Abstract
The first example of metal-free regioselective hydrazination of imidazo[1,2-a]pyridine with diethyl azodicarboxylate was accomplished. This procedure is chemically appealing due to the high degree of functional group tolerance and efficiency in expanding molecule diversity.
Introduction
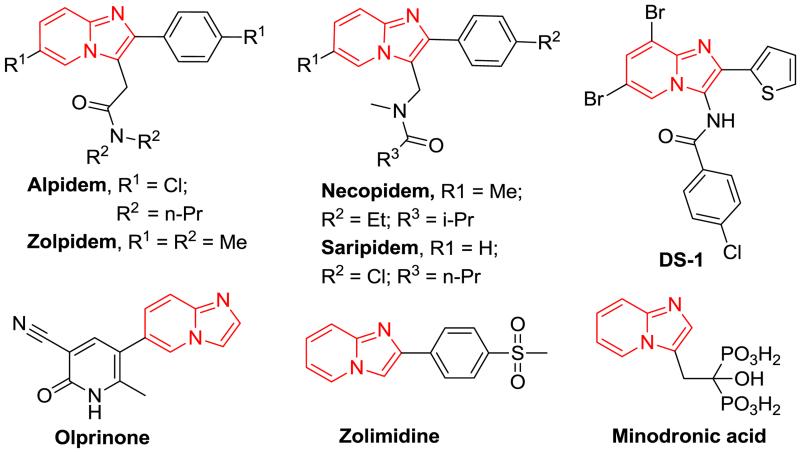
Imidazo[1,2-a]pyridine scaffolds have been widely investigated among chemists and medicinal chemists due to their remarkable biological and pharmacological activities.1 For example, they exhibit good anti-cancer,2 anti-inflammatory,3 anti-bacterial,4 anti-protozonal,5 anti-viral,6 anti-ulcer,7 anti-fungal,8 and anxiolytic properties. In addition, imidazo[1,2-a]pyridines have been found to be the core structure of many natural products and marketed drugs, including alpidem,9 necopidem,10 saripidem,11 zolpidem (Ambien ®),12 olprinone,13 DS-1,14 minodronic acid,15 divalpon,16 and Zolimidine.17 In fact, imidazo[1,2-a]pyridines are key scaffolds in the non-benzodiazepine drug class, which bind the GABA-A receptor and are first line treatments for insomnia and benzodiazepine-resistant anxiety disorders. The imidazo[1,2-a]pyridine scaffold is very important for pharmaceutical chemistry and novel synthetic methods to analogue the core structure could produce pharmacological distant drug candidates.
Over the past decade, in order to develop more bioactive imidazo[1,2-a]pyridines, novel synthetic approaches have been investigated to access imidazo[1,2-a]pyridine derivatives. For example, transition-metal catalyzed direct C-3 arylations of imidazo[1,2-a]pyridines with aryl halides18 or oxidative cross-coupling of imidazo[1,2-a]pyridine with arenes.19 Adimurthy’s lab20 recently reported the regioselective C-3 sulfenylation of imidazo[1,2-a]pyridines with thiophenols under the promotion of N-chlorosuccinimide, while Zhou’s research group21 achieved the chalcogenylation of imidazo[1,2-a]pyridine with dichalcogenides by using CuI as catalyst under air. Koubachi et al22 developed a direct and regioselective Pd/Cu-catalyzed intermolecular oxidative coupling of imidazo[1,2-a]pyridines with alkenes to access 3-alkenylimidazo[1,2-a]pyridine derivatives. While in the presence of ruthenium catalysts, Cao and co-workers23 developed an efficient regioselective C-3 alkenylation of substituted imidazo[1,2-a]pyridines with diverse acrylates. To the best of our knowledge, there has been no report involving C-3 regioselective hydrazination of imidazo[1,2-a]pyridines. The synthesis of C-3 aminated imidazo[1,2-a]pyridines has gained much attention owing to their various biological properties.24 However, few reports are available for the expeditious synthesis of these molecules, which employ toxic isocyanides or trimethylsilanecarbonitrile (TMSCN) in multiple component reactions.25 Therefore, a more practical method allowing for an expeditious access to C-3 aminated imidazo[1,2-a]pyridines is still needed. In continuation of our efforts on the development of expeditious methods for the synthesis of imidazo[1,2-a]pyridine analogues to generate kinase inhibitors,26 we report herein an efficient metal-free hydrazination of imidazo[1,2-a]pyridine with diethyl azodicarboxylate (DEAD) in neutral media.
Results and discussion
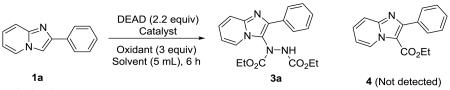
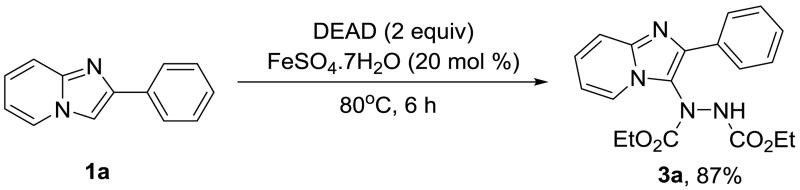
Originally, Muñiz et al27 reported a new coupling of aryl boronic acids and dialkyl azodicarboxylates to provide N-aryl hydrazines under palladium catalysis. Following this idea, Yu’s lab28 developed a palladium-catalyzed oxidative ethoxycarbonylation of aromatic C-H bond with diethyl azodicarboxylate. Therefore, we tested the possibility of Yu’s28 catalyst system for the hydrazination in imidazo[1,2-a]pyridine with DEAD. First, we carried out a coupling between 2-phenylimidazo[1,2-a]pyridine (1a) and DEAD in DMF at 100 °C for 6 h, with the use of 5 mol % Pd(OAc)2 as catalyst and 3 equivalents of Oxone as the oxidant. However, only 8% yield of product 3a was obtained. Notably, the ethoxycarbonylation product (4) was not detected. To improve the yield, the reaction was performed using several other common oxidants including DDQ, CAN, Cu(OAc)2, and TBHP. Unfortunately, no significant yield was obtained, although the best yield (29%) was achieved with TBHP in the reaction (Table 1, entry 1). When shifting the catalyst to other palladium sources, such as PdCl2 or Pd2(dba)3 (entry 2-3), no improvements were observed. By using iron catalysts,29 53% and 65% yields were obtained with FeCl2·4H2O and FeSO4·7H2O respectively (entry 5-6), although no product 3a was detected when FeCl3·6H2O was used (entry 4). Acetonitrile was investigated as an alternative solvent to increase yield. Acetonitrile was identified as a better solvent system producing an excellent yield of 88% (entry 7), when DEAD, FeSO4·7H2O, and TBHP were combined in the reaction.
Table 1.
| Entry | Catalyst | Equiv of Catalsyt | Oxidant | Solvent | T [°C] | 3a Yield[a] |
|---|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | 5 mol % | TBHP | DMF | 100 | 29 |
| 2 | PdCl2 | 5 mol % | TBHP | DMF | 100 | 11 |
| 3 | Pd2(dba)3 | 5 mol % | TBHP | DMF | 100 | 9 |
| 4 | FeCl3.6H2O | 20 mol % | TBHP | DMF | 100 | 0 |
| 5 | FeCl2.4H2O | 20 mol % | TBHP | DMF | 100 | 53 |
| 6 | FeSO4.7H2O | 20 mol % | TBHP | DMF | 100 | 65 |
| 7 | FeSO4.7H2O | 20 mol % | TBHP | MeCN | 80 | 88 |
| 8 | FeSO4.7H2O | 20 mol % | -- | MeCN | 80 | 91 |
| 9 | -- | -- | -- | MeCN | 80 | 92 |
| 10 | -- | -- | -- | Acetone | 80 | 99 |
| 11 | -- | -- | -- | EtOAc | 80 | 78 |
| 12 | -- | -- | -- | CHCl3 | 80 | 81 |
| 13 | -- | -- | -- | Toluene | 80 | 99 |
| 14 | -- | -- | -- | DCE | 80 | 99 |
| 15 | -- | -- | -- | DMA | 80 | 77 |
| 16 | -- | -- | -- | DMSO | 80 | 50 |
| 17 | -- | -- | -- | Xylenes | 80 | 93 |
| 18 | -- | -- | -- | NMP | 80 | 64 |
| 19 | -- | -- | -- | DMF | 80 | 59 |
| 20 | -- | -- | -- | MeOH | 80 | Incomplete |
Yields are those of isolated products, unless indicated otherwise. DEAD = Diethyl Azodicarboxylate, DMF = N,N-dimethylformamide, TBHP = tert-Butyl hydroperoxide, DCE = 1,2-dichloroethane, EtOAc = Ethyl Acetate, DMA = dimethylacetamide, DCE = dichloroethane, NMP = n-methylpyrrolidone
The oxidative radical reactions under TBHP as a radical initiator and iron as a catalyst are widely described in the chemistry literature,30 while dialkyl azodicarboxylates are renowned as radical acceptors.31 Consequently, we hypothesized that the hydrazination of imidazo[1,2-a]pyridine with DEAD fell into the iron/TBHP catalyzing oxidative radical reaction mechanism. To confirm our suspicion, a control experiment was conducted without the oxidant, TBHP. To our surprise, the reaction provided 3a in 91% yield.
This result suggested that the reaction does not proceed through a radical reaction mechanism. Considering the high electrophilic nature of dialkyl azodicarboxylates,32 we presumed that DEAD may form the C-3 adduct without the need of any catalyst. Based on this hypothesis, we examined the hydrazination reaction in the absence of any metal catalyst. The reaction afforded 3a in 92% yield. Therefore, DEAD acts as a strong electrophile and forms adducts with the most nucleophilic carbon (C-3) similar to non-catalyzed nitration or halogenation reactions (Table 1, entry 9). The reaction seems to be driven solely by overcoming a thermodynamic barrier, as reactions completed at 25 °C displayed incomplete conversion.
In the absence of catalyst, a variety of solvents were tested to determine solvent effects (Table 1, entries 9-20). It was found that non-polar, aprotic solvents such as toluene, DCE, and xylenes produced the highest yield. Even polar solvents, such as acetone or acetonitrile, produced high yields as well. However, in polar, protic solvents, such as MeOH, incomplete conversion was observed (Table 1, entry 20). This is likely a result of DEAD reacting with the solvent preferentially to the starting material. Other hydrazination agents, such as di-tert-butyl azodicarboxylate (DBAD), caused incomplete conversion to 3a.
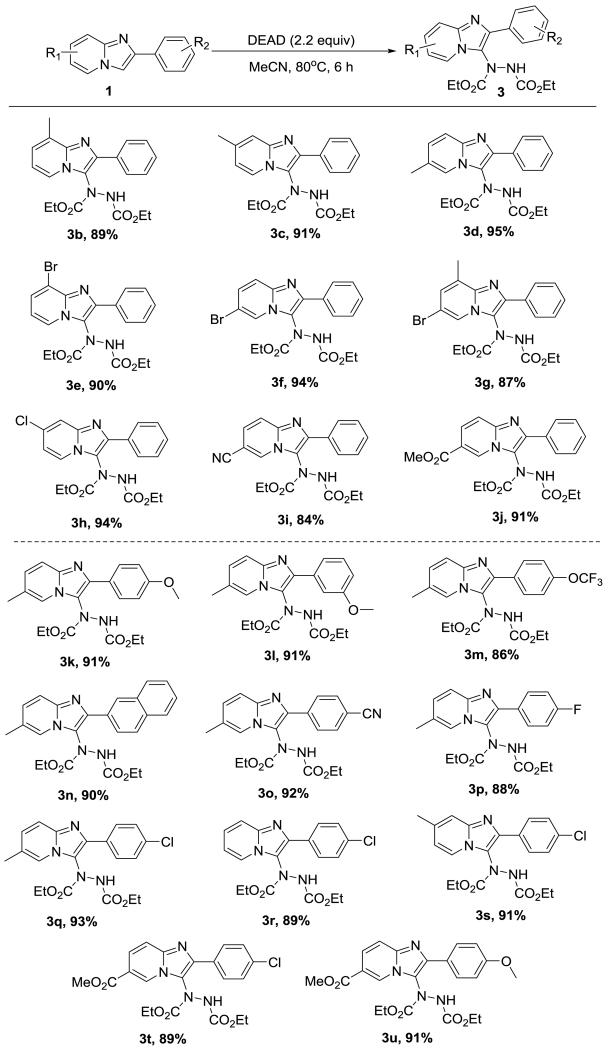
Next, we studied the scope of the regioselective hydrazination of imidazo[1,2-a]pyridine with DEAD using acetonitrile as solvent. Initially, we fixed the phenyl function on the C-2 position of imidazo[1,2-a]pyridine and then examined the efficiency of the reaction with various substituents on the pyridine scaffold. As shown in scheme 3, the hydrazination tolerated a large number of substrates, furnishing the corresponding title compounds (3b-3j) in good to excellent yields. Incorporation of a methyl group to the C-8, C-7, and C-6 position of imidazo[1,2-a]pyridine afforded 3b, 3c, 3d in yields of 89%, 91%, and 95%, respectively. Similarly, the introduction of an electron-withdrawing group (Br) at either the C-8/C-7 position of the imidazo[1,2-a]pyridine provided the corresponding products in excellent yields (3e-3f, 90% and 94%). Interestingly, the presence of both methyl and bromo (3g) was still suitable for this reaction. We also tested other electron-withdrawing groups (Cl, CN, and CO2Me), and they also reacted adequately producing yields between 84-94% (3h-3j).
Scheme 3.
Substrate scope of hydrazination reaction. Reaction condition: 2-phenylimidazo[1,2-a]pyridine (1 mmol, 1.0 equiv), DEAD (2 mmol, 2.0 equiv), MeCN (5 mL), 80 °C, 6 h. Yields reported are that of the isolated material.
Afterward, with methyl as the optimal substituent at the C-6 position of imidazo[1,2-a]pyridine, a series of functional groups at the para and ortho positions of the C-2 phenyl ring were explored (3k-3q). It was found that all substituents either with electron-donating (OMe and naphthyl) or electron-withdrawing properties (OCF3, CN, F, and Cl) offered excellent efficiency for this reaction with yields between 86-93%. As evident from the yields of products 3a-3q, we concluded that electronic effects associated with electron-donating/withdrawing substituents on the C-2 phenyl ring and pyridine scaffold of the imidazo[1,2-a]pyridine do not affect the efficiency of the reaction. To confirm this conclusion, we incorporated methoxycarbonyl to C-6 of imidazo[1,2-a]pyridine with 4-chloro-phenyl at the C-2 position, which was hydrazinated to afford 3t in a yield of 89%, which was similar to product 3r and 3s with yields of 89% and 91%, respectively. The reaction also produced the hydrazinated product 3u substituted by methoxycarbonyl and methoxyl in a yield of 91%.
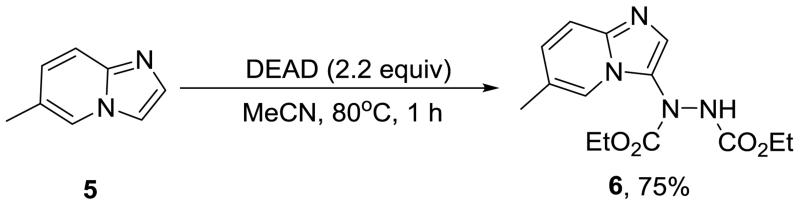
Furthermore, the optimized reaction conditions for hydrazination was successfully extended to C-2 unsubstituted imidazo[1,2-a]pyridine to expand the scope of the methodology. Under the same reaction conditions, 6-methylimidazo[1,2-a]pyridine (5) was hydrazinated with DEAD to achieve product 6 in 75% yield in 1 h at 80 °C (Scheme 4).
Scheme 4.
Hydrazination of 2-unsubstituted imidazo[1,2-a]pyridine
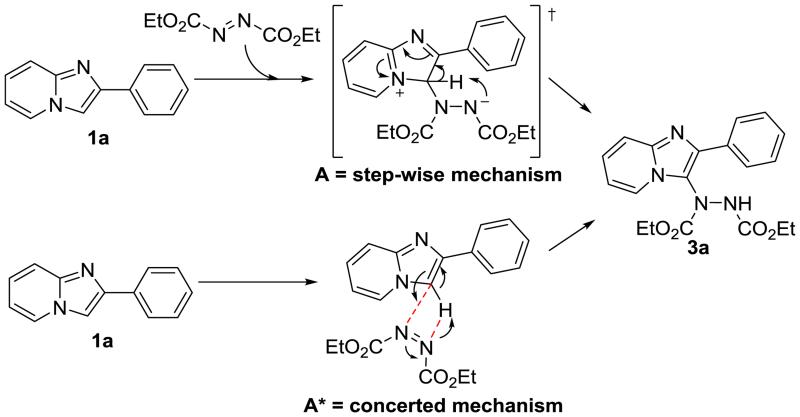
Based on the above experimental results, two plausible mechanisms are proposed (scheme 5). Similar to its role in the Mitsunobu reaction, DEAD can attack the C-3 position of 1a through a pseudo-Michael reaction to produced intermediate A. Subsequently, prototropy and restoring of conjugation provides the final product 3a. A concerted mechanism is also plausible where no transition state is formed as seen in A*. A step-wise mechanism is likely the preferred route because the reaction produces lower yields in polar solvents such as DMSO, DMF, DMA, and NMP. Low yields in Table-1 in entries with metal catalysts are likely due to the negative impact on the transition state or obstruction of adequate molecular orbital overlap. The proposed mechanisms are further validated because DBAD produced incomplete conversions. The tert-butyl group of DBAD will cause steric hindrance making C-3 nucleophilic attack more difficult.
Scheme 5.
Plausible reaction mechanisms
Conclusions
In conclusion, we have developed an efficient strategy for the regioselective hydrazination of imidazo[1,2-a]pyridine with DEAD in the absence of metal catalysts in neutral media, with a high degree of solvent and functional group tolerance, making this method a beneficial supplement to imidazo[1,2-a]pyridine derivative synthesis. Although it is known that DEAD can react with strong nucleophiles, such as phosphine, nitrogen, copper enolate of the β-dicarbonyl through the Michael reaction mechanism, this is the first time that DEAD has demonstrated its reactivity with the C-3 position of imidazole pyridines. Further investigations of the reactivity of DEAD with other nucleophiles as well as therapeutic evaluations of the hydrazinated products are currently underway in our laboratory and will be reported in due course.
Experimental section
General
Solvents were purchased from Aldrich or Acros and used without further purification. Other reagents were used as obtained from commercial providers except when otherwise noted. Analytical thin layer chromatography (TLC) was performed on pre-coated silica gel plates available from EMD. Visualization was accomplished with UV light. Column chromatography was performed using Biotage chromatographic systems. 1H NMR and 13C NMR spectra were recorded on a Varian Inova instrument (400 MHz). Chemical shifts were quoted in parts per million (ppm) referenced to the residual undeuterated solvent peak or 0.0 ppm for tetramethylsilane. The following abbreviations were used to explain multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. Coupling constants, J, were reported in Hertz unit (Hz). Low and high resolution mass spectra were obtained using ESI methods.
General procedure for the preparation of compounds 3 and 5
In a 25 mL tube imidazo[1,2-a]pyridines (1, 1 mmol), and diethyl azodicarboxylate (DEAD, 2 mmol) were taken in 5 mL MeCN. The tube was sealed with a pressure cap and heated to 80 °C for 6 h. After cooling to room temperature, the mixture was diluted with ethyl acetate (20 mL) and washed with water, brine, and dried over anhydrous Na2SO4. The organic solvent was removed under vacuum to get the crude product, which is purified using Biotage chromatographic systems.
Diethyl 1-(2-phenylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3a)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.71 (s, 1H), 7.79 (d, J = 7.3 Hz, 3H), 7.61 (d, J = 9.0 Hz, 1H), 7.39 (t, J = 7.5, 7.5 Hz, 2H), 7.33 −7.24 (m, 2H), 6.89 (t, J = 6.8, 6.8 Hz, 1H), 4.21 −4.15 (m, 4H), 1.22 −1.06 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 157.2, 155.2, 142.8, 139.2, 132.5, 128.5, 128.1, 126.9, 125.8, 124.6, 118.4, 117.1, 112.4, 63.8, 62.3, 14.2; HRMS (ESI+, m/z) calculated for C19H21N4O4 [M + H]+ 369.1557; found 369.1561.
Diethyl 1-(8-methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3b)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.55 (s, 1H), 7.77 (d, J = 7.6 Hz, 2H), 7.43 (t, J = 7.5, 7.5 Hz, 2H), 7.35 (d, J = 7.6 Hz, 3H), 6.72 (d, J = 7.0 Hz, 1H), 4.22 −4.11 (m, 4H), 2.42 (s, 3H), 1.23 (t, J = 7.1 Hz, 3H), 1.09 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.1, 155.3, 143.3, 139.0, 136.7, 132.7, 128.5, 127.9, 126.8, 123.8, 117.9, 115.5, 114.9, 63.7, 62.2, 21.2, 14.2; HRMS (ESI+, m/z) calculated for C20H23N4O4 [M + H]+ 383.1714; found 383.1718.
Diethyl 1-(7-methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3c)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.56 (s, 1H), 7.77 (d, J = 7.3 Hz, 2H), 7.48 (s, 1H), 7.41 (t, J = 7.5, 7.5 Hz, 2H), 7.33 (dd, J = 13.6, 6.0 Hz, 2H), 6.72 (dd, J = 7.0, 1.6 Hz, 1H), 4.19 (dt, J = 14.3, 6.9, 6.9 Hz, 4H), 2.41 (s, 3H), 1.22 (t, J = 7.1, 7.1 Hz, 3H), 1.09 (t, J = 7.2, 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.0, 154.9, 143.4, 139.1, 136.8, 132.9, 128.8, 128.2, 127.6, 126.7, 123.8, 115.6, 115.0, 63.93, 62.53, 21.36, 14.31; HRMS (ESI+, m/z) calculated for C20H23N4O4 [M + H]+ 383.1714; found 383.1712.
Diethyl 1-(6-methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3d)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.44 (s, 1H), 7.77 (d, J = 7.4 Hz, 2H), 7.52 (d, J = 9.2 Hz, 1H), 7.45 −7.37 (m, 3H), 7.34 −7.30 (m, 1H), 7.11 (d, J = 11.9 Hz, 1H), 4.26 −4.13 (m, 4H), 2.37 (s, 3H), 1.23 (t, J = 7.1, 7.1 Hz, 3H), 1.08 (t, J = 7.2, 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 156.9, 155.2, 142.0, 139.0, 133.1, 128.9, 128.7, 128.1, 126.7, 122.6, 122.1, 118.1, 116.6, 63.8, 62.4, 18.4, 14.3; HRMS (ESI+, m/z) calculated for C20H23N4O4 [M + H]+ 383.1714; found 383.1715.
Diethyl 1-(8-bromo-2-phenylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3e)
Yellow solid. 1H NMR (400 MHz, CDCl3) δ 8.71 (s, 1H), 7.79 (d, J = 7.6 Hz, 2H), 7.59 (s, 1H), 7.53 (d, J = 7.3 Hz, 1H), 7.39 (t, J = 7.5, 7.5 Hz, 2H), 7.32 (d, J = 7.3 Hz, 1H), 6.76 (t, J = 7.1, 7.1 Hz, 1H), 4.34 −4.08 (m, 4H), 1.22 (t, J = 7.1, 7.1 Hz, 3H), 1.05 (t, J = 7.1, 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.0, 154.9, 140.7, 140.3, 132.2, 128.7, 128.5, 128.1, 127.0, 124.0, 119.6, 112.7, 111.4, 64.0, 62.6, 14.2; [M+H]+ = 447; HRMS (ESI+, m/z) calculated for C19H20BrN4O4 [M + H]+ 447.0662; found 447.0665.
Diethyl 1-(6-bromo-2-phenylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3f)
Yellow solid. 1H NMR (400 MHz, CDCl3) δ 8.87 (s, 1H), 7.76 (d, J = 7.4 Hz, 2H), 7.53 −7.44 (m, 3H), 7.39 (d, J = 6.1 Hz, 1H), 7.37 −7.32 (m, 1H), 7.19 (s, 1H), 4.24 (dd, J = 12.3, 5.4 Hz, 4H), 1.27 (d, J = 6.5 Hz, 3H), 1.11 (t, J = 7.0, 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 156.6, 154.9, 141.4, 140.1, 132.3, 129.3, 129.0, 128.6, 126.9, 124.8, 118.7, 117.9, 107.3, 64.2, 62.7, 14.3; HRMS (ESI+, m/z) calculated for C19H20BrN4O4 [M + H]+ 447.0662; found 447.0667.
Diethyl 1-(6-bromo-8-methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3g)
Yellow solid. 1H NMR (400 MHz, CDCl3) δ 8.73 (s, 1H), 7.97 (d, J = 7.2 Hz, 1H), 7.74 (d, J = 7.8 Hz, 2H), 7.45 −7.15 (m, 3H), 7.13 (s, 1H), 4.34 −3.95 (m, 64H), 2.61 (s, 3H), 1.24 (t, J = 7.1, 7.1 Hz, 3H), 1.04 (t, J = 7.1, 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 156.9, 154.9, 146.5, 141.6, 139.5, 132.4, 128.6, 128.2, 127.8, 126.9, 122.5, 118.8, 107.1, 64.0, 62.5, 16.4, 14.3, 14.2; HRMS (ESI+, m/z) calculated for C20H22BrN4O4 [M + H]+ 461.0819; found 416.0812.
Diethyl 1-(7-chloro-2-phenylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3h)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.67 (s, 1H), 7.75 (d, J = 7.8 Hz, 3H), 7.60 (d, J = 2.0 Hz, 1H), 7.36 (dt, J = 19.7, 4.3, 4.3 Hz, 3H), 6.87 (dd, J = 7.3, 2.0 Hz, 1H), 4.26 −4.12 (m, 4H), 1.21 (t, J = 7.1, 7.1 Hz, 3H), 1.08 (t, J = 7.3, 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.0, 155.0, 142.8, 140.2, 132.6, 128.8, 128.5, 128.0, 126.8, 125.2, 118.6, 116.1, 114.1, 64.1, 62.6, 14.3; HRMS (ESI+, m/z) calculated for C19H20ClN4O4 [M + H]+ 403.1168; found 403.1166.
Diethyl 1-(6-cyano-2-phenylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3i)
White solid. 1H NMR (400 MHz, CDCl3) δ 9.23 (s, 1H), 7.79 (d, J = 7.2 Hz, 2H), 7.68 (d, J = 9.2 Hz, 1H), 7.62 −7.27 (m, 5H), 4.43 −4.13 (m, 4H), 1.26 (t, J = 7.1, 7.1 Hz, 3H), 1.11 (t, J = 7.3, 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 156.9, 154.6, 142.2, 131.7, 131.0, 129.2, 129.1, 127.1, 125.8, 119.5, 118.3, 116.6, 110.0, 64.5, 63.1, 14.3; HRMS (ESI+, m/z) calculated for C20H20N5O4 [M + H]+ 394.1510; found 394.1515.
Diethyl 1-(6-(methoxycarbonyl)-2-phenylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3j)
White solid. 1H NMR (400 MHz, CDCl3) δ 9.42 (s, 1H), 7.83 (t, J = 9.1, 9.1 Hz, 3H), 7.63 (d, J = 9.2 Hz, 1H), 7.44 (t, J = 7.5, 7.5 Hz, 2H), 7.37 (dd, J = 8.4, 6.2 Hz, 1H), 6.85 (s, 1H), 4.24 −4.17 (m, 4H), 3.97 (s, 3H), 1.25 (t, J = 7.1, 7.1 Hz, 3H), 1.09 (t, J = 7.0, 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 165.3, 156.7, 154.9, 143.6, 140.9, 132.2, 128.9, 128.8, 126.9, 125.5, 119.4, 116.7, 116.5, 64.2, 62.1, 52.5, 14.3, 14.2; HRMS (ESI+, m/z) calculated for C21H23N4O6 [M + H]+ 427.1612; found 427.1615.
Diethyl 1-(2-(4-methoxyphenyl)-6-methylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3k)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 7.70 (d, J = 10.6 Hz, 2H), 7.49 (d, J = 5.7 Hz, 1H), 7.10 (d, J = 9.6 Hz, 1H), 7.02 −6.81 (m, 3H), 4.22 (dq, J = 14.0, 7.0, 7.0, 6.9 Hz, 4H), 3.83 (s, 3H), 2.37 (s, 3H), 1.24 (t, J = 6.8, 6.8 Hz, 3H), 1.09 (t, J = 6.9, 6.9 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.6, 156.6, 155.4, 142.0, 139.1, 129.7, 128.9, 127.9, 125.5, 122.2, 116.4, 114.3, 113.9, 63.9, 62.4, 55.2, 18.4, 14.3; HRMS (ESI+, m/z) calculated for C21H25N4O5 [M + H]+ 413.1819; found 413.1814.
Diethyl 1-(2-(3-methoxyphenyl)-6-methylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3l)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.44 (s, 1H), 7.66 (s, 1H), 7.52 (d, J = 9.1 Hz, 1H), 7.39 (s, 1H), 7.33 −7.21 (m, 2H), 7.11 (d, J = 9.2 Hz, 1H), 6.86 (dt, J = 6.5, 2.9, 2.9 Hz, 1H), 4.23 −4.17 (m, 4H), 3.81 (s, 3H), 2.37 (s, 3H), 1.22 (t, J = 7.1, 7.1 Hz, 3H), 1.09 (t, J = 7.0, 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.8, 156.9, 155.2, 141.8, 138.9, 134.1, 129.6, 128.9, 122.2, 119.0, 118.2, 116.5, 114.4, 111.8, 63.8, 62.3, 55.1, 18.3, 14.2; HRMS (ESI+, m/z) calculated for C21H25N4O5 [M + H]+ 413.1819; found 413.1823.
Diethyl 1-(6-methyl-2-(4-(trifluoromethoxy)phenyl)imidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3m)
Off-white solid. 1H NMR (400 MHz, CDCl3) δ 8.44 (s, 1H), 7.83 (s, 1H), 7.74 (dd, J = 8.4, 5.5 Hz, 2H), 7.50 (d, J = 9.1 Hz, 1H), 7.12 (dd, J = 9.1, 1.7 Hz, 1H), 7.05 (t, J = 8.7, 8.7 Hz, 2H), 4.21 −4.17 (m, 4H), 2.37 (s, 3H), 1.23 (t, J = 7.2, 7.2 Hz, 3H), 1.08 (t, J = 7.2, 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 162.6 (d, J = 246 Hz), 156.9, 155.3, 142.0, 138.4, 129.1, 128.6 (d, J = 7.0 Hz), 122.3, 122.1, 117.9, 116.5, 115.7 (d, J = 21 Hz), 64.0, 62.5, 18.4, 14.3; HRMS (ESI+, m/z) calculated for C21H22F3N4O5 [M + H]+ 467.1537; found 467.1539.
Diethyl 1-(6-methyl-2-(naphthalen-2-yl)imidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3n)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.61 (s, 1H), 8.44 (s, 1H), 8.28 (s, 1H), 7.87 (d, J = 9.2 Hz, 1H), 7.78 −7.67 (m, 3H), 7.48 −7.39 (m, 3H), 6.99 (d, J = 11.0 Hz, 1H), 4.12 (dt, J = 22.9, 7.4, 7.4 Hz, 4H), 2.28 (s, 3H), 1.16 (t, J = 7.2, 7.2 Hz, 3H), 0.97 (t, J = 7.1, 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.1, 155.2, 142.1, 138.8, 133.3, 132.8, 130.0, 129.0, 128.3, 128.1, 127.4, 126.1, 124.4, 122.2, 118.3, 116.3, 63.8, 62.3, 18.3, 14.3; HRMS (ESI+, m/z) calculated for C24H25N4O4 [M + H]+ 433.1870; found 433.1876.
Diethyl 1-(2-(4-cyanophenyl)-6-methylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3o)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.56 (s, 1H), 7.90 −7.78 (m, 3H), 7.63 (d, J = 8.4 Hz, 2H), 7.36 (s, 1H), 6.80 −6.73 (m, 1H), 4.31 −4.07 (m, 4H), 2.44 (s, 3H), 1.24 (t, J = 7.2, 7.2 Hz, 3H), 1.08 (t, J = 7.2, 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.0, 155.0, 143.6, 137.9, 137.2, 132.3, 132.0, 130.4, 128.4, 127.2, 124.2, 118.7, 115.6, 111.2, 64.1, 62.6, 21.4, 14.3; HRMS (ESI+, m/z) calculated for C21H22N5O4 [M + H]+ 408.1666; found 408.1666.
Diethyl 1-(2-(4-fluorophenyl)-6-methylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3p)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.44 (s, 1H), 7.83 (s, 1H), 7.74 (dd, J = 8.4, 5.5 Hz, 2H), 7.50 (d, J = 9.1 Hz, 1H), 7.12 (dd, J = 9.1, 1.7 Hz, 1H), 7.05 (t, J = 8.7, 8.7 Hz, 2H), 4.21 −4.17 (m, 4H), 2.37 (s, 3H), 1.23 (t, J = 7.2, 7.2 Hz, 3H), 1.08 (t, J = 7.2, 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 162.6 (d, J = 246 Hz), 156.9, 155.3, 142.0, 138.4, 129.1, 128.6 (d, J = 7.0 Hz), 122.3, 122.1, 117.9, 116.5, 115.7 (d, J = 21 Hz), 64.0, 62.5, 18.4, 14.3; HRMS (ESI+, m/z) calculated for C20H22FN4O4 [M + H]+ 401.1620; found 401.1625
Diethyl 1-(2-(4-chlorophenyl)-6-methylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3q)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.71 (s, 1H), 8.11 (s, 1H), 7.72 (d, J = 8.2 Hz, 2H), 7.60 (d, J = 9.1 Hz, 1H), 7.31 −7.28 (m, 3H), 6.90 (t, J = 6.8, 6.8 Hz, 1H), 4.22 −4.16 (m, 4H), 1.20 (t, J = 7.2, 7.2 Hz, 3H), 1.07 (t, J = 7.2, 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.0, 155.1, 143.0, 138.3, 134.1, 131.2, 128.9, 128.1, 126.1, 124.6, 118.5, 117.2, 112.6, 64.0, 62.5, 14.3; HRMS (ESI+, m/z) calculated for C20H22ClN4O4 [M + H]+ 417.1324; found 417.1327.
Diethyl 1-(2-(4-chlorophenyl)imidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3r)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.56 (s, 1H), 8.02 (s, 1H), 7.69 (d, J = 8.2 Hz, 2H), 7.32 −7.26 (m, 3H), 6.73 −6.71 (m, 1H), 4.22 −4.16 (m, 4H), 2.41 (s, 3H), 1.22 (t, J = 7.2, 7.2 Hz, 3H), 1.07 (t, J = 7.2, 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.0, 155.1, 143.5, 138.0, 137.2, 133.9, 131.3, 128.8, 128.0, 123.9, 118.0, 115.6, 115.2, 64.0, 62.5, 21.3, 14.3; HRMS (ESI+, m/z) calculated for C19H20ClN4O4 [M + H]+ 403.1168; found 403.1166.
Diethyl 1-(2-(4-chlorophenyl)-7-methylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3s)
White solid. 1H NMR (400 MHz, DMSO-d6) δ 10.49 (s, 1H), 8.47 (s, 1H), 8.10 (d, J = 8.2 Hz, 2H), 7.54 (dd, J = 17.1, 8.7 Hz, 3H), 7.24 (d, J = 9.2 Hz, 1H), 4.19 −4.05 (m, 4H), 2.33 (s, 3H), 1.22 (t, J = 7.2, 7.2 Hz, 3H), 0.88 (d, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3 + CD3OD) δ 157.5, 154.4, 141.4, 137.1, 133.0, 132.1, 129.4, 129.2, 128.8, 122.5, 122.2, 118.4, 116.8, 63.7, 61.9, 18.2, 14.7, 14.4; HRMS (ESI+, m/z) calculated for C20H22ClN4O4 [M + H]+ 417.1324; found 417.1325.
Diethyl 1-(2-(4-chlorophenyl)-6-(methoxycarbonyl)imidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3t)
Off-white solid. 1H NMR (400 MHz, CDCl3 + CD3OD) δ 9.43 (s, 1H), 7.88 (d, J = 9.4 Hz, 1H), 7.79 (d, J = 6.8 Hz, 2H), 7.61 (d, J = 9.3 Hz, 1H), 7.44 (d, J = 8.5 Hz, 3H), 4.21 (dd, J = 15.6, 8.1 Hz, 4H), 3.99 (s, 3H), 1.27 (t, J = 7.1, 7.1 Hz, 3H), 1.07 (d, J = 7.1, 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3 + CD3OD) δ 165.2, 156.9, 154.6, 143.4, 139.7, 134.8, 130.3, 129.0, 128.4, 125.9, 119.7, 116.8, 116.4, 64.1, 62.5, 52.5, 14.1; HRMS (ESI+, m/z) calculated for C21H22ClN4O6 [M + H]+ 461.1222; found 461.1227.
Diethyl 1-(6-(methoxycarbonyl)-2-(4-methoxyphenyl)imidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (3u)
Off-white solid. 1H NMR (400 MHz, CDCl3) δ 9.37 (s, 1H), 7.82 (d, J = 9.5 Hz, 1H), 7.74 (d, J = 8.6 Hz, 2H), 7.59 (d, J = 9.4 Hz, 1H), 7.32 (s, 1H), 7.02 −6.96 (m, 2H), 4.23 (tt, J = 10.0, 10.0, 5.3, 5.3 Hz, 4H), 3.97 (s, 3H), 3.86 (s, 3H), 1.28 −1.22 (m, 3H), 1.22 −1.03 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 165.4, 160.0, 156.6, 155.0, 143.4, 140.8, 129.7, 128.4, 125.4, 124.7, 118.5, 116.4, 116.3, 114.4, 64.18, 62.6, 55.2, 52.4, 14.2; HRMS (ESI+, m/z) calculated for C22H25N4O7 [M + H]+ 457.1718; found 457.1714.
Diethyl 1-(6-methylimidazo[1,2-a]pyridin-3-yl)hydrazine-1,2-dicarboxylate (6)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.73 (s, 1H), 8.23 (s, 1H), 7.55 (s, 1H), 7.50 (d, J = 9.2 Hz, 1H), 7.08 (dd, J = 9.2, 1.7 Hz, 1H), 4.22 (t, J = 7.3, 7.3 Hz, 4H), 2.32 (s, 3H), 1.37 −1.10 (m, 6H); 13C NMR (100 MHz, CDCl3) 156.6, 155.2, 142.8, 129.7, 128.5, 122.9, 122.4, 121.6, 116.9, 63.6, 62.1, 18.2, 14.4, 14.3; HRMS (ESI+, m/z) calculated for C14H19N4O4 [M + H]+ 307.1401; found 307.1404.
Supplementary Material
Scheme 1.
Imidazo[1,2-a]pyridine-based therapeutic agents.
Scheme 2.
Control experiment
Acknowledgements
This work was supported by a training grant from The National Institutes of Health (T32 GM008804) and University of Arizona startup funding.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedure (3 and 6) and characterization of all compounds. See DOI: 10.1039/b000000x/
Notes and references
- 1.For a review, see: Enguehard-Gueiffier C, Gueiffier A. Mini-Rev. Med. Chem. 2007;7:888. doi: 10.2174/138955707781662645.
- 2.(a) Byth KF, Geh C, Forder CL, Oakes SE, Thomas AP. Mol. Cancer Ther. 2006;5:655. doi: 10.1158/1535-7163.MCT-05-0205. [DOI] [PubMed] [Google Scholar]; (b) El-Sayed WM, Hussin WA, Al-Faiyz YS, Ismail MA. Eur. J. Pharmacol. 2013;715:212. doi: 10.1016/j.ejphar.2013.05.018. [DOI] [PubMed] [Google Scholar]; (c) Kim O, Jeong Y, Lee H, Hong SS, Hong S. J. Med. Chem. 2011;54:2455. doi: 10.1021/jm101582z. [DOI] [PubMed] [Google Scholar]; (d) Kamal A, Reddy JS, Ramaiah MJ, Dastagiri D, Bharathi EV, Sagar MVP, Pushpavalli SNCVL, Ray P, Pal-Bhadra M. Med. Chem. Commun. 2010;1:355. [Google Scholar]; (e) Colletti SL, Frie JL, Dixon EC, Singh SB, Choi BK, Scapin G, Fitzgerald CE, Kumar S, Nichols EA, O’Keefe SJ, O’Neill EA, Porter G, Samuel K, Schmatz DM, Schwartz CD, Shoop WL, Thompson CM, Thompson JE, Wang R, Woods A, Zaller DM, Doherty JB. J. Med. Chem. 2003;46:349. doi: 10.1021/jm025585h. [DOI] [PubMed] [Google Scholar]; (f) Rupert KC, Henry JR, Dodd JH, Wadsworth SA, Cavender DE, Olini GC, Fahmy B, Siekierka JJ. Bioorg. Med. Chem. Lett. 2003;13:347. doi: 10.1016/s0960-894x(02)01020-x. [DOI] [PubMed] [Google Scholar]; (g) Follot S, Debouzy JC, Crouzier D, Enguehard-Gueiffier C, Gueiffier A, Nachon F, Lefebvre B, Fauvelle F. Eur. J. Med. Chem. 2009;44:3509. doi: 10.1016/j.ejmech.2008.12.026. [DOI] [PubMed] [Google Scholar]; (h) Enguehard C, Renou JL, Allouchi H, Leger JM, Gueiffier A. Chem. Pharm. Bull. 2000;48:935. doi: 10.1248/cpb.48.935. [DOI] [PubMed] [Google Scholar]; (i) Enguehard-Gueiffier C, Fauvelle F, Debouzy JC, Peinnequin A, Thery I, Dabouis V, Gueiffier A. Eur. J. Pharm. Sci. 2005;24:219. doi: 10.1016/j.ejps.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Lacerda RB, de Lima CK, da Silva LL, Romeiro NC, Miranda AL, Barreiro EJ, Fraga CA. Bioorg. Med. Chem. 2009;17:74. doi: 10.1016/j.bmc.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 4.(a) Shukla NM, Salunke DB, Yoo E, Mutz CA, Balakrishna R, David SA. Bioorg. Med. Chem. 2012;20:5850. doi: 10.1016/j.bmc.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Al-Tel TH, Al-Qawasmeh RA, Zaarour R. Eur. J. Med. Chem. 2011;46:1874. doi: 10.1016/j.ejmech.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 5.(a) Ismail MA, Arafa RK, Wenzler T, Brun R, Tanious FA, Wilson WD, Boykin DW. Bioorg. Med. Chem. 2008;16:683. doi: 10.1016/j.bmc.2007.10.042. [DOI] [PubMed] [Google Scholar]; (b) Biftu T, Feng D, Fisher M, Liang GB, Qian X, Scribner A, Dennis R, Lee S, Liberator PA, Brown C, Gurnett A, Leavitt PS, Thompson D, Mathew J, Misura A, Samaras S, Tamas T, Sina JF, McNulty KA, McKnight CG, Schmatz DM, Wyvratt M. Bioorg. Med. Chem. Lett. 2006;16:2479. doi: 10.1016/j.bmcl.2006.01.092. [DOI] [PubMed] [Google Scholar]
- 6.(a) Véron JB, Allouchi H, Enguehard-Gueiffier C, Snoeck R, Andrei G, De Clercq E, Gueiffier A. Bioorg. Med. Chem. 2008;16:9536. doi: 10.1016/j.bmc.2008.09.027. [DOI] [PubMed] [Google Scholar]; (b) Gudmundsson KS, Williams JD, Drach JC, Townsend LB. J. Med. Chem. 2003;46:1449. doi: 10.1021/jm020339r. [DOI] [PubMed] [Google Scholar]; (c) Gudmundsson KS, Johns BA. Org. Lett. 2003;5:1369. doi: 10.1021/ol0343616. [DOI] [PubMed] [Google Scholar]; (d) Gudmundsson KS, Johns BA. Bioorg. Med. Chem. Lett. 2007;17:2735. doi: 10.1016/j.bmcl.2007.02.079. [DOI] [PubMed] [Google Scholar]
- 7.Kaminski JJ, Doweyko AM. J. Med. Chem. 1997;40:427. doi: 10.1021/jm950700s. [DOI] [PubMed] [Google Scholar]
- 8.Rival Y, Grassy G, Taudon A, Ecalle R. Eur. J. Med. Chem. 1991;26:13. [Google Scholar]
- 9.George PG, Rossey G, Sevrin M, Arbilla S, Depoortere H, Wick AELERS. Monograph Ser. 1993;8:49. [Google Scholar]
- 10.Depoortere H, George P. US 5064836. 1991 [Google Scholar]
- 11.Sanger DJ. Behav. Pharmacol. 1995;6:116. [PubMed] [Google Scholar]
- 12.Du B, Shan A, Zhang Y, Zhong X, Chen D, Cai K. Am. J. Med. Sci. 2014;347:178. doi: 10.1097/MAJ.0b013e318287c79c. [DOI] [PubMed] [Google Scholar]
- 13.Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. Neuropharmacology. 2009;56:182. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Uemura Y, Tanaka S, Ida S, Yuzuriha T. J. Pharm. Pharmacol. 1993;45:1077. doi: 10.1111/j.2042-7158.1993.tb07184.x. [DOI] [PubMed] [Google Scholar]
- 15.Sorbera LA, Castaner J, Leeson PA. Drugs Fut. 2002;27:935. [Google Scholar]
- 16.Pellón R, Ruíz A, Lamas E, Rodríguez C. Behav. Pharmacol. 2007;18:81. doi: 10.1097/FBP.0b013e3280143212. [DOI] [PubMed] [Google Scholar]
- 17.Belohlavek D, Malfertheiner P. Scand. J. Gastroenterol Suppl. 1979;54:44. [PubMed] [Google Scholar]
- 18.(a) Marhadour S, Marchand P, Pagniez F, Bazin MA, Picot C, Lozach O, Ruchaud S, Antoine M, Meijer L, Rachidi N, Le Pape P. Eur. J. Med. Chem. 2012;58:543. doi: 10.1016/j.ejmech.2012.10.048. [DOI] [PubMed] [Google Scholar]; (b) Fu HY, Chen L, Doucet H. J. Org. Chem. 2012;77:4473. doi: 10.1021/jo300528b. [DOI] [PubMed] [Google Scholar]; (c) Liu Y, He L, Yin G, Wu G, Cui Y. Bull. Korean Chem. Soc. 2013;34:2340. [Google Scholar]; (d) Cao H, Zhan H, Lin Y, Lin X, Du Z, Jiang H. Org. Lett. 2012;14:1688. doi: 10.1021/ol300232a. [DOI] [PubMed] [Google Scholar]; (e) Bagdi AK. Adv. Synth. Catal. 2013;355:1741. [Google Scholar]
- 19.Wang SH, Liu WJ, Cen JH, Liao JQ, Huang JP, Zhan HY. Tetrahedron Lett. 2014;55:1589. [Google Scholar]
- 20.Ravi C, Mohan DD, Adimurthy S. Org. Lett. 2014;16:2978. doi: 10.1021/ol501117z. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Hong JQ, Zhou XG. Tetrahedron. 2011;67:3690. [Google Scholar]
- 22.Koubachia J, Berteina-Raboin S, Mouaddibb A, Guillaumet G. Synthesis. 2009:271. [Google Scholar]
- 23.Zhan HY, Zhao LM, Li NY, Chen LB, Liu JY, Liao JQ, Cao H. RSC Adv. 2014;4:32013. [Google Scholar]
- 24.(a) Nordqvist A, Nilsson MT, Lagerlund O, Muthas D, Gising J, Yahiaoui S, Odell LR, Srinivasa BR, Larhed M, Mowbray SL, Karlén A. Med. Chem. Commun. 2012;3:620. [Google Scholar]; (b) Hamdouchi C, Keyser H, Collins E, Jaramillo C, De Diego JE, Spencer CD, Dempsey JA, Anderson BD, Leggett T, Stamm NB, Schultz RM, Watkins SA, Cocke K, Lemke S, Burke TF, Beckmann RP, Dixon JT, Gurganus RM, Rankl NB, Houck KA, Zhang F, Vieth M, Espinosa J, Timm DE, Campbell RM, Patel BK, Brooks HB. Mol Cancer Ther. 2004;3:1. [PubMed] [Google Scholar]; (c) Elleder D, Baiga TJ, Russell RL, Naughton JA, Hughes SH, Noel JP, Young JA. Virol J. 2012;9:305. doi: 10.1186/1743-422X-9-305. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Odell LR, Nilsson MT, Gising J, Lagerlund O, Muthas D, Nordqvist A, Karlén A, Larhed M. Bioorg Med Chem Lett. 2009;19:4790. doi: 10.1016/j.bmcl.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 25.(a) Blackburn C, Guan B. Tetrahedron Lett. 2000;41:1495. [Google Scholar]; (b) Guchhait SK, Chaudhary V, Madaan C. Org. Biomol. Chem. 2012;10:9271. doi: 10.1039/c2ob26733k. [DOI] [PubMed] [Google Scholar]; (c) Venkatesham R, Manjula A, Vittal Rao B. J. Heterocyclic Chem. 2011;48:942. [Google Scholar]
- 26.(a) Wang YX, Saha B, Li F, Frett B, Li HY. Tetrahedron Lett. 2014;55:1281. [Google Scholar]; (b) Wang YX, Frett B, Li HY. Org. Lett. 2014;16:3016. doi: 10.1021/ol501136e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muñiz K, Iglesias A. Angew. Chem., Int. Ed. 2007;46:6350. doi: 10.1002/anie.200700288. [DOI] [PubMed] [Google Scholar]
- 28.Yu WY, Sit WN, Lai KM, Zhou ZY, Chan ASC. J. Am. Chem. Soc. 2008;130:3304. doi: 10.1021/ja710555g. [DOI] [PubMed] [Google Scholar]
- 29.(a) Li YM, Jia F, Li ZP. Chem. Eur. J. 2013;19:82. [Google Scholar]; (b) Ouyang XH, Song RJ, Li JH. Eur. J. Org. Chem. 2014:3395. doi: 10.1021/jo5005982. [DOI] [PubMed] [Google Scholar]; (c) Cahiez G, Foulgoc L, Moyeux A. Angew. Chem., Int. Ed. 2009;48:2969. doi: 10.1002/anie.200900175. [DOI] [PubMed] [Google Scholar]; (d) Nakanishi M, Bolm C. Adv. Synth. Catal. 2007;349:861. [Google Scholar]; (e) kshirsagar U, Regev C, Parnes R, Pappo D. Org. Lett. 2013;15:3174. doi: 10.1021/ol401532a. [DOI] [PubMed] [Google Scholar]; (f) Rohlmann R, Stopka T, Richter H, mancheno OG. J. Org. Chem. 2013;78:6050. doi: 10.1021/jo4007199. [DOI] [PubMed] [Google Scholar]
- 30.(a) Cheng Y, Dong WR, Wang L, Parthasarathy K, Bolm C. Org. Lett. 2014;16:2000. doi: 10.1021/ol500573f. [DOI] [PubMed] [Google Scholar]; (b) Xu XS, Tang YC, Li XQ, Hong G, Fang MW, Du XH. J. Org. Chem. 2014;79:446. doi: 10.1021/jo402529r. [DOI] [PubMed] [Google Scholar]; (c) Maity S, Pramanik A. Tetrahedron Lett. 2014 http://dx.doi.org/10.1016/j.tetlet.2014.08.074.; (d) Barton DHR, Gloahec VNL, Patin H. New J. Chem. 1998;22:565. [Google Scholar]
- 31.(a) Chudasama V, Ahern JM, Dhokia DV, Fitzmaurice RJ, Caddick S. Chem. Commun. 2011;47:3269. doi: 10.1039/c0cc04520a. [DOI] [PubMed] [Google Scholar]; (b) Ni B, Zhang Q, Garre S, Headley AD. Adv. Synth. Catal. 2009;351:875. [Google Scholar]; (c) Amaoka Y, Kamijo S, Hoshikawa T, Inoue M. J. Org. Chem. 2012;77:9959. doi: 10.1021/jo301840e. [DOI] [PubMed] [Google Scholar]; (d) Schmidt VA, Alexanian EJ. J. Am. Chem. Soc. 2011;133:11402. doi: 10.1021/ja204255e. [DOI] [PubMed] [Google Scholar]; (e) Ryu I, Tani A, Fukuyama T, Ravelli D, Montanaro S, Fagnoni M. Org. Let. 2013;15:2554. doi: 10.1021/ol401061v. [DOI] [PubMed] [Google Scholar]
- 32.(a) Nair V, Biju AT, Mathew SC, Babu BP. Chem. Asian J. 2008;3:810. doi: 10.1002/asia.200700341. [DOI] [PubMed] [Google Scholar]; (b) Vallribera A, Sebastian RM, Shafir A. Curr. Org. Chem. 2011;15:1539. [Google Scholar]; (c) Kanzian T, Mayr H. Chem.Eur. J. 2010;16:11670. doi: 10.1002/chem.201001598. [DOI] [PubMed] [Google Scholar]; (d) Huisgen R, Jakob F. Liebigs Ann. Chem. 1954;590:37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.