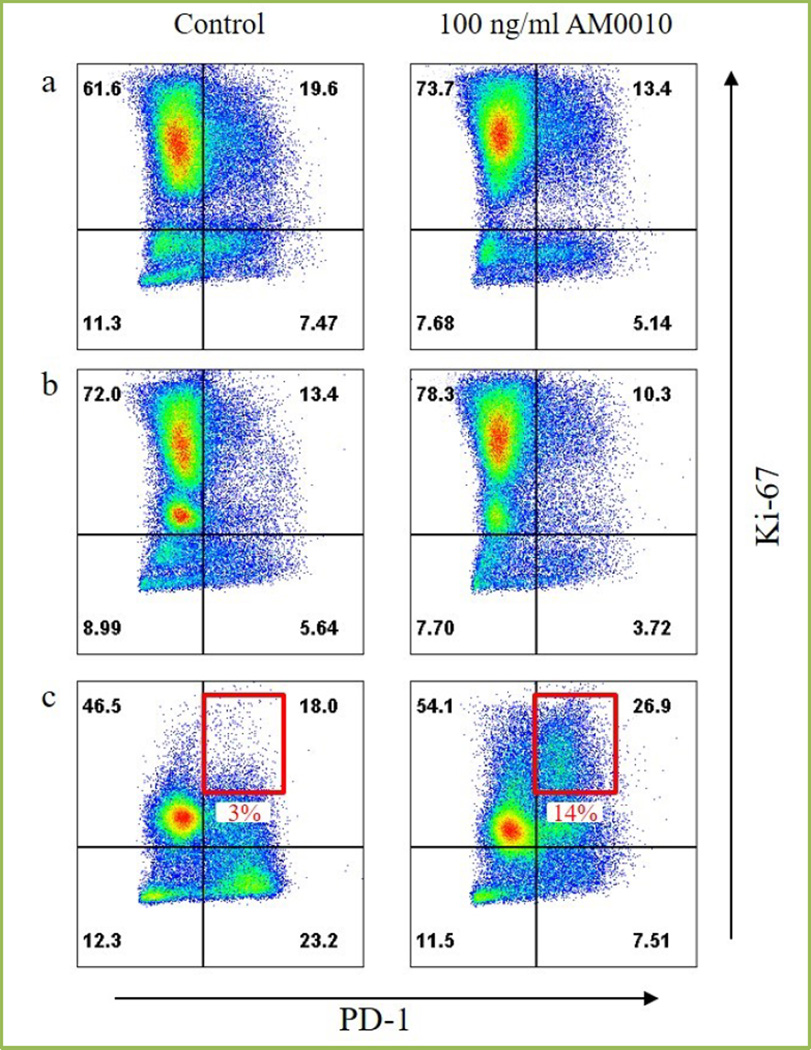
Lastly, we’ve reported that mice cured of their tumors by PEG-rMuIL-10 exposure exhibit long term anti-tumor immunity[46]. These data suggest that exposure to PEG-IL-10 facilitates the persistence of tumor antigen specific CD8+ T cells, thereby overcoming the third challenge of immunoncology. Recent evidence[44], indicates in vitro treatment of CD8+ T cells with AM0010 facilitates the enhanced response to T cell receptor engagement. This enhanced response of both increased IFNγ and Granzyme B secretion is predominantly dependent on AM0010’s increase of intra-nuclear AP1. AP1 is one of the dominant pathways activated upon T cell receptor engagement[48]. However, in context of T cell persistence, AM0010 also induces the phosphorylation of STAT3. STAT3 is a known anti-apoptotic survival factor[49, 50]. Given the possibility that T cell persistence and, by proxy, prevention of activation induced cell death may represent the solution to the third challenge of immunoncology, we tested whether AM0010 exposure of serially stimulated CD8+ T cells resulted in increased numbers of activated, cytotoxic, CD8+ T cells. Figure 4 illustrates that the stimulation of CD8+ T cells with AM0010 during multiple rounds of in vitro stimulation resulted in less apoptotic cells (not shown) and more Ki67+ cells. This in turn led to an increase of a PD1+Ki67+ double positive CD8+ T cells (4c). These data are in keeping with the previous report[45] where treatment of tumor bearing mice with PEG-rMuIL-10 lead to increases in intratumoral proliferation of antigen specific CD8+ T cells. In addition, these data corroborate AM0010 clinical data which show that increases in PD1+ CD8+ peripheral T cells[47] correspond with substantial anti-tumor immunity. High expression of PD1 is thought to target cells for apoptosis[51]. It is therefore counterintuitive that treatment with AM0010 would lead to an increase in PD1+ CD8+ T cells. However, the data shown in Figure 4c suggest that AM0010 solves the final challenge in immunoncology. We hypothesize that by persistent induction of STAT3, AM0010 treatment rescues tumor antigen specific, cytotoxic CD8+ T cells from self-limiting activation induced cell death.
Fig 4. Effect of AM0010 on CD8+ T cell activation induced cell death.
Human CD8+ T cells from human peripheral blood were isolated using magnetic (Miltenyi) bead separation. CD8+ T cells were activated with plate-bound anti-CD3 (eBioscience) and anti-CD28 (eBioscience) for 3 days. Following activation, CD8+ T cells were re-plated and treated with 100 ng/ml AM0010 for 3 days (4a). After AM0010 treatment, CD8+ T cells were re-stimulated with plate-bound anti-CD3 and anti-CD28 for 3 days in the presence or absence of 100 ng/ml AM0010 (4b). Following re-stimulation, CD8+ T cells were re-plated and treated with 100 ng/ml AM0010 for 3 days (4c). Cells were stained for cell surface PD1 (BioLegend) and Ki67 (eBioscience) and analyzed by FACS.
Conclusion
In summary, there are three current challenges to immunoncology. These challenges are the activation and expansion, the induction of tumor infiltration, and facilitating the persistence of tumor antigen specific, highly cytotoxic CD8+ T cells. In regards to these three challenges, exposure of both tumor bearing mice and cancer patients to PEGylated rIL-10 appears to engage a multiplicity of related biology’s which culminate to overcome these specific challenges. PEG-IL-10 exposure enhances CD8+ T cell activation shown by increased expression of IFNγ, Granzymes and Perforin. PEG-IL-10 increases intratumoral density through promoting intratumoral antigen specific proliferation. And lastly, PEG-IL-10, through a combination of receptor mediated signal transduction cascades, prevents the apoptosis associated with activation induced cell death while promoting proliferation, leading to the persistence of PD1+ high expressing CD8+ T cells. While IL-10 has been primarily studied as an anti-inflammatory molecule[52], this is a myeloid specific biology[53]. Treatment of tumor bearing mice[46, 54–56] and cancer patients[47] with IL-10/PEGylated IL-10 clearly illustrate that IL-10 engagement of its receptor predominantly leads to CD8+ T cell centric immune activation. This biology is currently being harnessed to dramatically and positively impact the lives of cancer patients.
Acknowledgements
JBM received two NIH Predoctoral training grants, (PI E. Grimm) T32-CA60440 and (PI H. Annanthaswamy) T32-CA009598 which funded the initial investigation into IL-10’s regulation of CD8+ T cell cytotoxic biology. Data in Figure 1d was acquired in EAG lab at UTMDACC by JBM.
Footnotes
Authors’ contributions
IHC critically reviewed the manuscript, conducted the in life portion of the in vivo studies, and designed the activation induced cell death assay system. VW critically reviewed the manuscript and worked with IHC to design and conduct the activation induced cell death assays and perform the FACs analysis. SM critically reviewed the manuscript and worked with JBM to strategically focus the review. JBM conceptualized and conducted the antigen specific assays, conceptualized and conducted the ELISPOTs, requested interrogation of AM0010 in the activation induced cell death system and wrote the review.
All authors read and approved the final manuscript.
References
- 1.Gresser IA, Chekhov MD. Coley's toxins. N Engl J Med. 1987;317:457. doi: 10.1056/NEJM198708133170717. [DOI] [PubMed] [Google Scholar]
- 2.Coley WB., II Contribution to the Knowledge of Sarcoma. Ann Surg. 1891;14:199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62. doi: 10.3389/fonc.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenz HJ. Cetuximab in the management of colorectal cancer. Biologics. 2007;1:77–91. [PMC free article] [PubMed] [Google Scholar]
- 7.Ascierto PA, Marincola FM, Ribas A. Anti-CTLA4 monoclonal antibodies: the past and the future in clinical application. J Transl Med. 2011;9:196. doi: 10.1186/1479-5876-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ascierto PA, Marincola FM. 2015: The Year of Anti-PD-1/PD-L1s Against Melanoma and Beyond. EBioMedicine. 2015;2:92–93. doi: 10.1016/j.ebiom.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27:39–46. doi: 10.1093/intimm/dxu095. [DOI] [PubMed] [Google Scholar]
- 10.Han EQ, Li XL, Wang CR, Li TF, Han SY. Chimeric antigen receptor-engineered T cells for cancer immunotherapy: progress and challenges. J Hematol Oncol. 2013;6:47. doi: 10.1186/1756-8722-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–1212. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardoll D. T cells take aim at cancer. Proc Natl Acad Sci U S A. 2002;99:15840–15842. doi: 10.1073/pnas.262669499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardoll DM. Tumor reactive T cells get a boost. Nat Biotechnol. 2002;20:1207–1208. doi: 10.1038/nbt1202-1207. [DOI] [PubMed] [Google Scholar]
- 15.Jung IK, Kim SS, Suh DS, Kim KH, Lee CH, Yoon MS. Tumor-infiltration of T-lymphocytes is inversely correlated with clinicopathologic factors in endometrial adenocarcinoma. Obstet Gynecol Sci. 2014;57:266–273. doi: 10.5468/ogs.2014.57.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jochems C, Schlom J. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp Biol Med (Maywood) 2011;236:567–579. doi: 10.1258/ebm.2011.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 18.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 19.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–526. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadrup S, Donia M, Thor Straten P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013;6:123–133. doi: 10.1007/s12307-012-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reissfelder C, Stamova S, Gossmann C, Braun M, Bonertz A, Walliczek U, et al. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015;125:739–751. doi: 10.1172/JCI74894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aust S, Horak P, Pils D, Pils S, Grimm C, Horvat R, et al. The prognostic value of estrogen receptor beta and proline-, glutamic acid- and leucine-rich protein 1 (PELP1) expression in ovarian cancer. BMC Cancer. 2013;13:115. doi: 10.1186/1471-2407-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu WH, Miyai K, Cajas-Monson LC, Luo L, Liu L, Ramamoorthy SL. Tumor-infiltrating CD8 T lymphocytes associated with clinical outcome in anal squamous cell carcinoma. J Surg Oncol. 2015 doi: 10.1002/jso.23998. [DOI] [PubMed] [Google Scholar]
- 26.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharpe M, Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8:337–350. doi: 10.1242/dmm.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 29.Stern AM, Markel H. The history of vaccines and immunization: familiar patterns, new challenges. Health Aff (Millwood) 2005;24:611–621. doi: 10.1377/hlthaff.24.3.611. [DOI] [PubMed] [Google Scholar]
- 30.Bodey B, Bodey B, Jr, Siegel SE, Kaiser HE. Failure of cancer vaccines: the significant limitations of this approach to immunotherapy. Anticancer Res. 2000;20:2665–2676. [PubMed] [Google Scholar]
- 31.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104:599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumgaertner P, Jandus C, Rivals JP, Derre L, Lovgren T, Baitsch L, et al. Vaccination-induced functional competence of circulating human tumor-specific CD8 T-cells. Int J Cancer. 2012;130:2607–2617. doi: 10.1002/ijc.26297. [DOI] [PubMed] [Google Scholar]
- 33.Payne R, Glenn L, Hoen H, Richards B, Smith JW, 2nd, Lufkin R, et al. Durable responses and reversible toxicity of high-dose interleukin-2 treatment of melanoma and renal cancer in a Community Hospital Biotherapy Program. J Immunother Cancer. 2014;2:13. doi: 10.1186/2051-1426-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amin A, White RL., Jr High-dose interleukin-2: is it still indicated for melanoma and RCC in an era of targeted therapies? Oncology (Williston Park) 2013;27:680–691. [PubMed] [Google Scholar]
- 35.McDermott DF. Update on the application of interleukin-2 in the treatment of renal cell carcinoma. Clin Cancer Res. 2007;13:716s–720s. doi: 10.1158/1078-0432.CCR-06-1872. [DOI] [PubMed] [Google Scholar]
- 36.Fang L, Lonsdorf AS, Hwang ST. Immunotherapy for advanced melanoma. J Invest Dermatol. 2008;128:2596–2605. doi: 10.1038/jid.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 38.Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers (Basel) 2011;3:3856–3893. doi: 10.3390/cancers3043856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGray AJ, Hallett R, Bernard D, Swift SL, Zhu Z, Teoderascu F, et al. Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumor. Mol Ther. 2014;22:206–218. doi: 10.1038/mt.2013.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arakaki R, Yamada A, Kudo Y, Hayashi Y, Ishimaru N. Mechanism of activation-induced cell death of T cells and regulation of FasL expression. Crit Rev Immunol. 2014;34:301–314. doi: 10.1615/critrevimmunol.2014009988. [DOI] [PubMed] [Google Scholar]
- 42.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 43.Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y. Cytokines in cancer immunity and immunotherapy. Immunol Rev. 2004;202:275–293. doi: 10.1111/j.0105-2896.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 44.Chan IH, Wu V, Bilardello M, Mar E, Oft M, Van Vlasselaer P, et al. The Potentiation of IFN-gamma and Induction of Cytotoxic Proteins by Pegylated IL-10 in Human CD8 T Cells. J Interferon Cytokine Res. 2015 doi: 10.1089/jir.2014.0221. [DOI] [PubMed] [Google Scholar]
- 45.Emmerich J, Mumm JB, Chan IH, LaFace D, Truong H, McClanahan T, et al. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 2012;72:3570–3581. doi: 10.1158/0008-5472.CAN-12-0721. [DOI] [PubMed] [Google Scholar]
- 46.Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell. 2011;20:781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Infante JR, Naing A, Papadopoulos KP, Autio KA, Ott PA, Wong DJL, et al. A first-in-human dose escalation study of PEGylated recombinant human IL-10 (AM0010) in advanced solid tumors. ASCO Meeting Abstracts. 2015;33:3017. [Google Scholar]
- 48.Auphan N, Ghosh S, Flavell RA, Schmitt-Verhulst AM. Differential requirements for NF-kappaB and AP-1 trans-activation in response to minimal TCR engagement by a partial agonist in naive CD8 T cells. J Immunol. 1999;163:5219–5227. [PubMed] [Google Scholar]
- 49.Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18:254–267. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 51.Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37. doi: 10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- 52.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 53.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujii S, Shimizu K, Shimizu T, Lotze MT. Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ. Blood. 2001;98:2143–2151. doi: 10.1182/blood.v98.7.2143. [DOI] [PubMed] [Google Scholar]
- 55.Groux H, Cottrez F, Rouleau M, Mauze S, Antonenko S, Hurst S, et al. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J Immunol. 1999;162:1723–1729. [PubMed] [Google Scholar]
- 56.Zheng LM, Ojcius DM, Garaud F, Roth C, Maxwell E, Li Z, et al. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J Exp Med. 1996;184:579–584. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]