Abstract
Social anxiety often develops in adolescence, and precedes the onset of depression and substance use disorders. The link between social anxiety and use of behaviors to minimize distress in social situations (i.e., safety behaviors) is strong and for some patients, this link poses difficulty for engaging in, and benefiting from, exposure-based treatment. Yet, little is known about whether individual differences may moderate links between social anxiety and safety behaviors, namely variations in genetic alleles germane to anxiety. We examined the relation between adolescent social anxiety and expressions of safety behaviors, and whether allelic variation for anxiety moderates this relation. Adolescents (n=75; ages 14–17) were recruited from two larger studies investigating measurement of family relationships or adolescent social anxiety. Adolescents completed self-report measures about social anxiety symptoms and use of safety behaviors. They also provided saliva samples to assess allelic variations for anxiety from two genetic polymorphisms (BDNF rs6265; TAQ1A rs1800497). Controlling for adolescent age and gender, we observed a significant interaction between social anxiety symptoms and allelic variation (β=0.37, t=2.41, p=.02). Specifically, adolescents carrying allelic variations for anxiety evidenced a statistically significant and relatively strong positive relation between social anxiety symptoms and safety behaviors (β=0.73), whereas adolescents not carrying allelic variation evidenced a statistically non-significant and relatively weak relation (β=0.22). These findings have important implications for treating adolescent social anxiety, in that we identified an individual difference variable that can be used to identify people who evidence a particularly strong link between use of safety behaviors and expressing social anxiety.
Keywords: Adolescents, Social anxiety, Safety behaviors, Taq1A, BDNF
Adolescence is a critical time period of development. Many disorders have their onset in adolescence, particularly disorders with biological underpinnings (Paus et al. 2008) including social anxiety disorder. Intervening in social anxiety at an early stage is critical because the disorder tends to precede other mental disorders, such as depression and substance use (Beesdo et al. 2007; Marmorstein 2012). To treat social anxiety disorder, researchers often recommend interventions focused, in part, on exposing patients to anxiety provoking social situations (Kendall et al. 2006), yet not all patients respond well to this treatment (Hedtke et al. 2009). One group of treatment non-responders includes individuals who use behaviors to minimize distress within social situations (e.g., safety behaviors; Hedtke et al. 2009). It is possible that the extent to which a person evidences a strong link between their level of social anxiety and their use of safety behaviors may be moderated by individual differences, namely biological factors tied to expressions of anxiety.
As the field of psychology begins to incorporate multiple factors of etiology into research and treatment for mental disorders (e.g., Insel et al. 2010), investigating biological factors such as genetic individual difference variables linked to social anxiety and safety behaviors may usefully inform methods for personalizing treatments to optimize treatment response (for a review, see De Los Reyes and Aldao 2015). In fact, there is a growing body of studies investigating genetic factors as moderators of psychological phenomena (e.g., Bau et al. 2000; Belsky and van IJzendoorn 2015; Caspi et al. 2002; Mandelli and Serretti 2013). Further, the use of genetic moderation, which may distinguish groups who are more or less vulnerable to situational factors (e.g., social stress) depending on their genes, is supported by the theory of differential susceptibility to environmental influences (Belsky and Pluess 2009). Specific to anxiety, much of this work is informed by neurobehavioral models such as Reinforcement Sensitivity Theory (Gray 1970, 1976; Corr 2004, 2008; Gray and McNaughton 2000). Reinforcement Sensitivity Theory posits that variations in neurobiological risks for anxiety (e.g., allelic risk factors for anxiety) underlie individual differences in the expression of stable traits of personality related to anxiety (Eysenck 1990). In line with this prior research and theory, the purpose of this study is to investigate the extent to which an individual difference allelic risk factor linked to anxiety moderates the relation between social anxiety symptoms and use of safety behaviors.
Safety Behaviors and Social Anxiety
Social anxiety disorder is characterized by a fear of evaluation or embarrassment in social or performance situations (American Psychiatric Association 2000, 2013). Socially anxious individuals express these fears behaviorally by avoiding social situations, or otherwise enduring these situations with intense distress. The cognitive model of social anxiety (Clark and Wells 1995) posits a cycle that begins with an individual's negative beliefs about their social abilities and social world, and that is maintained by safety behaviors, internal focus, and negative attributions. These factors impact the individual's behavior in social situations and as a result, may elicit negative reactions from other people, further contributing to this cycle of social anxiety and providing evidence of their (perceived) lack of social ability. Thus, social anxiety is broadly characterized by increased negative affect (Watson et al. 2005) and a heightened self-focused attention (Bögels and Mansell 2004; Mathews and MacLeod 2005).
The nature and extent of safety behavior use have important implications for the development, maintenance, and treatment of social anxiety (Clark and Wells 1995; Morrison and Heimberg 2013). Socially anxious individuals may engage in a variety of safety behaviors in order to reduce the acute distress they experience in social situations (e.g., Salkovskis 1991; Wells et al. 1995), and often in an attempt to avoid evaluation from others before or during feared situations (Cuming et al. 2009; Kashdan et al. 2011). According to Helbig-Lang and Petermann (2010, pgs. 219–220):
Safety behaviors are dysfunctional emotion regulation strategies. They can be differentiated from adaptive coping depending both on the situation in which they occur (actual threat versus overrated or no real threat) as well as their function (preventing feared outcomes that are unlikely to happen versus habitual behavior or behavior unrelated to the occurrence of anxiety).
Examples of safety behaviors relevant to social anxiety include avoiding eye contact, saying as little as possible, wearing clothes that conceal signs of physiological arousal (e.g., baggy clothes to mask sweating), and privately rehearsing beforehand what to say in a situation. These behaviors may temporarily alleviate socially anxious individuals' negative thoughts and physiological experiences (e.g., perspiration and increased heart rate), but tend to maintain anxiety over time (Lovibond et al. 2009). In fact, the use of safety behaviors has an acute negative reinforcement property by leading to short-term reductions of discomfort in social situations (e.g., reduced distress as a function of avoiding speaking at a social gathering; Salkovskis 1991). Specifically, long-term use of safety behaviors (e.g., regularly refraining from mingling at social gatherings) may result in lost opportunities for disconfirmation of social fears, and thus, leads to increased risk for maintenance of social anxiety (see Thomas et al. 2012). Children and adolescents with social anxiety disorder exhibit a greater variety and higher rate of safety behaviors than socially anxious and non-anxious children and adolescents (Kley et al. 2012), providing evidence for the association between safety behaviors and social anxiety disorder (Clark and Wells 1995).
Findings from prior work in both youth and adults examining the outcomes of exposure-based treatments suggest 1) that some individuals use safety behaviors much more frequently than other individuals during exposure to social situations, and 2) the use of safety behaviors during exposures impedes treatment gains (Hedtke et al. 2009; Helbig-Lang and Petermann 2010; Kim 2005; Schmidt et al. 2012; Taylor and Alden 2010; Wells et al. 1995). Therefore, research strongly suggests that the use of safety behaviors by socially anxious individuals may represent a maladaptive coping strategy that prevents successful treatment of their symptoms and maintains corresponding impairments in social functioning.
Biological Bases for Links Between Social Anxiety Symptoms and Safety Behaviors
Thus, paradoxically, the use of safety behaviors appears to interfere with exposure-based treatment, and tends to result in a more negative impression to others in social situations—yet, individuals with social anxiety persist in using these strategies. Biological predispositions toward expressing features of social anxiety symptoms may serve to moderate the strength of links between social anxiety and safety behaviors. There is a robust literature underlying how genetic factors both impact the trajectory of the development of psychopathology and may even inform targeted interventions (Bau et al. 2000; Belsky and Pluess 2009; Belsky and van IJzendoorn 2015; Caspi et al. 2002; Hayden et al. 2010; Mandelli and Serretti 2013), and more specifically, supports the investigation of the biological bases for anxiety (e.g., Dillon et al. 2014; Flint 2004; Woody and Szechtman 2011). Influenced by these findings, we posit that investigating an individual differences variable of anxiety-related personality traits (Flint 2004; Kennis et al. 2013; McNaughton and Corr 2004, 2008) as a moderator may elucidate why the link between social anxiety and safety behaviors is strong for some adolescents and not others. In particular, personality is conceptualized here as the biological underpinnings of an individual's response to the environment (Allport 1937; Corr 2002; Davidson 2001), for which an individual's genetic make-up is known to make a substantial contribution (Eysenck 1990). In fact, the Reinforcement Sensitivity Theory provides a framework for a neurobehavioral etiology of anxiety, or an explanation for how anxiety-related behaviors are linked to biological systems of punishment and reward (Gray 1970, 1976; Corr 2004; Gray and McNaughton 2000). Specifically, allelic variation for dopamine as a genetic moderator both provides support for individual differences in expressions of anxious behavior (Freitas-Ferrari et al. 2010; Koven and Carr 2012; Schneier et al. 2000) and a potential illustration of how genes confer vulnerability to environmental factors like exposure to social situations (Belsky and Pluess 2009).
Allelic Variations Related to Social Anxiety
Two relevant allelic variation factors in the study of adolescent social anxiety and safety behaviors are the single nucleotide polymorphism (SNP) dopamine D2 receptor gene (DRD2/ANKK1 Taq1A rs1800497), and brain-derived neurotrophic factor gene SNP (BDNF rs6265). First, reduced dopaminergic functioning (i.e., decreased numbers of dopamine receptors) appears to be a factor related to social anxiety (Freitas-Ferrari et al. 2010; Gillath et al. 2008; Schneier et al. 2000; Stein et al. 2002). The polymorphism Taq1A (rs1800497) on the Anakin repeat transporter gene (ANKK1) next to the dopamine D2 receptor gene (see Dick et al. 2007; Neville et al. 2004) is associated with risk for anxiety (Hayden et al. 2010) and alcohol dependence (Blum et al. 1991; Preuss et al. 2007). Biologically, the presence of the A1 allele of Taq1A is associated with reduced dopamine receptors (Jönsson et al. 1999; Noble et al. 1991; Pohjalainen et al. 1998), and hypothesized to index difficulty coping with stress (Bau et al. 2000). For individuals carrying the A1 allele, the presence of this allele is related to characteristics of social anxiety. For example, relative to adolescents without the A1 allele, adolescents with the A1 allele undergoing electroencephalography while completing a probabilistic learning task exhibited greater sensitivity to negative feedback and less focus on positive feedback on their task performance (Althaus et al. 2009). The presence of the A1 allele in a sample of children was significantly related to increased anxiety and depression, measured by both maternal report and structured interview (Hayden et al. 2010). Further, there is a significant link between carrying the A1 allele and increased social problems (Marino et al. 2004). In fact, individuals who carry one or both copies of the A1 allele appear to be at greater risk for concerns often linked with increased social anxiety and/or social problems (i.e., substance use problems), relative to individuals without the A1 allele (Berggren et al. 2006; Huang et al. 2009; van der Zwaluw et al. 2010; van der Zwaluw et al. 2011).
Second, and also linked to dopaminergic pathways, BDNF is a nerve growth factor protein related to neural plasticity (Berton et al. 2006; Guillin et al. 2001; Hyman et al. 1991; Seroogy et al. 1994). Of relevance to social anxiety, allelic variations of BDNF tend to relate with variations in functional impairments in the domains of fear-related learning, memory, and extinction (Casey et al. 2011; Soliman et al. 2010; Yu et al. 2009). The BDNF SNP Val66Met (rs6265) has two alleles (Methionine [Met], Valine [Val]), comprising 3 genotypes: Val/Val, Val/Met, and Met/Met. The Met allele has been linked to stress reactivity, mood and anxiety disorders and related behaviors (Chen et al. 2006; Colzato et al. 2011; Duman and Monteggia 2006; Gadow et al. 2009; Jiang et al. 2005; Verhagen et al. 2010), alcohol dependence (Su et al. 2011), smoking behavior (Lang et al. 2007), memory (Egan et al. 2003; Yu et al. 2009), and introversion (Terracciano et al. 2008, 2009; Stein and Gelernter 2010), many of which also relate to social anxiety. In a sample of young adults completing a stressor task (i.e., cold pressure task), carriers of the Met allele, relative to the Val/Val genotype, experienced greater nervousness, social anxiety, stress response and weekly alcohol consumption (Colzato et al. 2011). Given BDNF's association with risk for anxiety and sequelae (e.g., addiction), and its possible influence on the ability to learn the difference between safety and threat (Casey et al. 2010), it is an important gene to study in relation to social anxiety.
Present Study
The purpose of this study was to advance knowledge on the influence of an individual difference allelic risk factor on the relation between social anxiety symptoms and safety behaviors in adolescents. Specifically, we applied research and theory on allelic variations related to anxiety symptoms to improve our understanding of individual differences in the strength of relations between adolescent social anxiety and safety behaviors. One way to investigate the risk for social anxiety is to create a dichotomous variable indexing allelic variation related to neurotransmitters germane to anxiety, namely dopamine (see Conner et al. 2010). Allelic variation variables are often used in other fields of study in the general medical sciences (e.g., to examine breast cancer, gout, and heart disease; Aulchenko et al. 2009; Dehghan et al. 2008; De Jager, et al. 2009; Jacobsen et al. 1997; Kathiresan et al. 2008; Morrison et al. 2007). We expected that high levels of social anxiety would be related to high usage of safety behaviors, and that this relation would be moderated by the presence of allelic variation for anxiety symptoms. Specifically, we hypothesized that adolescents carrying allelic variation for anxiety symptoms would evidence a stronger positive association between social anxiety symptoms and safety behaviors, relative to adolescents carrying no such allelic variation.
Method
Participants
Participants were families who lived in a large metropolitan area in the Mid-Atlantic United States. In order to participate in the study, families had to: (a) speak English fluently, (b) understand the consent and assent process, (c) have an adolescent currently living in the home whom the parent did not report as having a history of learning or developmental disabilities, and (d) have an adolescent and parent present for the assessment. Seventy-nine families participated in the study; however, four families were excluded from the analyses because either the adolescent did not provide sufficient saliva to assess the two allelic variation variables described below or did not provide proper survey measure data. Recent studies using sampling approaches similar to our own support the use of samples of this size in genetics research (e.g., Althaus et al. 2009; Anderson et al. 2012; Beevers et al. 2007; Carlson et al. 2012; Mueller et al. 2013; Way and Taylor 2011).
Thus, the analytic sample of 75 families included adolescents aged 14-to-17 years (32 males and 43 females; M age= 15.37 years [SD=1.09]). The parents identified family ethnicity/race as African American or Black (64.9 %); White, Caucasian American, or European (28.4 %); Hispanic or Latino/a (4.1 %); Asian American (2.7 %); American Indian (2.7 %); or “Other” (three [4.1 %] participants entered “Bi-Racial,” “Indian,” and “Jewish”; one participant did not report ethnicity/race data). The composition of family ethnicity/race totals above 100 % because there was overlap among the ethnic/racial categories, resulting from participants having the option of selecting more than one ethnic/racial category. Based on parent report, over one quarter (28.8 %) of the families had a weekly household income of $500 or less; 49.3 % earned $901 or more per week (two participants did not provide income data).
The 75 families participating in this study were recruited from two larger studies, conducted at the same time and recruited from the same geographic location, that focused on measurement of family relationships (e.g., parent-adolescent conflict; see De Los Reyes et al. 2012b) or adolescent social anxiety (e.g.,De Los Reyes et al. 2012a). Specifically, 36 adolescents had a parent who contacted the laboratory in response to an advertisement for a non-clinic study about family relationships. Additionally, we augmented the sample by including 39 adolescents who had a parent who contacted the laboratory in response to an advertisement for a social anxiety clinical screening evaluation. We included these adolescents to increase variability in the assessment of adolescent safety behaviors, consistent with prior work (see Thomas et al. 2012), thus increasing our statistical power to detect hypothesized effects. Importantly, demographic comparisons of these two samples of adolescents yielded no significant differences on any of the demographic characteristics reported previously (i.e., adolescent age, adolescent gender, family ethnicity/race, and family income, all ps>.11). Similarly, the two samples did not significantly differ on frequency distributions for the genetic allele variations reported below (i.e., DRD2/ANKK1, and BDNF), ps>.11. In sum, these samples did not significantly differ on demographic characteristics and genetic allele variation frequencies, thus providing a justification for combining them to address our study aims.
Procedure
All procedures were approved by the Internal Review Board of the large Mid-Atlantic university at which the study was conducted. We recruited participants through community agencies and events, as well as via advertisements posted online (e.g., Craigslist) and in newspapers in qualifying neighborhoods (i.e., neighborhoods targeted because of demographic and income variability). Additionally, we recruited participants who were referred for social anxiety evaluations through the offices of pediatricians, mental health professionals, and other health care providers.
After respondents were screened for eligibility over the telephone, we scheduled them for an assessment. After the parents provided written consent and the adolescents provided assent, adolescents provided a saliva sample for the genotyping analyses described below. Participants were then led to a separate room to complete a counterbalanced battery of assessments via individual computer-based questionnaires. Specifically, for all survey assessments, participants provided computer-based responses to items that were recorded using IBM SPSS Data Collection survey administration software (Version 5.6; IBM Corporation 2009). Following completion of the study, participants were debriefed as to the overall goals of the study and monetarily compensated for their time.
Measures
Adolescent and Parent Survey Measures
Adolescents and parents completed measures assessing domains of adolescent and family demographics, adolescent social anxiety, and adolescent safety behaviors:
Adolescent and family demographics
Demographic data were obtained through parent reports of child age and gender, family/ethnicity/race, and family income.
Self-reports of adolescent social anxiety concerns
We assessed adolescent self-reported social anxiety using the Multidimensional Anxiety Scale for Children (MASC; March 1997), a 39-item scale that assesses various domains of anxiety functioning in youths: physical symptoms, harm avoidance, social anxiety, and separation anxiety/panic. Adolescents rated each symptom on a scale from 0 (Never true about me) to 3 (Often true about me). Total scores can range from 0 to 117, with higher scores reflecting greater anxiety symptoms. The MASC has been used extensively, with strong evidence for its reliability and validity (March 1997; Silverman and Ollendick 2005). For the current study, we examined the nine-item Social Anxiety subscale, which yielded a high internal consistency estimate for the sample, α=.88.
Self-reports of adolescent safety behaviors
Use of safety behaviors was assessed by having the adolescents complete the Subtle Avoidance Frequency Examination (SAFE; Cuming et al. 2009). The SAFE is a 32-item scale originally developed for adults that assesses subtle safety-seeking behaviors that an individual may use prior to or during a social situation. Adolescents rated each item by determining how often they would engage in the behavior if they were in a social situation by using a scale from 1 (Never) to 5 (Always). Total scores can range from 32 to 160, with higher scores reflecting greater use of safety behaviors. The SAFE distinguishes clinical (i.e., social anxiety disorder patients) from non-clinical adult samples and evidences high construct validity (Cuming et al. 2009). Additionally, a recent age- and gender-matched case-control study of socially anxious and non-socially anxious adolescents supported the internal consistency discriminant, and convergent validity of the SAFE when administered to adolescents, most of which are included in the current study (Thomas et al. 2012). Specifically, the SAFE correlated significantly with the MASC (r=.49) and exhibited low-to-moderate, non-significant correlations with measures related to attention/hyperactivity symptoms and depression. Further, the SAFE successfully distinguished referral status in the sample by differentiating between adolescents referred for anxiety concerns from adolescents recruited for a study on family behavior (Thomas et al. 2012). The SAFE yielded a high internal consistency estimate for the current sample, α =.90. Sample items in which adolescents rate how often they engage in safety behaviors include “Speak softly” and “Hold your arms still”.
Genetic Analysis
Through the sampling procedures described below, we assessed for the presence of multiple gene alleles which previous work indicates are associated with anxiety symptoms.
Saliva sampling and storage
Adolescent participants provided a single saliva sample via a sublingual Salimetrics© (State College, PA) oral cotton swab (Part No. 5001.02). Specifically, study personnel instructed the adolescents to hold the swab under their tongue for two minutes' time, and to deposit the swab into a plastic vial (Salimetrics© swab storage tube, Part No. 5001.05), within which the swab remained for storage. This is in line with the manufacturer's instructions. We stored saliva samples in a Biomedical Solutions Incorporated Upright Freezer (Model SCGP17OW1AF) before packaging and shipping the samples in dry ice for genetic extraction and analysis.
Genetic extraction and analysis
Extraction and analysis of genetic information was performed by personnel at the Salimetrics© laboratory (State College, PA). Specifically a modified Puregene (Gentra) extraction method was used to isolate the DNA from the saliva samples we provided. The samples were treated with Cell Lysis Solution (Qiagen) and Proteinase K and then incubated. After cell lysis, the samples were treated with Protein Precipitation Solution and then centrifuged. The supernatant was removed and samples were treated with Isopropanol to precipitate the DNA. Finally, the samples were washed to remove any remaining impurities, and the DNA was suspended in Nuclease Free Water. Following this, a GE NanoVue Spectrophotometer was used to measure the absorbance of the purified DNA, and to determine the quantity and quality of nucleic acid recovered in each sample. A Taqman Genotyping Assay (Applied Biosystems) was employed to amplify and evaluate the DRD2/ANKK1 and BDNF alleles described below.
DRD2/ANKK1
To assess for gene allele variation for social anxiety characteristics, we assessed for genotype frequencies on the DRD2/ANKK1 Taq1A rs1800497 gene. In line with previous work (e.g., Preuss et al. 2007), we assessed for the presence of the A1 allele. Specifically, we identified adolescents who were either homozygous (A1/A1) or heterozygous for A1 (A1/A2), hereafter referred to as “A1+”. There were 6 adolescents with the A1/A1 genotype, 26 adolescents with the A1/A2 genotype, and 43 adolescents with the A2/A2 genotype. The measured genotype frequencies corresponded to the Hardy-Weinberg equilibrium (N=78, χ2=0.88, df=1, p=346).
BDNF
To assess for gene allele variation for fear-related learning, memory, and extinction, we assessed for genotype frequencies on the BDNF rs6265 gene. Consistent with prior research (Colzato et al. 2011; Gadow et al. 2009; Lang et al. 2007), we identified participants with the Met allele (i.e., Val/Met or Met/Met; hereafter collectively referred to as “Met+”). There were 6 adolescents with the Val/Met genotype, 2 adolescents with the Met/Met genotype, and 67 adolescents with the Val/Val genotype. The measured genotype frequencies were not in Hardy-Weinberg equilibrium (N=77, χ2=9.88, df=1, p=.002), which could be due to the rarity of the Met/Met genotype (Shimizu et al. 2004).
Allelic variation
We were interested in identifying allelic variation for dopaminergic effects on social anxiety which involved identifying adolescents who were A1+ (n=32) and/or Met+(n=8). From these allele variation frequencies, we calculated a dichotomous allelic variation score (Conner et al. 2010), based on whether an adolescent carried a risk on either of the two alleles (n=38) or carried no risk (n=37). We considered creating a count variable with a possible range of 0-to-2 but decided against it because only 2 adolescents carried allele variation for more than one gene, thus limiting our statistical power to detect interaction effects using a range of 0-to-2.
Data-Analytic Plan
We first conducted preliminary analyses to detect deviations from normality. We also computed bivariate correlations between our main independent and dependent variables. Further, we conducted independent samples t-tests to compare allele variation conditions on safety behavior usage and social anxiety symptoms. These univariate tests allowed us to examine the utility of an allelic risk score for interpreting scores on a well-established measure of social anxiety (i.e., MASC Social Anxiety subscale), as well as our criterion variable measure of safety behaviors (i.e., the SAFE). To test our main hypothesis, we conducted a hierarchical regression analysis in which the adolescent SAFE total scores served as the criterion variable. We entered child age (centered) and gender (coded “0” for male and “1” for female) in the first step as independent variables. In the second step, we entered as independent variables the MASC Social Anxiety scores (centered) and a dichotomous variable representing the presence (coded “1”) or absence (coded “0”) of any of the aforementioned allelic variations for DRD2/ANKK1 and BDNF. In the third step, we entered the interaction term for the MASC Social Anxiety scores and allelic variations scores. In the presence of a significant interaction effect, we used Holmbeck's (2002) guidelines for post-hoc probing of significant moderator effects. This included: (a) computation of slope estimates using centered variables (reducing multicollinearity) and (b) examining the statistical significance of these slopes for the presence versus absence of allelic variations for anxiety symptoms (i.e., the moderator variable).
Importantly, the general cognitive model of social anxiety posits that symptoms and maladaptive behaviors are often cyclical (e.g., increased social anxiety prompts increased use of safety behaviors, eliciting negative reactions from other individuals in the interaction, thus increasing social anxiety; Clark and Wells 1995). Thus, consistent with the bidirectional relations between safety behaviors and social anxiety, we constructed a second hierarchical regression in which the MASC Social Anxiety scores served as the criterion variable and we entered SAFE total scores (centered) as an independent variable. All other independent variables, regression equation steps, and processes for testing interaction effects remained the same as the first equation described previously.
Results
Preliminary Analyses
Continuous Variables
The MASC Social Anxiety score (M=12.02; SD=6.46) and the SAFE total score (M=72.61; SD= 19.02) both met the statistical assumptions for the analyses (i.e., acceptable ranges of skewness and kurtosis statistics [≈ +/−1.0]). Consistent with prior work (Cuming et al. 2009), the MASC Social Anxiety score and the SAFE total score correlated in the moderate-to-large range, r=.52, p<.001 (Cohen 1988).
Univariate Analyses
To compare social anxiety scores and safety behavior scores in adolescents with and without allelic variation for anxiety symptoms, we conducted independent samples t-tests. There were no significant differences on scores of safety behavior use (t(73)=−1.26, p=.21) for adolescents with (M=75.34, SD=20.03) and without allelic variation for anxiety (M=69.81, SD=17.76). However, scores of social anxiety symptoms did significantly differ (t(73)= −2.47, p=.016, d=−0.58) between adolescents with allelic variation for anxiety (M=13.79, SD=6.61) and without allelic variation risk (M=10.21, SD=5.85). Importantly, the mean level of social anxiety symptoms we observed for adolescents with allelic variation of risk for anxiety (13.79) was in line with previously recommended clinical cutoffs for the MASC Social Anxiety scale (13.5; Wood et al. 2002).
Interaction Between Allelic Variations and Adolescent Social Anxiety Symptoms in Relation to Adolescent Safety Behaviors
Hierarchical regression analyses were conducted to test our main hypothesis. In Step 1, we observed non-significant effects for adolescent age and gender, F(2, 72)=0.41, p=.66. In Step 2, the main effect of social anxiety (b=1.51, SE=0.31, β=0.51, p<.001), but not the allelic variation score (b=0.11, SE=4.01, β=0, p=.97), were significant, F(4, 70)=6.60, p<.001. Consistent with our hypothesis, as presented in Table 1, in Step 3 we observed a significant interaction between the dichotomous allelic variation score and the MASC Social Anxiety scale score in relation to the SAFE total score. As seen in Table 1, this interaction explained variance in the relation between social anxiety symptoms and safety behaviors over the unique contributions of the independent variables, F(5, 69)=6.81, p<.001. Graphically depicted in Fig. 1, post-hoc probing analyses revealed that adolescents carrying allelic variations for anxiety evidenced a statistically significant and relatively strong positive relation between the MASC Social Anxiety scale score and the SAFE total score, whereas adolescents who did not carry allelic variation of risk for anxiety symptoms evidenced a statistically non-significant and relatively weak relation (see also Table 1).1
Table 1. Hierarchical regression analyses examining the interaction between allelic variation of risk for anxiety symptoms and adolescent safety behaviors in relation to adolescent social anxiety symptoms.
| Main regression model | Post-hoc tests of moderation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Variable | ΔR2 | B | SeB | β | Variable | ΔR2 | B | SeB | β |
| Step 1 | 0.01 | Step 3 (when “0” = at least one allele risk) | 0.05* | ||||||
| Adolescent age | −0.33 | 1.73 | −0.02 | Allele risk score | 0.54 | 3.88 | 0.01 | ||
| Adolescent gender | 3.48 | 3.81 | 0.09 | MASC social anxiety score | 2.14 | 0.40 | 0.73** | ||
| Step 2 | .26** | Allele risk × MASC social anxiety score | 1.48 | 0.61 | 0.32* | ||||
| Allele risk score | 0.54 | 3.88 | 0.01 | ||||||
| MASC social anxiety score | 0.66 | 0.46 | 0.22 | Step 3 (when “0” = no allele risk) | .05* | ||||
| Step 3 | 0.05* | Allele risk score | 0.54 | 3.88 | 0.01 | ||||
| Allele risk × MASC social anxiety score | 1.48 | 0.61 | 0.37* | MASC social anxiety score | 0.66 | 0.46 | 0.22 | ||
| Allele risk × MASC social anxiety score | 1.48 | 0.61 | 0.37* | ||||||
For the main regression model (left side of table), regression terms for variables entered at steps 1, 2, and 3 are displayed, based on terms observed for these variables in step 3 of the model; ΔR2 statistics for each step were based on variables entered in that step; for the post-hoc moderation tests (right side of table), only Step 3 is reported, with the moderator variable (i.e., allele risk) manipulated to reflect instances in which “0” equaled “at least one allele risk”, and instances in which “0” equaled “no allele risk”) (see Holmbeck 2002); MASC Multidimensional Anxiety Scale for Children
p<.05;
p<.001
Fig. 1.
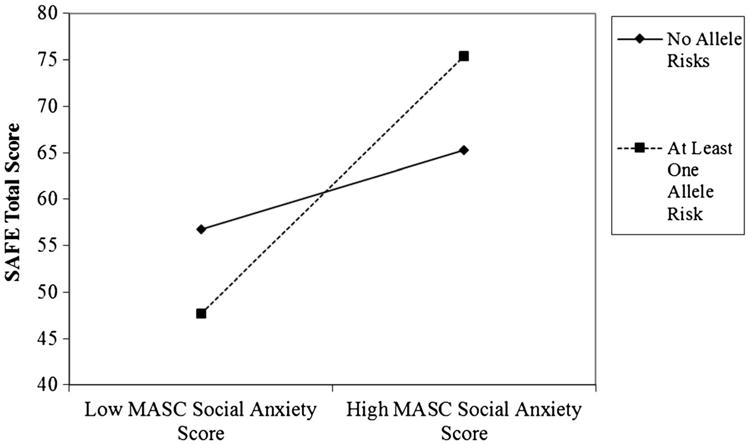
Interaction effect between Multidimensional Anxiety Scale for Children (MASC) Social Anxiety scale scores and allelic variation of risk for anxiety symptoms, positively relating to Subtle Avoidance Frequency Examination (SAFE). Post-hoc probing analyses indicated that adolescents carrying at least one allele risk experienced positive relations between MASC Social Anxiety scale scores and SAFE total scores (see Table 1)
As shown in Table 2, when the criterion variable was the MASC social anxiety subscale and we tested the interaction between allelic variation and the SAFE total score, the interaction effect was also significant. Specifically, in Step 1 we observed non-significant effects for adolescent age and gender, F(2, 72)=0.19, p=.82. In Step 2, the main effects of both SAFE total score (b=0.16, SE=0.03, β=0.48, p<.001) and the allelic variation score (b=2.67, SE=1.29, β=0.21, p=.04) were significant, F(4, 70)=7.93, p<.001. In Step 3, both the main effect of the allelic variation score and the interaction between the dichotomous allelic variation score and the SAFE total score in relation to MASC Social Anxiety scale score were significant, F(5, 69)=7.83, p<.001. Post-hoc probing analyses indicated that adolescents carrying allelic variations for anxiety evidenced a statistically significant and relatively strong positive relation between the SAFE total score and the MASC Social Anxiety scale score, whereas adolescents who did not carry allelic variation of risk for anxiety symptoms evidenced a statistically non-significant and relatively weak relation (see Table 2).
Table 2. Hierarchical regression analyses examining the interaction between allelic variation of risk for anxiety symptoms and adolescent social anxiety symptoms in relation to adolescent safety behaviors.
| Main regression model | Post-hoc tests of moderation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Variable | ΔR2 | B | SeB | β | Variable | ΔR2 | B | SeB | β |
| Step 1 | 0 | Step 3 (when “0” = at least one allele risk) | 0.05* | ||||||
| Adolescent age | −0.05 | 0.57 | −0.01 | Allele risk score | 2.72 | 1.25 | 0.21* | ||
| Adolescent gender | 0.40 | 1.26 | 0.03 | SAFE total score | 0.23 | 0.04 | 0.68** | ||
| Step 2 | 0.30** | Allele risk × SAFE total score | 0.15 | 0.06 | 0.30* | ||||
| Allele risk score | 2.72 | 1.25 | 0.21* | ||||||
| SAFE total score | 0.07 | 0.05 | 0.22 | Step 3 (when “0” = no allele risk) | 0.05* | ||||
| Step 3 | 0.05* | Allele risk score | 2.72 | 1.25 | 0.21* | ||||
| Allele risk × SAFE total score | 0.15 | 0.06 | 0.34* | SAFE total score | 0.07 | 0.05 | 0.22 | ||
| Allele risk × SAFE total score | 0.15 | 0.06 | 0.34* | ||||||
For the main regression model (left side of table), regression terms for variables entered at steps 1, 2, and 3 are displayed, based on terms observed for these variables in step 3 of the model; ΔR2 statistics for each step were based on variables entered in that step; for the post-hoc moderation tests (right side of table), only Step 3 is reported, with the moderator variable (i.e., allele risk) manipulated to reflect instances in which “0” equaled “at least one allele risk”, and instances in which “0” equaled “no allele risk”) (see Holmbeck 2002); SAFE Subtle Avoidance Frequency Examination
p<.05;
p<.001
Discussion
Main Findings
The purpose of this study was to extend the literature on adolescent safety behaviors and adolescent social anxiety symptoms. In a sample of adolescents enriched for variability in social anxiety and safety behaviors, we advanced the literature by incorporating recent work on allelic variations of risk for anxiety symptoms and examining how individual differences on alleles relevant to anxiety moderate the relation between social anxiety and use of safety behaviors. Consistent with prior research and theory related to anxiety (Colzato et al. 2011; Hayden et al. 2010; Wells et al. 1995) and genetic moderators of psychopathology (e.g., Bau et al. 2000;Belsky and van IJzendoorn 2015; Caspi et al. 2002; Mandelli and Serretti 2013), allelic variation of risk for anxiety symptoms moderated this relation such that we observed a large-magnitude and positive relation between social anxiety symptoms and safety behaviors, but only for those adolescents carrying allelic variation for anxiety symptoms. Interestingly, the average MASC social anxiety score for adolescents carrying the allelic variation of risk for anxiety symptoms was slightly above previously reported clinical cutoffs for the MASC social anxiety scale (Wood et al. 2002), thus corroborating our use of the allelic variation score in this study.
Limitations
Conducting psychological research involving genes is a developing area for which recommendations have recently been developed (Johnston et al. 2013). Along those lines, this study had several limitations. First, the sample size was relatively low compared to other studies on genetic correlates of risk. Although we were well-powered to detect our hypothesized effects, our sample size nonetheless may have contributed to BDNF genotype frequencies not corresponding to the Hardy-Weinberg equilibrium (e.g., Wittke-Thompson et al. 2005), particularly since the Met/Met genotype is rare (Shimizu et al. 2004). Importantly, our sample size is in line with some recent work taking an enriched sampling approach similar to our own (i.e., patients and non-clinic participants from the community) to study genes related to individuals with clinical disorders (Althaus et al. 2009; Anderson et al. 2012; Beevers et al. 2007; Carlson et al. 2012; Mueller et al. 2013; Way and Taylor 2011). Second, Taq1A and BDNF have been associated with several psychological domains besides anxiety, such as memory and depression (Duman and Monteggia 2006; Egan et al. 2003). At the same time, the genes we investigated are both linked to the regulation of dopamine in the brain, which has been implicated in the development and maintenance of anxiety concerns (Freitas-Ferrari et al. 2010; Gillath et al. 2008; Schneier et al. 2000; Stein et al. 2002). Further, both genes have extensive empirical support for their relation to symptoms related to social anxiety, in line with recommendations for genetic research (Johnston et al. 2013).
Third, our survey measures were administered via self-report. One recommendation regarding gene-environment interactions is to use separate informants to assess independent and criterion variables (Johnston et al. 2013). However, in light of the often-covert nature of safety behaviors and social anxiety symptoms, our findings might not generalize to measurements based on observers' reports (e.g., parents and teachers; see De Los Reyes 2013; De Los Reyes et al. 2015; De Los Reyes et al. 2013). Fourth, and similarly, although we assessed multiple allele variations in this study, it is likely that many other genetic expressions relate to safety behaviors and social anxiety symptoms. Therefore, we encourage future research to extend our findings using study designs that are multi-informant, multi-allele, and wherever possible, prospective, in order to monitor how genetic factors may relate to the development of other maladaptive coping strategies similar to safety behaviors.
Implications for Clinical Research and Practice
Biological Underpinnings of Social Anxiety
Results from the present study support the utility of investigating the biological underpinnings of social anxiety. Indeed, this work may inform future research on the identification of key intervention targets that facilitate reductions in anxiety-related impairments (see also De Los Reyes and Aldao 2015). The genetic polymorphisms we investigated have links to social anxiety characteristics, and only those adolescents with the presence of the allelic risk exhibited a significant relation between social anxiety and safety behaviors. These findings further support current initiatives for investigating multiple domains of functioning and their links with psychopathology (e.g., Research Domain Criteria; Insel et al. 2010), as well as provide further support for biological factors linked to anxiety-related behavior. As mentioned previously, the genotypes we examined in this study are related to other mechanisms such as number of dopamine receptors and activity in brain regions linked to social anxiety. This suggests promising future directions for further investigation into what aspects of these allelic risks may be encoding for the strong link between social anxiety and safety behaviors. It is possible that these biological factors pose risk for the development of cognitive and behavioral symptoms of social anxiety, and may also make typical exposure treatment less successful. However, these notions are speculative and thus merit further study.
Treatment of Social Anxiety Disorder in Adolescents
Our findings have two important implications for treating adolescent social anxiety. First, a large body of literature supports the efficacy of exposure-based treatments for reducing adolescent social anxiety symptoms (e.g., Alfano and Beidel 2011). However, when children and adolescents engage in safety behaviors during behavioral exposures administered during these treatments, this predicts poor treatment outcomes (see Hedtke et al. 2009). Conversely, treatment research indicates that eliminating use of safety behaviors during exposures results in decreased social anxiety (Kim 2005; Taylor and Alden 2010; Wells et al. 1995). Thus, it is possible that assessing safety behaviors regularly in adolescents presenting with social anxiety may provide an indicator of possible maladaptive coping, which could be a potential target in treatment for social anxiety.
Second, and of direct relevance according to our findings, a biological characteristic that may impact exposure treatment is the allelic risk association with fear-related learning and memory. To illustrate, research on Taq1A suggests that during a probabilistic learning task, individuals with the A1 allele have difficulty learning from the negative results of their responses to stimuli in order to modify their future responses (Klein et al. 2007). Thus, individuals with the Taq1A allele may be less likely to observe and modify their behavior when their coping strategies are not working—which could include the use of safety behaviors to ameliorate distress. Prior studies have been successful in developing targeted psychosocial interventions based on genetic profiles, so this suggests a possible future avenue of research with this population (Belsky and van IJzendoorn 2015; Brody et al. 2009).
Prevention of Conditions that Co-Occur with Social Anxiety
It is important to note that both of the genotypes investigated in the present study also have links to concerns that commonly co-occur with social anxiety, namely substance use (Berggren et al. 2006; Blum et al. 1991; Colzato et al. 2011; Huang et al. 2009; Preuss et al. 2007; Su et al. 2011; van der Zwaluw et al. 2010; van der Zwaluw et al. 2011). Understanding the relation between safety behaviors and social anxiety, and how allelic variation may moderate this relation, may help prevent substance use in adolescence from developing into substance use disorders. This is particularly important given the fact that substances such as alcohol have been associated with short-term reductions in anxiety in feared situations (Abrams et al. 2001; Battista et al. 2012), which could potentially lead to a pattern of increasing usage over time. Given the strong association between social anxiety and substance use, and the temporal ordering of the two disorders (i.e., social anxiety tends to precede substance use; Marmorstein 2012), intervening during adolescence may be essential in preventing future substance problems.
Concluding Comments
The present study sought to investigate the moderating effects of allelic variation of risk for anxiety symptoms on the association between adolescent social anxiety and safety behaviors. We observed a large-magnitude and positive relation between adolescent social anxiety symptoms and safety behaviors, but only for adolescents carrying an allelic variation for anxiety symptoms. These findings have important implications for identifying adolescents at risk for experiencing reduced response to exposure-based treatments for social anxiety. Given the impairment in functioning associated with developing social anxiety disorder, the assessment of safety behaviors in adolescents with social anxiety may be a salient starting point to investigate and determine maladaptive coping behaviors.
Acknowledgments
This work was supported, in part, by an internal grant from the University of Maryland (College of Behavioral and Social Sciences Dean's Research Initiative) awarded to Andres De Los Reyes. This work was partially supported by a Predoctoral National Research Service Award to Sarah Thomas from the National Institute on Drug Abuse (F31-DA033913).
Footnotes
Prior work indicates that allele frequencies may covary with ethnicity in the general population. In our current study, ethnicity did not explain any variance in the criterion variable (F[2, 72]=0.19, p=.827) and thus was excluded from all analyses.
Conflict of Interest Sarah A. Thomas, Justin W. Weeks, Lea R. Dougherty, Melanie F. Lipton, Samantha E. Daruwala, Kathryn Kline, and Andres De Los Reyes report no conflicts of interest.
Experiment Participants The study reported in this article involved human participants, and as such we obtained approval for administration of study protocols from the Internal Review Board of the large Mid-Atlantic University at which we conducted the study. We obtained informed consent from all participants before administration of study protocols.
Contributor Information
Sarah A. Thomas, Email: thomas82@umd.edu.
Justin W. Weeks, Email: weeksj@ohio.edu.
Lea R. Dougherty, Email: ldougher@umd.edu.
Melanie F. Lipton, Email: mlipton@umd.edu.
Samantha E. Daruwala, Email: sdaruwal@gmail.com.
Kathryn Kline, Email: kkline1@terpmail.umd.edu.
Andres De Los Reyes, Email: adlr@umd.edu.
References
- Abrams K, Kushner M, Medina K, Voight A. The pharmacologic and expectancy effects of alcohol on social anxiety in individuals with social phobia. Drug and Alcohol Dependence. 2001;64(2):219–231. doi: 10.1016/S0376-8716(01)00125-9. [DOI] [PubMed] [Google Scholar]
- Alfano CA, Beidel DC. Social anxiety in adolescents and young adults: Translating developmental science into practice. Washington, DC: American Psychological Association; 2011. [Google Scholar]
- Allport GW. Personality: A psychological interpretation. New York: Holt; 1937. [Google Scholar]
- Althaus M, Groen Y, Wijers AA, Mulder LM, Minderaa RB, Kema IP, Dijck JD, Hartman CA, Hoekstra PJ. Differential effects of 5-HTTLPR and DRD2/ANKK1 polymorphisms on electrocortical measures of error and feedback processing in children. Clinical Neurophysiology. 2009;120(1):93–107. doi: 10.1016/j.clinph.2008.10.012. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4, text revth. Washington, DC: Author; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Washington, DC: Author; 2013. [Google Scholar]
- Anderson DE, Bell TA, Awh E. Polymorphisms in the 5-HTTLPR gene mediate storage capacity of visual working memory. Journal of Cognitive Neuroscience. 2012;24(5):1069–1076. doi: 10.1162/jocn_a_00207. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Aulchenko YS, Ripatti S, Lindqvist I, Boomsma D, Heid IM, Pramstaller PP, Penninx BW, Janssens AC, Wilson JF, Spector T, Martin NG, Pedersen NL, Kyvik KO, Kaprio J, Hofman A, Freimer NB, Jarvelin MR, Gyllensten U, Campbell H, Rudan I. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nature Genetics. 2009;41(1):47–55. doi: 10.1038/ng.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista SR, MacDonald D, Stewart SH. The effects of alcohol on safety behaviors in socially anxious individuals. Journal of Social and Clinical Psychology. 2012;31(10):1074–1094. doi: 10.1521/jscp.2012.31.10.1074. [DOI] [Google Scholar]
- Bau CHD, Almeida S, Hutz MH. The TaqI A1 allele of the dopamine D2 receptor gene and alcoholism in Brazil: association and interaction with stress and harm avoidance on severity prediction. American Journal of Medical Genetics. 2000;96(3):302–306. doi: 10.1002/1096-8628(20000612)96:3<302::aid-ajmg13>3.0.co;2-i. doi:1096-8628(20000612)96/1096-8628(20000612)96:%3C302∷AID-AJMG13%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Hofler M, Lieb R, Wittchen H. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry. 2007;64(8):903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Gibb BE, McGeary JE, Miller IW. Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. Journal of Abnormal Psychology. 2007;116(1):208–212. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, van IJzendoorn MH. What works for whom? Genetic moderation of intervention efficacy. Development and Psychopathology. 2015;27(1):1–6. doi: 10.1017/S0954579414001254. [DOI] [PubMed] [Google Scholar]
- Berggren U, Fahlke C, Aronsson E, Karanti A, Eriksson M, Blennow K, Thelle D, Zetterberg H, Balldin J. The TAQI DRD2 A1 allele is associated with alcohol-dependence although its effect size is small. Alcohol and Alcoholism. 2006;41(5):479–485. doi: 10.1093/alcalc/agl043. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble EP, Sheridan PJ, Finley O. Association of the A1 allele of the D2 dopamine receptor gene with severe alcoholism. Alcohol. 1991;8(5):409–416. doi: 10.1016/0741-8329(91)90693-Q. [DOI] [PubMed] [Google Scholar]
- Bögels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clinical Psychology Review. 2004;24(7):827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Brody GH, Beach SH, Philibert RA, Chen Y, Murry V. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: gene × environment hypotheses tested via a randomized prevention design. Child Development. 2009;80(3):645–661. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. The orienting of spatial attention to backward masked fearful faces is associated with variation in the serotonin transporter gene. Emotion. 2012;12(2):203–207. doi: 10.1037/a0025170. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, Soliman F, Somerville LH. The storm and stress of adolescence: insights from human imaging and mouse genetics. Developmental Psychobiology. 2010;52(3):225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Ruberry EJ, Libby V, Glatt CE, Hare T, Soliman F, Duhoux S, Frielingsdorf H, Tottenham N. Transitional and translational studies of risk for anxiety. Depression and Anxiety. 2011;28(1):18–28. doi: 10.1002/da.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt T, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing DQ, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social phobia Diagnosis, assessment, and treatment. New York, NY: Guilford Press; 1995. pp. 69–93. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Colzato LS, Van der Does A, Kouwenhoven C, Elzinga BM, Hommel B. BDNF Val66Met polymorphism is associated with higher anticipatory cortisol stress response, anxiety, and alcohol consumption in healthy adults. Psychoneuroendocrinology. 2011;36(10):1562–1569. doi: 10.1016/j.psyneuen.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Conner BT, Hellemann GS, Ritchie TL, Noble EP. Genetic, personality, and environmental predictors of drug use in adolescents. Journal of Substance Abuse Treatment. 2010;38(2):178–190. doi: 10.1016/j.jsat.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Corr PJ. J. A. Gray's reinforcement sensitivity theory: Tests of the joint subsystems hypothesis of anxiety and impulsivity. Personality and Individual Differences. 2002;33(4):511–532. doi: 10.1016/S0191-8869(01)00170-2. [DOI] [Google Scholar]
- Corr PJ. Reinforcement sensitivity theory and personality. Neuroscience and Biobehavioral Reviews. 2004;28(3):317–332. doi: 10.1016/j.neubiorev.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Corr PJ. The reinforcement sensitivity theory of personality. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Cuming S, Rapee RM, Kemp N, Abbott MJ, Peters L, Gaston JE. A self-report measure of subtle avoidance and safety behaviors relevant to social anxiety: development and psychometric properties. Journal of Anxiety Disorders. 2009;23(7):879–883. doi: 10.1016/j.janxdis.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Toward a biology of personality and emotion. In: Damasio AR, Harrington A, Kagan J, McEwen BS, Moss H, Shaikh R, editors. Unity of knowledge: The convergence of natural and human science. New York, NY: New York Academy of Sciences; 2001. pp. 191–207. [DOI] [PubMed] [Google Scholar]
- De Jager PL, Chibnik LB, Cui J, Reischl J, Lehr S, Simon K, Aubin C, Bauer D, Heubach JF, Sandbrink R, Tyblova M, Lelkova P, Steering committee of the BENEFIT study, Steering committee of the BEYOND study, Steering committee of the LTF study, Steering committee of the CCR1 study. Havrdova E, Pohl C, Horakova D, Ascherio A, Hafler DA, Karlson EW. Integration of genetic risk factors into a clinical algorithm for multiple sclerosis susceptibility: a weighted genetic risk score. Lancet Neurology. 2009;8(12):1111–1119. doi: 10.1016/S1474-4422(09)70275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Reyes A. Strategic objectives for improving understanding of informant discrepancies in developmental psychopathology research. Development and Psychopathology. 2013;25(3):669–682. doi: 10.1017/S0954579413000096. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, Aldao A. Introduction to the special issue. Toward implementing physiological measures in clinical child and adolescent assessments. Journal of Clinical Child and Adolescent Psychology. 2015;44(2):221–237. doi: 10.1080/15374416.2014.891227. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, Aldao A, Thomas SA, Daruwala S, Swan AJ, Van Wie M, Goepel KA, Lechner WV. Adolescent self-reports of social anxiety: can they disagree with objective psychophysiological measures and still be valid? Journal of Psychopathology and Behavioral Assessment. 2012a;34(3):308–322. doi: 10.1007/s10862-012-9289-2. [DOI] [Google Scholar]
- De Los Reyes A, Thomas SA, Swan AJ, Ehrlich KB, Reynolds EK, Suarez L, Dougherty LR, MacPherson L, Pabón SC. “It depends on what you mean by ‘disagree’”: differences between parent and child perceptions of parent—child conflict. Journal of Psychopathology and Behavioral Assessment. 2012b;34(3):293–307. doi: 10.1007/s10862-012-9288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Reyes A, Thomas SA, Goodman KL, Kundey SMA. Principles underlying the use of multiple informants' reports. Annual Review of Clinical Psychology. 2013;9:123–149. doi: 10.1146/annurev-clinpsy-050212-185617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Reyes A, Augenstein TM, Wang M, Thomas SA, Drabick DAG, Burgers D, Rabinowitz J. The validity of the multi-informant approach to assessing child and adolescent mental health. Psychological Bulletin. 2015;141 doi: 10.1037/a0038498. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan A, Köttgen A, Qiong Y, Shih-Jen H, Kao W, Rivadeneira F, Boerwinkle E, Levy D, Hofman A, Astor BC, Benjamin EJ, van Duijn CM, Witteman JC, Coresh J, Fox CS. Association of three genetic loci with uric acid concentration and risk of gout: A genome-wide association study. Lancet. 2008;372(9654):1953–1961. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Wang JC, Plunkett J, Aliev F, Hinrichs A, Bertelsen S, Budde JP, Goldstein EL, Kaplan D, Edenberg HJ, Nurnberger J, Jr, Hesselbrock V, Schuckit M, Kuperman S, Tischfield J, Porjesz B, Begleiter H, Bierut LJ, Goate A. Family-based association analyses of alcohol dependence phenotypes across DRD2 and neighboring gene ANKK1. Alcoholism: Clinical and Experimental Research. 2007;31(10):1645–1653. doi: 10.1111/j.1530-0277.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Rosso IM, Pechtel P, Killgore WS, Rauch SL, Pizzagalli DA. Peril and pleasure: an RDOC-inspired examination of threat responses and reward processing in anxiety and depression. Depression and Anxiety. 2014;31(3):233–249. doi: 10.1002/da.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. Genetic and environmental contributions to individual differences: the three major dimensions of personality. Journal of Personality. 1990;58(1):245–261. doi: 10.1111/j.1467-6494.1990.tb00915.x. [DOI] [PubMed] [Google Scholar]
- Flint J. The genetic basis of neuroticism. Neuroscience and Biobehavioral Reviews. 2004;28(3):307–316. doi: 10.1016/j.neubiorev.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Freitas-Ferrari M, Hallak JC, Trzesniak C, Filho A, Machado-de-Sousa J, Chagas MN, Nardi AE, Crippa JS. Neuroimaging in social anxiety disorder: a systematic review of the literature. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34(4):565–580. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Roohi J, DeVincent CJ, Kirsch S, Hatchwell E. Association of COMT (Val158met) and BDNF (Val66met) gene polymorphisms with anxiety, ADHD and tics in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;39(11):1542–1551. doi: 10.1007/s10803-009-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillath O, Shaver PR, Baek J, Chun DS. Genetic correlates of adult attachment style. Personality and Social Psychology Bulletin. 2008;34(10):1396–1405. doi: 10.1177/0146167208321484. [DOI] [PubMed] [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extra-version. Behaviour Research and Therapy. 1970;8(3):249–266. doi: 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- Gray JA. The behavioural inhibition system: A possible substrate for anxiety. In: Feldman MP, Broadhurst AM, editors. Theoretical and experimental bases of behaviour modification. London: Wiley; 1976. pp. 3–41. [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. 2nd. Oxford: Oxford University Press; 2000. [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz J, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411(6833):86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Dougherty LR, Olino TM, Laptook RS, Dyson MW, Bufferd SJ, Emily Durbin C, Sheikh HI, Singh SM. The dopamine D2 receptor gene and depressive and anxious symptoms in childhood: associations and evidence for gene-environment correlation and gene-environment interaction. Psychiatric Genetics. 2010;20(6):304–310. doi: 10.1097/YPG.0b013e32833adccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedtke KA, Kendall PC, Tiwari S. Safety-seeking and coping behavior during exposure tasks with anxious youth. Journal of Clinical Child and Adolescent Psychology. 2009;38(1):1–15. doi: 10.1080/15374410802581055. [DOI] [PubMed] [Google Scholar]
- Helbig-Lang S, Petermann F. Tolerate or eliminate? A systematic review on the effects of safety behavior across anxiety disorders. Clinical Psychology: Science and Practice. 2010;17(3):218–233. doi: 10.1111/j.1468-2850.2010.01213.x. [DOI] [Google Scholar]
- Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27(1):87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Huang W, Payne TJ, Ma JZ, Beuten J, Dupont RT, Inohara N, Li MD. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology. 2009;34(2):319–330. doi: 10.1038/npp.2008.37. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic-neurons of the substantia nigra. Nature. 1991;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- IBM Corporation. IBM SPSS Data Collection (Version 5.6) [Computer Software] Somers, NY: IBM Corporation; 2009. [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB, Valdimarsdottir HB, Brown KL, Offit K. Decision-making about genetic testing among women at familial risk for breast cancer. Psychosomatic Medicine. 1997;59(5):459–466. doi: 10.1097/00006842-199709000-00001. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xu K, Hoberman J, Tian F, Marko AJ, Waheed JF, Harris CR, Marini AM, Enoch MA, Lipsky RH. BDNF variation and mood disorders: A novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30(7):1353–1361. doi: 10.1038/sj.npp.1300703. [DOI] [PubMed] [Google Scholar]
- Johnston C, Lahey BB, Matthys W. Editorial policy for candidate gene studies. Journal of Abnormal Child Psychology. 2013;41(4):511–514. [Google Scholar]
- Jönsson EG, Nöthen MM, Grünhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopa-mine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular Psychiatry. 1999;4(3):290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Weeks JW, Savostyanova AA. Whether, how, and when social anxiety shapes positive experiences and events: a self-regulatory framework and treatment implications. Clinical Psychology Review. 2011;31(5):786–799. doi: 10.1016/j.cpr.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Kathiresan S, Melander O, Anevski D, Guiducci C, Burtt NP, Roos C, Hirschhorn JN, Berglund G, Hedblad B, Groop L, Altshuler DM, Newton-Cheh C, Orho-Melander M. Polymorphisms associated with cholesterol and risk of cardiovascular events. New England Journal of Medicine. 2008;358(12):1240–1249. doi: 10.1056/NEJMoa0706728. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Robin JA, Hedtke KA, Suveg C, Flannery-Schroeder E, Gosch E. Considering CBT with anxious youth? Think exposures. Cognitive and Behavioral Practice. 2006;12(1):136–148. [Google Scholar]
- Kennis M, Rademaker AR, Geuze E. Neural correlates of personality: An integrative review. Neuroscience and Biobehavioral Reviews. 2013;37(1):73–95. doi: 10.1016/j.neubiorev.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Kim E. The effect of the decreased safety behaviors on anxiety and negative thoughts in social phobics. Journal of Anxiety Disorders. 2005;19(1):69–86. doi: 10.1016/j.janxdis.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Klein TA, Neumann J, Reuter M, Hennig J, von Cramon D, Ullsperger M. Genetically determined differences in learning from errors. Science. 2007;318(5856):1642–1645. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- Kley H, Tuschen-Caffier B, Heinrichs N. Safety behaviors, self-focused attention and negative thinking in children with social anxiety disorder, socially anxious and non-anxious children. Journal of Behavior Therapy and Experimental Psychiatry. 2012;43(1):548–555. doi: 10.1016/j.jbtep.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Koven NS, Carr LH. Differential relationships among facets of alexithymia and BDNF-and dopamine-related polymorphisms. Neuroscience and Medicine. 2012;3(1):7–13. [Google Scholar]
- Lang UE, Sander T, Lohoff FW, Hellweg R, Bajbouj M, Winterer G, Gallinat J. Association of the met66 allele of brain-derived neurotrophic factor (BDNF) with smoking. Psychopharmacology. 2007;190(4):433–439. doi: 10.1007/s00213-006-0647-1. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Mitchell CJ, Minard E, Brady A, Menzies RG. Safety behaviours preserve threat beliefs: protection from extinction of human fear conditioning by an avoidance response. Behaviour Research and Therapy. 2009;47(8):716–720. doi: 10.1016/j.brat.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Mandelli L, Serretti A. Gene environment interaction studies in depression and suicidal behavior: an update. Neuroscience and Biobehavioral Reviews. 2013;37(10, Part 1):2375–2397. doi: 10.1016/j.neubiorev.2013.07.011. [DOI] [PubMed] [Google Scholar]
- March JS. Manual for the Multidimensional Anxiety Scale for Children (MASC) Toronto: Multi-Health Systems; 1997. [Google Scholar]
- Marino C, Vanzin L, Giorda R, Frigerio A, Lorusso M, Nobile M, Massimo M, Battaglia M. An assessment of transmission disequilibrium between quantitative measures of childhood problem behaviors and DRD2/Taq1 and DRD4/48 bp-repeat polymorphisms. Behavior Genetics. 2004;34(5):495–502. doi: 10.1023/B:BEGE.0000038487.80597.7e. [DOI] [PubMed] [Google Scholar]
- Marmorstein NR. Anxiety disorders and substance use disorders: Different associations by anxiety disorder. Journal of Anxiety Disorders. 2012;26(1):88–94. doi: 10.1016/j.janxdis.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1(1):167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neuroscience and Biobehavioral Reviews. 2004;28(3):285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. The neuropsychology of fear and anxiety: A foundation for reinforcement sensitivity theory. In: Corr PJ, editor. The reinforcement sensitivity theory of personality. Cambridge: Cambridge University Press; 2008. pp. 44–94. [Google Scholar]
- Morrison AS, Heimberg RG. Social anxiety and social anxiety disorder. Annual Review of Clinical Psychology. 2013;9:249–274. doi: 10.1146/annurev-clinpsy-050212-185631. [DOI] [PubMed] [Google Scholar]
- Morrison AC, Bare LA, Chambless LE, Ellis SG, Malloy M, Kane JP, Pankow JS, Devlin JJ, Willerson JT, Boerwinkle E. Prediction of coronary heart disease risk using a genetic risk score: The Atherosclerosis Risk in Communities Study. American Journal of Epidemiology. 2007;166(1):28–35. doi: 10.1093/aje/kwm060. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Aouidad A, Gorodetsky E, Goldman D, Pine DS, Ernst M. Gray matter volume in adolescent anxiety: an impact of the brain-derived neurotrophic factor Val66Met polymorphism? Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(2):184–195. doi: 10.1016/j.jaac.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: A novel kinase gene closely linked to DRD2 on chromosome band 11q23. 1. Human Mutation. 2004;23(6):540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Noble EP, Blum K, Ritchie T, Montgomery A. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Archives of General Psychiatry. 1991;48(7):648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Någren K, Lehikoinen P, Anttila K, Syvälahti EK, Hietala J. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Molecular Psychiatry. 1998;3(3):256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Zill P, Koller G, Bondy B, Soyka M. D2 dopamine receptor gene haplotypes and their influence on alcohol and tobacco consumption magnitude in alcohol-dependent individuals. Alcohol and Alcoholism. 2007;42(3):258–266. doi: 10.1093/alcalc/agm030. [DOI] [PubMed] [Google Scholar]
- Salkovskis PM. The importance of behaviour in the maintenance of anxiety and panic: a cognitive account. Behavioural Psychotherapy. 1991;19(1):6–19. doi: 10.1017/S0141347300011472. [DOI] [Google Scholar]
- Schmidt NB, Buckner JD, Pusser A, Woolaway-Bickel K, Preston JL, Norr A. Randomized controlled trial of false safety behavior elimination therapy: a unified cognitive behavioral treatment for anxiety psychopathology. Behavior Therapy. 2012;43(3):518–532. doi: 10.1016/j.beth.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Liebowitz MR, Abi-Dargham A, Zea-Ponce Y, Lin S, Laruelle M. Low dopamine D2 receptor binding potential in social phobia. The American Journal of Psychiatry. 2000;157(3):457–459. doi: 10.1176/appi.ajp.157.3.457. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Tran T, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain‐derived neurotrophic factor and neurotrophin‐3 mRNAs. Journal of Comparative Neurology. 1994;342(3):321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: The possibility to explain ethnic mental traits. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;126(1):122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- Silverman WK, Ollendick TH. Evidence-based assessment of anxiety and its disorders in children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34(3):380–411. doi: 10.1207/s15374424jccp3403_2. [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327(5967):863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Gelernter J. Genetic basis of social anxiety disorder. In: Hofmann SG, DiBartolo PM, editors. Social anxiety: Clinical, developmental, and social perspectives. 2nd. San Diego, CA: Elsevier Academic Press; 2010. pp. 313–322. [DOI] [Google Scholar]
- Stein DJ, Westenberg HM, Liebowitz MR. Social anxiety disorder and generalized anxiety disorder: Serotonergic and dopaminergic neurocircuitry. Journal of Clinical Psychiatry. 2002;63(Suppl6):12–19. [PubMed] [Google Scholar]
- Su N, Zhang L, Fei F, Hu H, Wang K, Hui H, Jiang XF, Li X, Zhen HN, Li J, Cao BP, Dang W, Qu Y, Zhou F. The brain-derived neurotrophic factor is associated with alcohol dependence-related depression and antidepressant response. Brain Research. 2011;1415:119–126. doi: 10.1016/j.brainres.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Alden LE. Safety behaviors and judgmental biases in social anxiety disorder. Behaviour Research and Therapy. 2010;48(3):226–237. doi: 10.1016/j.brat.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Terracciano A, Sanna S, Uda M, Deiana B, Usala G, Busonero F, Maschio A, Scally M, Patriciu N, Chen WM, Distel MA, Slagboom EP, Boomsma DI, Villafuerte S, Sliwerska E, Burmeister M, Amin N, Janssens AC, van Duijn CM, Schlessinger D, Abecasis GR, Costa PT. Genome-wide association scan for five major dimensions of personality. Molecular Psychiatry. 2008;15(6):647–656. doi: 10.1038/mp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Tanaka T, Sutin AR, Deiana B, Balaci L, Sanna S, Olla N, Maschio A, Uda M, Ferrucci L, Schlessinger D, Costa PT. BDNF Val66Met is associated with introversion and interacts with 5-HTTLPR to influence neuroticism. Neuropsychopharmacology. 2009;35(5):1083–1089. doi: 10.1038/npp.2009.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Daruwala SE, Goepel KA, De Los Reyes A. Using the Subtle Avoidance Frequency Examination in adolescent social anxiety assessments. Child & Youth Care Forum. 2012;41(6):547–559. doi: 10.1007/s10566-012-9181-y. [DOI] [Google Scholar]
- van der Zwaluw CS, Engels R, Vermulst AA, Franke BB, Buitelaar JJ, Verkes RJ, Scholte RJ. Interaction between dopamine D2 receptor genotype and parental rule-setting in adolescent alcohol use: evidence for a gene-parenting interaction. Molecular Psychiatry. 2010;15(7):727–735. doi: 10.1038/mp.2009.4. [DOI] [PubMed] [Google Scholar]
- van der Zwaluw CS, Kuntsche E, Engels RE. Risky alcohol use in adolescence: the role of genetics (DRD2, SLC6A4) and coping motives. Alcoholism: Clinical and Experimental Research. 2011;35(4):756–764. doi: 10.1111/j.1530-0277.2010.01393.x. [DOI] [PubMed] [Google Scholar]
- Verhagen MM, van der Meij AA, van Deurzen PM, Janzing JE, Arias-Vásquez AA, Buitelaar JK, Franke BB. Meta-analysis of the BDNF Val66met polymorphism in major depressive disorder: effects of gender and ethnicity. Molecular Psychiatry. 2010;15(3):260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- Watson D, Gamez W, Simms LJ. Basic dimensions of temperament and their relation to anxiety and depression: a symptom-based perspective. Journal of Research in Personality. 2005;39(1):46–66. doi: 10.1016/j.jrp.2004.09.006. [DOI] [Google Scholar]
- Way BM, Taylor SE. A polymorphism in the serotonin transporter gene moderates cardiovascular reactivity to psychosocial stress. Psychosomatic Medicine. 2011;73(4):310–317. doi: 10.1097/PSY.0b013e31821195ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A, Clark DM, Salkovskis P, Ludgate J, Hackmann A, Gelder M. Social phobia: the role of in-situation safety behaviors in maintaining anxiety and negative beliefs. Behavior Therapy. 1995;26(1):153–161. doi: 10.1016/S0005-7894(05)80088-7. [DOI] [PubMed] [Google Scholar]
- Wittke-Thompson JK, Pluzhnikov A, Cox NJ. Rational inferences about departures from Hardy-Weinberg equilibrium. The American Journal of Human Genetics. 2005;76(6):967–986. doi: 10.1086/430507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, Piacentini JC, Bergman R, McCracken J, Barrios V. Concurrent validity of the anxiety disorders section of the anxiety disorders interview schedule for DSM-IV: child and parent versions. Journal of Clinical Child and Adolescent Psychology. 2002;31(3):335–342. doi: 10.1207/153744202760082595. [DOI] [PubMed] [Google Scholar]
- Woody EZ, Szechtman H. Adaptation to potential threat: The evolution, neurobiology, and psychopathology of the security motivation system. Neuroscience and Biobehavioral Reviews. 2011;35(4):1019–1033. doi: 10.1016/j.neubiorev.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, Bath KG, Lee FS, Chen Z. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. The Journal of Neuroscience. 2009;29(13):4056–4064. doi: 10.1523/JNEUROSCI.5539-08.200. [DOI] [PMC free article] [PubMed] [Google Scholar]


