Abstract
Iron–sulfur (Fe–S) clusters are essential protein cofactors for most life forms. In human mitochondria, the core Fe–S biosynthetic enzymatic complex (called SDUF) consists of NFS1, ISD11, ISCU2, and frataxin (FXN) protein components. Few mechanistic details about how this complex synthesizes Fe–S clusters and how these clusters are delivered to targets are known. Here circular dichroism and Mössbauer spectroscopies were used to reveal details of the Fe–S cluster assembly reaction on the SDUF complex. SDUF reactions generated [2Fe-2S] cluster intermediates that readily converted to stable [2Fe-2S] clusters bound to uncomplexed ISCU2. Similar reactions that included the apo Fe–S acceptor protein human ferredoxin (FDX1) resulted in formation of [2Fe-2S]-ISCU2 rather than [2Fe-2S]-FDX1. Subsequent addition of dithiothreitol (DTT) induced transfer of the cluster from ISCU2 to FDX1, suggesting that [2Fe-2S]-ISCU2 is an intermediate. Reactions that initially included DTT rapidly generated [2Fe-2S]-FDX1 and bypassed formation of [2Fe-2S]-ISCU2. In the absence of apo-FDX1, incubation of [2Fe-2S]-ISCU2 with DTT generated [4Fe-4S]-ISCU2 species. Together, these results conflict with a recent report of stable [4Fe-4S] cluster formation on the SDUF complex. Rather, they support a model in which SDUF builds transient [2Fe-2S] cluster intermediates that generate clusters on sulfur-containing molecules, including uncomplexed ISCU2. Additional small molecule or protein factors are required for the transfer of these clusters to Fe–S acceptor proteins or the synthesis of [4Fe-4S] clusters.
Iron–sulfur (Fe–S) clusters are essential protein cofactors that have functions such as electron transfer, substrate binding and activation, and regulation of gene expression or enzyme activity.1 Common Fe–S clusters, including [2Fe-2S] ([Fe2S2]) and [4Fe-4S] ([Fe4S4]) structures, can be synthesized in nonenzymatic self-assembly reactions from iron, sulfide, and appropriate sulfur-containing ligands.2,3 Similar reaction conditions are frequently used to chemically reconstitute recombinant proteins with biological Fe–S clusters. In contrast, the in vivo synthesis of Fe–S clusters does not use this self-assembly chemistry but rather highly conserved assembly pathways that regulate the biosynthesis of Fe–S clusters and control the inherent reactivity and toxicity of cluster intermediates.4–9 In humans, such clusters are synthesized on an assembly complex composed of NFS1, ISD11, ISCU2, and frataxin (FXN) components.10,11 Many details of the mechanism for cluster synthesis, the types of clusters synthesized by the core assembly complex, and additional protein factors that provide regulation and/or target specificity are being actively investigated.
In eukaryotes, the primary Fe–S biosynthetic pathway is located in the mitochondria. This pathway not only builds [2Fe-2S] and [4Fe-4S] clusters but also delivers them to target proteins. This pathway is analogous to the bacterial ISC system.12 In humans, NFS1 (analogous to bacterial IscS) along with the accessory protein ISD11 generates the functional cysteine desulfurase complex (named SD) that catalyzes the conversion of l-cysteine to l-alanine.13–16 Sulfur is transferred to the Fe–S catalytic subunit ISCU2 (analogous to bacterial IscU) where it combines with iron and electrons to generate Fe–S clusters.17,18 Human FXN stimulates cysteine desulfurase and iron–sulfur assembly activities and may function as an allosteric activator to regulate the Fe–S assembly complex (DOI: 10.1021/bi5014497).10 DTT is often used as a surrogate electron donor for in vitro assays to replace the function of the ferredoxin/ferredoxin reductase/NADPH system in providing electrons for in vivo Fe–S cluster formation and/or transfer.19,20 Chaperone and Fe–S carrier proteins are thought to interact with uncomplexed [2Fe-2S]-IscU and facilitate the transfer of intact Fe–S clusters to target proteins.21–32
No intermediates in the biosynthesis or self-assembly of [2Fe-2S] clusters have been established, but some details of the conversion of [2Fe-2S] clusters to [4Fe-4S] clusters are known. Inorganic biomimetic chemistry reveals that [4Fe-4S] clusters can be formed by reductively coupling two [2Fe-2S] clusters.2,3 In a remarkable study, the Huynh, Dean, and Johnson groups demonstrated reductive coupling chemistry on an IscU dimeric species with the sequential formation of a single [2Fe-2S] cluster followed by a second [2Fe-2S] cluster, and then conversion of these [2Fe-2S] clusters to form one [4Fe-4S] cluster per IscU dimer.33 This reductive coupling chemistry occurs at an IscU dimeric interface with each monomer contributing one [2Fe-2S] cluster. The O2 sensor protein FNR employs a different [2Fe-2S]-to-[4Fe-4S] cluster conversion process in which persulfide-ligated [2Fe-2S] clusters convert to cysteine-ligated [4Fe-4S] clusters upon addition of iron.34
A crystal structure of a bacterial IscS–IscU complex reveals a [2Fe-2S] cluster bound by cysteine ligands that originated from both IscS and IscU subunits.35 The cluster is buried in the IscS–IscU protein interface, suggesting a reductive coupling mechanism in which two [2Fe-2S] species come together to form a [4Fe-4S] cluster on the IscS–IscU assembly complex that would require a different conformation. Recent Mössbauer studies of the murine Fe–S assembly system led to the proposal that FXN facilitates [4Fe-4S] cluster formation on the SDUF complex.36 It is unclear whether the [4Fe-4S] species is synthesized on the murine SDUF assembly complex or generated through reactions with uncomplexed [2Fe-2S]-IscU, similar to the bacterial systems.33
The initial objectives of this study were to link the protein assembly state with the type of cluster generated by the human Fe–S assembly complex. In the following paper (DOI: 10.1021/bi5014497), Fe–S assembly reactions using the human SDUF complex were shown to partition between biosynthetic [2Fe-2S] cluster synthesis and Fe–S mineral-like pathways. Fe–S mineral-like material elutes from size-exclusion columns as high-molecular weight species (HMWS) that readily separates from SDUF and uncomplexed ISCU2. Conditions that minimize the production of the reaction byproduct sulfide also suppress HMWS formation. This discovery allowed us to find that FXN accelerates the rate of [2Fe-2S] cluster synthesis by the SDUF complex. Here, Mössbauer, circular dichroism (CD), and electronic absorbance spectroscopies were used in conjunction with size-exclusion LC and ICP-MS to analyze the products of Fe–S biosynthetic reactions by the SDUF complex. Additional studies explored the requirements for cluster transfer and conversion chemistry by the human assembly system.
MATERIALS AND METHODS
Protein Expression and Purification
Human NFS1-ISD11 (named SD), ISCU2, FXN, and FDX1 were expressed and purified as described (DOI: 10.1021/bi5014497).10,37 Buffer A [50 mM HEPES (pH 7.8) and 250 mM NaCl] was used for all experiments unless otherwise stated. The [2Fe-2S] cluster was removed from FDX1 as described (DOI: 10.1021/bi5014497).38 Unless otherwise stated, anaerobic experiments were performed in an Mbraun glovebox at ~12 °C with an argon atmosphere and <1 ppm O2 as monitored by a Teledyne Model 310 analyzer.
Fe–S Cluster Formation Assays
Fe–S cluster assembly assay mixtures under four experimental conditions (DTT-free, Stoichiometric, Catalytic, and Acceptor) were prepared anaerobically and transferred to a 1 cm path length anaerobic cuvette (250 or 400 μL) for circular dichroism (CD) (Chirascan) data collection at 20 °C. DTT-free assay conditions included 10 μM SD, 30 μM ISCU2, 30 μM FXN, 1 mM l-cysteine, and 250 μM Fe(NH4)2(SO4)2 (final concentrations). Reactions were initiated by the addition of cysteine. Stoichiometric conditions were identical to those of the DTT-free experiment except that the ISCU2 concentration was decreased from 30 to 10 μM. Catalytic conditions were identical to those of the DTT-free experiment except that the SD concentration was decreased from 10 to 0.5 μM. Acceptor conditions included 4 μM SD, 30 μM ISCU2, 30 μM FXN, 90 μM apo-FDX1, 250 μM Fe(NH4)2(SO4)2, and 1 mM l-cysteine with or without 4 mM DTT. Control Fe–S reactions included 250 μM Na2S and either 250 μM Fe(NH4)2(SO4)2 or 250 μM FeCl3. These control reaction mixtures were incubated with or without 1 mM l-cysteine or 4 mM DTT. All reaction mixtures turned brown within a few minutes. Control reaction mixtures were incubated for an additional 10–60 min in the glovebox, placed in a sealed anaerobic cuvette, and analyzed by CD spectroscopy.
Mössbauer Analysis of Fe–S Species
Sample M1 was prepared by combining 10 μM SD, 1300 μM ISCU2, 1.5 mM 57Fe2+, and 10 mM l-cysteine in a total volume of 1600 μL. Ferrous ions were slowly added to the Fe–S assembly reaction (~375 μM/h). The sample was incubated for 1 h at 12 °C and then split in half. Half of the sample was incubated with 10 mM DTT for 1 h, and then both samples were separately analyzed by LC–ICP-MS and Mössbauer spectroscopy. Sample M2 was prepared by combining 8 μM SD, 200 μM ISCU2, and 8 μM FXN with 500 μM 57Fe2+ and 1 mM l-cysteine in a total volume of 8 mL. A portion was removed to monitor reaction progress via CD spectroscopy. The 57Fe2+ was prepared by reducing a 80 mM 57Fe3+-citrate stock solution with dithionite that was carefully monitored by Mössbauer spectroscopy to quantitatively generate reduced iron with no excess dithionite. The M2 sample was incubated for 1 h at 12 °C; half was removed and concentrated to 800 μL, diluted to 3 mL with buffer A, and reconcentrated to 800 μL three times (buffer exchanged) before analysis by LC–ICP-MS and Mössbauer spectroscopy. The remaining portion of the M2 sample was incubated with 10 mM DTT for an additional 1.5 h at 12 °C, buffer exchanged, and then separately analyzed by LC–ICP-MS and Mössbauer spectroscopy. Low-field Mössbauer spectra were recorded using a model MS4 WRC spectrometer (SEE Co., Edina, MN) at 5 K and 0.05 T (field applied parallel to the γ radiation), and analyzed using WMOSS software (SEE Co.). Parameters are quoted relative to those of α-Fe foil at 298 K.
LC–ICP-MS Analysis
Buffer consisting of 50 mM Tris-HCl and 150 mM NaCl (pH 7.4) was prepared in doubly distilled deionized trace-metal-free water. This buffer was used to equilibrate a Superdex 200 (10/300, GE Healthcare) column. The elution flow rate was 0.5 mL/min, controlled by a Bio-Inert high-performance liquid chromatography system (Agilent Technologies). A variable-wavelength diode array detector (Agilent Technologies) was positioned postcolumn and set to 280 nm. The entire system was housed in an ~7 °C Mbraun glovebox with <3 ppm O2. The postdetector flow was split using a micro splitter valve (Upchurch Scientific) with ~30% directed to an ICP-MS (model 7700x, Agilent Technologies) and the remainder to a model 1260 Infinity Analytical-Scale Fraction Collector (Agilent Technologies). The ICP-MS was used in collision cell mode (He, 4.3 mL min−1) with platinum cones and a skimmer to minimize polyatomic inferences. The instrument was optimized daily using the manufacturer’s tuning solution. The sample was introduced through a standard Micromist nebulizer (Glass Expansion, West Melbourne, Australia). 56Fe and 57Fe were monitored with a dwell time of 100 ms.
RESULTS
Anaerobic Fe–S assembly reactions were performed for the human SDUF complex under four conditions called DTT-free, Stoichiometric, Catalytic, and Acceptor (see Materials and Methods). The DTT-free, Stoichiometric, and Catalytic conditions differed in the amount of excess ISCU2 relative to NFS1 in the reaction mixture.10,11 The 300–600 nm region of the CD spectrum was used to monitor the kinetics of [2Fe-2S] cluster synthesis and/or transfer reactions. Fe–S mineral-like materials (DOI: 10.1021/bi5014497) that form from Fe, sulfide, and DTT do not significantly contribute to this spectral region (see below). Moreover, [4Fe-4S] clusters and [2Fe-2S] clusters bound to small molecule thiols such as glutathione (GSH) also exhibit very weak features in this region of the CD spectrum.39,40 In other experiments, Fe–S assembly reactions were subjected to size-exclusion chromatography to evaluate the protein assembly state, ICP-MS to determine the iron content, and UV–vis, CD, and Mössbauer spectroscopies to define the generated Fe–S species.
The SDUF Complex Generates [2Fe-2S] Clusters Associated with Uncomplexed ISCU2
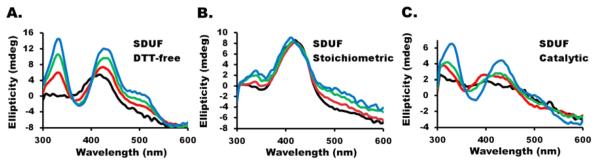
Under DTT-free conditions, Fe–S assembly reactions generated CD features at 330 and 430 nm due to a bound [2Fe-2S] cluster (Figure 1A), consistent with results from the following paper (DOI: 10.1021/bi5014497) and with results obtained using other proteins.25,41,42 The feature at 420 nm is due to the PLP cofactor. Here we explored additional reaction conditions to probe whether the assembled [2Fe-2S] clusters were bound exclusively to the ISCU2 component of the SDUF complex or whether they might also be bound to ISCU2 proteins that were not associated with the complex. Under Stoichiometric conditions (equimolar NSF1:ISCU2 ratios achieved by decreasing the ISCU2 concentration from 30 to 10 μM), the [2Fe-2S]-associated CD signal did not develop (Figure 1B). Many scenarios can explain the lack of a [2Fe-2S] cluster CD signal under Stoichiometric conditions (see Discussion). Regardless, this suggested that the excess ISCU2 present under DTT-free conditions allowed the buildup of [2Fe-2S] clusters. This implies that [2Fe-2S]-bound ISCU2 can exist independently of the SDUF complex. Further evidence of this was obtained using Catalytic reaction conditions in which the SD concentration was decreased from 10 μM (as is present in DTT-free conditions) to 0.5 μM, thus creating a 1:60 NSF1:ISCU2 molar ratio. Time-dependent formation of [2Fe-2S] clusters under catalytic conditions (Figure 1C) provided additional evidence that [2Fe-2S] clusters can exist on “free” ISCU2 (or possibly as a FXN–ISCU243 complex). The change of ~4 mdeg for the 330 nm ellipticity corresponds to a [2Fe-2S] cluster concentration of ~6 μM,24 which is 10-fold higher than the SD concentration of 0.5 μM used in these experiments. This raises interesting mechanistic issues regarding the transfer of Fe–S clusters from the SDUF complex to downstream targets (see Discussion).
Figure 1.
CD spectra of SDUF reaction mixtures prepared under various conditions. (A) DTT-free reaction measured 0 (black), 20 (red), 40 (green), and 90 (blue) min after the reaction was initiated. (B and C) Stoichiometric and Catalytic conditions, respectively. Time points in panels B and C are color-coded as in panel A.
Mössbauer spectroscopy combined with LC–ICP-MS analysis was also used to evaluate whether the assembly complex can generate [2Fe-2S] clusters associated with uncomplexed ISCU2. In addition, these experiments were designed to evaluate the conditions necessary to generate [4Fe-4S] clusters by the SDUF complex. Scaling up the Fe–S reaction under DTT-free conditions to concentrations appropriate for Mössbauer spectroscopy resulted in HMWS formation (data not shown). We therefore decreased the SD concentration and performed Fe–S assembly reactions under conditions that included excess ISCU2 [Mössbauer experiment 1 (M1)]. The reaction was initiated by the slow addition of Fe2+. The sample was incubated at 12 °C for 60 min and split into two fractions. To evaluate whether DTT induces cluster conversion chemistry, 10 mM DTT was added to one of the fractions, and both were incubated for an additional 60 min. Each fraction was passed through a Superdex-200 size-exclusion column, and the eluate flowed into an online ICP-MS instrument such that eluted iron could be detected in real time (Figure 2A). Most of the iron in both fractions was associated with low-molecular weight species (LMWS; elution volume of >20 mL). Quadrupole doublets dominated both Mössbauer spectra (Figure 2B,C) and were assigned to high-spin Fe2+ species coordinated primarily to sulfur donor ligands (Table 1). This high-abundance species is most likely associated with the LMWS evident in the LC traces (Figure 2A), possibly complexes formed with the l-cysteine in the reaction mixture. The M1 fraction without DTT (Figure 2B) also exhibited two minor quadrupole doublets. One was typical of high-spin Fe2+ bound predominantly to oxygen/nitrogen ligands, while the other (5% of the spectral intensity) had parameters of a [2Fe-2S]2+ cluster (δ = 0.3 mm/s, and ΔEQ = 0.7 mm/s). Consistent with this, the absorbance spectrum associated with the peak that eluted at 17.6 mL of the DTT-free fraction (Figure 2A, inset) had features of a [2Fe-2S] cluster (DOI: 10.1021/bi5014497). The retention time of this peak suggested a monomeric ISCU2 species (Figure 2A). The M1 sample with DTT (Figure 2C) exhibited a minor species (10% spectral intensity) with Mössbauer parameters of [4Fe-4S]2+ clusters (δ = 0.45 mm/s, and ΔEQ = 1.17 mm/s). We conclude that Fe–S assembly reactions under Catalytic conditions produce [2Fe-2S] clusters associated with uncomplexed ISCU2 and that DTT facilitates the conversion from [2Fe-2S]2+ (Figure 2D) to [4Fe-4S]2+ (Figure 2E) species.
Figure 2.
SDUF reactions under Catalytic conditions generate [2Fe-2S]2+ clusters in the absence of DTT and [4Fe-4S]2+ clusters in the presence of DTT (Mössbauer experiment M1). (A) LC–ICP-MS chromatogram (57Fe detection) of reaction mixtures without (black) and with (red) DTT. The inset shows the absorbance spectrum of the DTT-free fraction at 17.6 mL (arrow). (B) Mössbauer spectrum (5 K, 0.05 T, parallel) of the DTT-free sample prior to LC–ICP-MS analysis. Green and blue lines are quadrupole doublets due to HS Fe2+ with S-based (80%) and O/N-based donors (12%), respectively. Arrows indicate a 5% contribution from a doublet due to [2Fe-2S]2+ clusters. (C) M1 Mössbauer spectrum (5 K, 0.05 T, parallel) of the reaction mixture containing 10 mM DTT. The green line is a quadrupole doublet due to HS Fe2+ with likely S coordination (85% of the spectral intensity). Arrows indicate a quadrupole doublet (10% intensity) due to [4Fe-4S]2+ clusters. (D and E) Same as panels B and C, respectively, after subtracting the quadruple doublets due to HS Fe2+. Red lines are simulations of the quadrupole doublets due to [2Fe-2S]2+ and [4Fe-4S]2+ clusters. Mössbauer parameters are listed in Table 1.
Table 1.
Mössbauer Parameters for Fe–S species
| experiment | species | spectral intensity (%) |
isomer shift (mm/s) |
quadrupole splitting (mm/s) |
|---|---|---|---|---|
| M1 | HS Fe2+ O/N ligand |
12 | 1.2 | 3.6 |
| HS Fe2+ S ligand |
80 | 0.72 | 3.3 | |
| [2Fe-2S]2+ | 5 | 0.3 | 0.7 | |
| M1 with DTT |
HS Fe2+ S ligand |
85 | 0.72 | 3.3 |
| [4Fe-4S]2+ | 10 | 0.45 | 1.17 | |
| M2 | HS Fe2+ O/N ligand |
50 | 1.3 | 3.1 |
| [2Fe-2S]2+ | 42 | 0.3 | 0.7 | |
| M2 with DTT |
[2Fe-2S]2+ | 65 | 0.3 | 0.7 |
| [4Fe-4S]2+ | 15 | 0.45 | 1.17 |
We repeated the coupled LC–ICP-MS/Mössbauer experiment at a 5-fold higher SD:ISCU2 ratio and included a buffer exchange step to remove iron associated with LMWS. The SDUF-catalyzed reaction was initiated by adding l-cysteine to a 57Fe-containing sample [Mössbauer experiment 2 (M2)]. The solution was incubated anaerobically at 12 °C for 1 h, and the reaction was limited by a buffer exchange step that removed unreacted reagents. Half of the sample was analyzed by CD, LC–ICP-MS (Figure 3A), and Mössbauer spectroscopy (Figure 3B). The CD spectrum (Figure 4A, black line) suggested a [2Fe-2S] cluster. Notably, control reactions with iron and sulfide did not significantly contribute to this region of the CD spectrum. The other half of the sample was incubated with 10 mM DTT at 12 °C for an additional 1.5 h. The buffer was exchanged, and the products of the reaction were also analyzed by LC–ICP-MS (Figure 3C) and Mössbauer spectroscopy (Figure 3D). The M2 sample lacking DTT (Figure 3B) exhibited two major quadrupole doublets (Table 1). One had parameters of [2Fe-2S]2+ clusters (δ = 0.3 mm/s, and ΔEQ = 0.7 mm/s; 42% of the spectral intensity, blue line). The other originated from a high-spin Fe2+ species with predominantly oxygen/nitrogen ligands (50% of the spectral intensity, green line). We tentatively assigned the [2Fe-2S]2+ cluster to uncomplexed ISCU2 (corresponding to the peak at ~16 mL) and the high-spin iron species to the LMWS (at ~28 mL) (Figure 3A). Iron was also associated with a minor species at ~12 mL that corresponds to the retention time of the SDUF assembly complex. The M2 sample containing DTT exhibited a LC–ICP-MS chromatogram with one major peak that corresponded to uncomplexed ISCU2 (Figure 3C). No LMWS were evident in this sample, possibly because of the additional buffer exchange step. The intensity of the peak at ~12 mL was decreased, which suggests that iron was not associated with the SDUF complex in this sample. Moreover, the peak for uncomplexed ISCU2 was shifted compared to the ISCU2-associated peak in the LC trace of the sample without DTT (Figure 3A). Further studies are required, but this shift may indicate a conversion from monomeric to dimeric ISCU2 species. The Mössbauer spectrum for the M2 sample with DTT (Figure 3D) revealed a quadrupole doublet due to [2Fe-2S]2+ clusters (δ = 0.3 mm/s, and ΔEQ = 0.7 mm/s; 65% of the spectral intensity, blue line) with a minor contribution of a [4Fe-4S]2+ cluster doublet (δ = 0.45 mm/s, and ΔEQ = 1.17 mm/s; 15% of the spectral intensity, orange line). Together, the Fe–S assembly reactions monitored by CD and Mössbauer spectroscopy indicate that the SDUF complex forms [2Fe-2S] clusters on the ISCU2 subunits of the assembly complex. These subunits dissociated to form cluster-bound ISCU2, or the complex transferred [2Fe-2S] clusters to uncomplexed ISCU2. DTT appears to be required for the conversion from [2Fe-2S] to [4Fe-4S] clusters.
Figure 3.
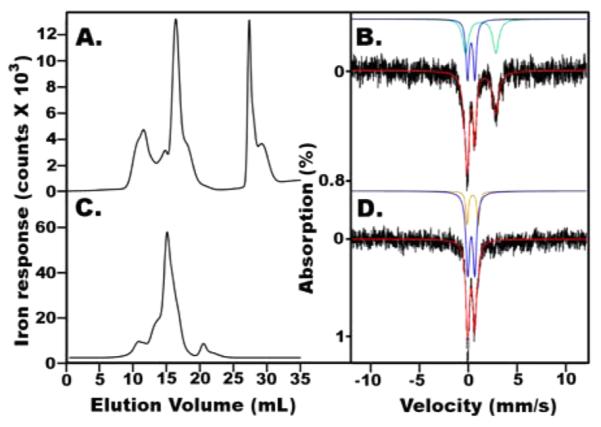
[2Fe-2S]2+ and [4Fe-4S]2+ clusters form in reaction mixtures involving SDUF under Catalytic conditions (Mössbauer experiment M2). (A and C) LC–ICP-MS (57Fe detection) of the mixture without and with 10 mM DTT, respectively. The HMWS, native SDUF, uncomplexed ISCU2, and LMWS eluted at ~9, 12, 16, and 28 mL, respectively. (B) Mössbauer spectrum (5 K, 0.05 T) of the sample without DTT. Green and blue lines are simulations of quadrupole doublets due to high-spin Fe2+ and [2Fe-2S]2+ clusters, respectively. The red line shows the overall simulation. (D) Mössbauer spectrum (5 K, 0.05 T, parallel) of the sample with DTT. Blue and gold lines are simulations of quadruple doublets due to [2Fe-2S]2+ and [4Fe-4S]2+ clusters, respectively. The red line shows the overall simulation.
Figure 4.
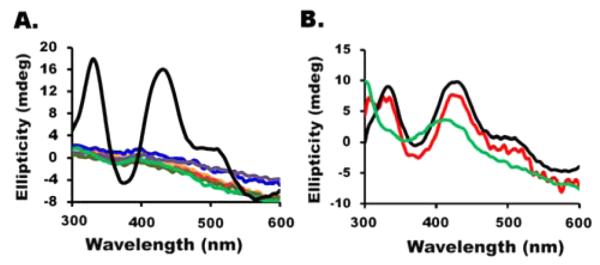
CD features for M2 (Figure 3), control, and DTT-spiked reaction mixtures. (A) The CD spectrum for [2Fe-2S]-ISCU2 for the M2 sample is colored black. CD spectra are overlaid for control reactions that include Fe(NH4)2(SO4)2 and Na2S (orange), FeCl3 and Na2S (red), Fe(NH4)2(SO4)2, Na2S, and DTT (blue), FeCl3, Na2S, and DTT (purple), Fe(NH4)2(SO4)2, Na2S, and cysteine (dark green), and FeCl3, Na2S, and cysteine (light green). (B) The [2Fe-2S]-ISCU2 signal was generated for the SDUF sample under DTT-free conditions (black). DTT (4 mM) was added, and the spectra were recorded after 2 (red) and 5 (green) min.
DTT-Mediated [2Fe-2S] Cluster Conversion and Transfer Chemistry
To further evaluate the effect of DTT on cluster conversion, we performed a reaction with the SDUF complex under DTT-free conditions to generate a CD signal typical of [2Fe-2S]-ISCU2. Then we added DTT and followed the CD spectra as a function of time. Our results revealed a DTT-dependent loss of the Fe–S cluster CD signal that resulted in spectra dominated by features assigned to the PLP cofactor (Figure 4B). The loss of the [2Fe-2S]-ISCU2 CD signal could have been due to conversion to a CD-silent [4Fe-4S] cluster (consistent with the Mössbauer experiments), transfer of the [2Fe-2S] cluster to DTT, or conversion of the [2Fe-2S] cluster into another species such as the Fe–S mineral. Although this cluster conversion/transfer process was generally reproducible, the rate varied with some unknown factor. Interestingly, reactions in which DTT was included in the initial reaction mixtures produced Fe–S cluster-associated HMWS (DOI: 10.1021/bi5014497), whereas reactions in which DTT was added after the formation of [2Fe-2S] clusters on uncomplexed ISCU2 generated [4Fe-4S] clusters (Figures 2 and 3).
We then explored the conditions necessary to facilitate transfer of the [2Fe-2S] cluster from ISCU2 to apo-FDX1. Although the absorbance properties for [2Fe-2S] clusters bound to ISCU2 and FDX1 are similar, the CD spectra exhibit distinct features. Notably, [2Fe-2S]-ISCU2 exhibits a positive CD peak at 330 nm that is greatly diminished in intensity for human [2Fe-2S]-FDX1. The CD spectrum for the PLP cofactor also contributes a positive peak at 330 nm that has an intensity between those of [2Fe-2S]-ISCU2 and [2Fe-2S]-FDX1.
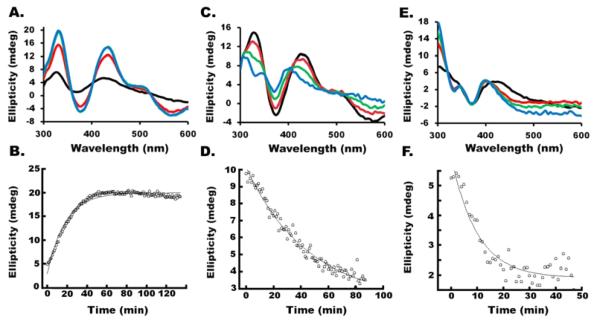
We explored whether the human SDUF complex is capable of building and transferring [2Fe-2S] clusters to apo-FDX1. Surprisingly, the Fe–S reaction under Acceptor conditions resulted in the development of the [2Fe-2S]-ISCU2 rather than the [2Fe-2S]-FDX1 CD signal (Figure 5A). The CD signal monitored at 330 nm developed slowly, and its intensity plateaued and did not decrease at longer time points (Figure 5B). The kinetic trace was fit as a pseudo-first-order process to an exponential association equation (rate constant of 0.055 min−1). Subsequent injection of DTT resulted in the development of the CD signal for the [2Fe-2S]-FDX1 species that coincided with the decrease in the intensity of the CD signal for [2Fe-2S]-ISCU2 (Figure 5C). The slow loss of intensity for the 330 nm CD peak was fit to an exponential decay process [rate constant of 0.022 min−1 (Figure 5D)]. As a control, we repeated the Fe–S assembly reaction under Acceptor conditions in the presence of DTT. In this case, the [2Fe-2S]-FDX1 signal appeared immediately and there was no evidence of a [2Fe-2S]-ISCU2 intermediate species (Figure 5E). The loss of the 330 nm signal was fit to an exponential decay [rate constant of 0.10 min−1 (Figure 5F)]. These results indicate DTT can mediate [2Fe-2S] cluster transfer chemistry from ISCU2 to apo-FDX1. In addition, the [2Fe-2S]-FDX1 signal developed faster for an SDUF sample, which has to both synthesize and transfer the cluster, than the transfer reaction from [2Fe-2S]-ISCU2 to apo-FDX1.
Figure 5.
Cluster conversion reactions for the SDUF complex monitored by CD spectroscopy. (A) The [2Fe-2S]-ISCU2 signal was generated for the SDUF sample under Acceptor conditions. Spectra were recorded at 0 (black), 20 (red), 40 (green), and 90 (blue) min. (B) Time-dependent changes in ellipticity at 330 nm for panel A. (C) DTT triggered the transfer of the [2Fe-2S] cluster from ISCU2 to FDX1. A sample prepared as described for panel A after reaching a plateau (at 90 min) was spiked with DTT. CD spectra were recorded at 0 (black), 20 (red), 40 (green), and 90 (blue) min. (D) Time-dependent changes in ellipticity at 330 nm for panel C. (E) A sample was prepared as described for panel A except that DTT was included at the start of the reaction. CD spectra were recorded at 0 (black), 10 (red), 20 (green), and 40 (blue) min. (F) Time-dependent changes in ellipticity at 330 nm for panel E. Exponential fits through the data (lines) were generated using apparent first-order rate constants of 0.055, 0.022, and 0.10 min−1 for panels B, D, and F, respectively.
DISCUSSION
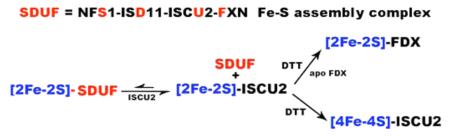
Mechanisms by which the prokaryotic Fe–S cluster bio-synthetic machinery assembles and transfers clusters to target proteins are poorly understood. Even less is known about eukaryotic systems. Here we explored reactions with the human Fe–S assembly complex in the presence and absence of an Fe–S cluster acceptor protein. Mössbauer spectroscopy coupled to LC–ICP-MS experiments indicates that Fe–S assembly reactions generate [2Fe-2S] clusters associated with uncomplexed ISCU2. CD experiments revealed a correlation between the [2Fe-2S] cluster signal intensity and the amount of excess ISCU2 in the reaction. Moreover, under Catalytic conditions, the amount of cluster formed was 10-fold higher than the concentration of SD. These results are consistent with a model in which a transitory [2Fe-2S] cluster intermediate is generated on the ISCU2 component of the SDUF complex (Figure 6, reaction A) and in which this species spontaneously generates an autonomous [2Fe-2S]-ISCU2 species (Figure 6, reaction B). The transitory nature of a [2Fe-2S]-SDUF species is consistent with it being an intermediate in the catalytic cycle. Regeneration of a cluster-free SDUF species is required to initiate another round of catalysis.
Figure 6.
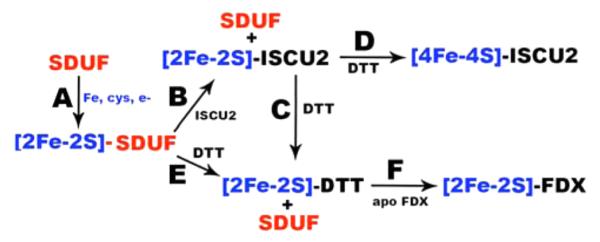
Model for human Fe–S cluster biosynthesis and cluster conversion reactions. (A) Synthesis of the [2Fe-2S] cluster intermediate on the SDUF complex. (B) Generation of [2Fe-2S]-ISCU2 species. (C) Ligand exchange reaction to generate [2Fe-2S]-DTT species. (D) DTT-mediated [2Fe-2S] to [4Fe-4S] cluster conversion. (E) Ligand substitution reaction with [2Fe-2S]-SDUF to form [2Fe-2S]-DTT species. (F) Cluster transfer to generate holo-FDX1. Protein complexes are colored red and iron-containing species blue. Reagents necessary for conversion chemistry are shown with arrows.
The mechanism by which [2Fe-2S]-SDUF generates [2Fe-2S]-ISCU2 is unclear. The [2Fe-2S]-ISCU2 component may simply dissociate from the SDUF complex. Consistent with this, the prokaryotic D39A IscU variant binds 20-fold weaker to IscS when it contains a cluster.44 In the human system, apo-ISCU2 binds tightly to the assembly complex and does not appreciably dissociate during size-exclusion chromatography.10,11 The impact of [2Fe-2S] cluster formation on the affinity of native ISCU2 for the human Fe–S assembly complex is not known. However, this subunit dissociation model does not explain the lack of a [2Fe-2S] cluster CD signal under Stoichiometric conditions.
A second [2Fe-2S]-ISCU2 formation model involves the direct or indirect transfer of the [2Fe-2S] cluster from SDUF to uncomplexed apo-ISCU2 through a ligand exchange process. The proposed [2Fe-2S]-SDUF intermediate is supported by a crystal structure of the prokaryotic IscS–IscU complex with a [2Fe-2S] cluster bound.35 Notably, this structure is of the IscU D35A variant, which appears to stabilize the cluster-bound state and/or inhibit Fe–S cluster transfer.45,46 Thus, the cluster may be significantly more exposed in the native complex than in the D35A variant crystal structure. This cluster transfer model implies that the [2Fe-2S] cluster is more stable when bound to uncomplexed ISCU2 than to the SDUF complex. An unstable [2Fe-2S]-SDUF species and a lack of excess (uncomplexed) apo-ISCU2 to function in a cluster transfer reaction might explain the absence of a CD signal under Stoichiometric conditions. The CD signal intensity might also be impacted by different [2Fe-2S] ligation properties when the cluster is bound to SDUF and uncomplexed ISCU2. The [2Fe-2S] cluster transfer model suggests the intriguing possibility that both the SDUF and uncomplexed ISCU2 may be capable of transferring clusters to acceptor molecules.
Next, we probed the ability of SDUF and ISCU2 to transfer clusters to the apo [2Fe-2S] cluster acceptor FDX1. Here we showed for the human system that [2Fe-2S] clusters are transferred from ISCU2 to apo-FDX1 only in the presence of DTT. We hypothesize that DTT mediates a substitution reaction and generates a [2Fe-2S]-DTT species (Figure 6, reaction C) that then transfers the cluster to apo-FDX1 (Figure 6, reaction F). The rate constant for this ISCU2 to FDX1 cluster transfer process indicates a reaction that is slower than the combined synthesis and cluster transfer reaction from SDUF to FDX1. Further, no evidence of a [2Fe-2S]-ISCU2 CD signal was observed for the SDUF reaction. Together, this indicates that the [2Fe-2S]-ISCU2 species is not an intermediate under these conditions. We therefore propose that the [2Fe-2S] cluster is transferred from SDUF to DTT (Figure 6, reaction E), followed by transfer from DTT to apo-FDX1 (Figure 6, reaction F). The ability of DTT to participate in a ligand exchange reaction is supported by similar reports of small molecule thiol reagents that are required for the removal47 or exchange48 of protein-bound [2Fe-2S] clusters. DTT may mimic a physiological Fe–S transfer agent such as a small molecule thiol (glutathione)39 or protein factors implicated as intermediate Fe–S carrier proteins (glutaredoxins).21,30 Importantly, DTT is regularly included in chaperone-mediated reaction mixtures, and it should be evaluated for a similar intermediate carrier role in those cluster transfer experiments. Our results are consistent with branched pathways from [2Fe-2S]-SDUF, one of which is mimicked by DTT (Figure 6, reaction E) and a second that generates [2Fe-2S]-ISCU2 (Figure 6, reaction B). In this model, uncomplexed ISCU2 may function as a semistable cluster storage species. Fe–S cluster transfer from uncomplexed ISCU2 would then proceed by well-established chaperone-mediated processes to intermediate carrier proteins or apo target proteins. Additional studies are required to evaluate conditions under which the SDUF complex can bypass [2Fe-2S]-ISCU2 and transfer clusters in a presumably chaperone-independent process (the chaperones compete with Nfs1 and Yfh1 binding to Isu1 in yeast).49,50 We disfavor but cannot rule out an alternate model to explain the kinetic data in which DTT facilitates a chemical reconstitution process that is faster than the transfer of the cluster from [2Fe-2S]-ISCU2 to apo-FDX1. Together, our data support the role of DTT in mediating transfer of the cluster from [2Fe-2S]-ISCU2, and possibly [2Fe-2S]-SDUF, to apo-FDX1.
Data presented here supporting a transitory [2Fe-2S]-SDUF intermediate model appear to be inconsistent with the proposal that the mammalian Fe–S assembly complex forms a stable [4Fe-4S] species.36 We find that reactions with the human SDUF complex can also generate [4Fe-4S] clusters. However, our LC–ICP-MS and Mössbauer results indicate these [4Fe-4S] clusters are associated with ISCU2 rather than the SDUF complex. The conversion of [2Fe-2S] to [4Fe-4S] clusters was incomplete and required the presence of DTT. Interestingly, the addition of DTT after in situ generation of [2Fe-2S]-ISCU2 resulted in the formation of [4Fe-4S]-ISCU2 (Figure 6, reaction D) or [2Fe-2S]-FDX1 (Figure 6, reactions C and F) depending on the absence or presence of apo-FDX1, respectively. In contrast, adding DTT upon initiation of the Fe–S assembly reaction resulted in HMWS or [2Fe-2S]-FDX1 in the absence or presence of apo-FDX1, respectively [see the following paper (DOI: 10.1021/bi5014497)]. Slow DTT-dependent conversion of [2Fe-2S] to [4Fe-4S] clusters has been previously observed in bacterial systems.51,52 Overall, the cluster conversion chemistry for the human system is consistent with reactions for uncomplexed prokaryotic IscU in which [2Fe-2S] clusters are reductively coupled to form [4Fe-4S] clusters.33
In summary, we have provided details of [2Fe-2S] cluster synthesis and transfer reactions for the human Fe–S assembly complex. Our data support the formation of a transitory [2Fe-2S]-SDUF intermediate in Fe–S cluster biosynthesis that readily generates [2Fe-2S]-ISCU2 species. Here we propose two possible mechanisms, subunit dissociation and cluster transfer, for the generation of the [2Fe-2S]-ISCU2 species. We also provide evidence of DTT-mediated cluster transfer and cluster conversion reactions. Additional studies are required to evaluate if these DTT-mediated processes mimic physiological reactions. Recently, in vitro kinetic studies monitored by CD spectroscopy were performed for the yeast Fe–S assembly system.53 As seen for the human system, the authors concluded [2Fe-2S]-Isu1 species are generated under catalytic conditions. Despite these similar conclusions, there are interesting differences in the human and yeast systems. First, the yeast [2Fe-2S]-Isu1, in contrast to human [2Fe-2S]-ISCU2, is stable in the presence of DTT. Second, the FXN homologue Yfh1 appears to be required for [2Fe-2S] cluster synthesis in yeast,53 whereas FXN accelerates but is not required for [2Fe-2S] cluster synthesis in humans (DOI: 10.1021/bi5014497). Next, these in vitro studies need to be expanded to evaluate complete cluster synthesis and transfer reactions that include components such as the chaperones, intermediate carrier proteins, and multiple Fe–S target proteins. Overall, these studies and the following paper reveal that Fe–S assembly and transfer reactions are complicated by competing mineralization and branched biosynthetic pathways, and they support a model in which [2Fe-2S] cluster intermediates are generated by the SDUF assembly complex that can be used to produce cluster-bound ISCU2 or FDX1.
ACKNOWLEDGMENTS
We thank Christopher D. Putnam, James Vranish, and Seth Cory for helpful discussion and suggestions.
Funding
This work was supported in part by Texas A&M University, Robert A. Welch Foundation Grant A-1647 (D.P.B.), and National Institutes of Health Grants GM096100 (D.P.B.) and GM084266 (P.A.L.).
ABBREVIATIONS
- CD
circular dichroism
- DTT
dithiothreitol
- FDX1
ferredoxin
- FXN
frataxin
- GSH
glutathione
- HMWS
high-molecular weight species
- IPTG
isopropyl β-d-1-thiogalactopyranoside
- ISC
iron–sulfur cluster
- LC–ICP-MS
liquid chromatography interfaced to inductively coupled plasma mass spectrometry
- LMWS
low-molecular weight species
- SD
protein complex composed of NFS1 and ISD11
- SDU
protein complex composed of SD and ISCU2
- SDUF
protein complex composed of SDU and frataxin
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Johnson DC, Dean DR, Smith AD, Johnson M. Structure, function, and formation of biological iron-sulfur clusters. Annu. Rev. Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- (2).Beinert H, Holm RH, Münck E. Iron-sulfur clusters: Nature’s modular, multipurpose structures. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- (3).Rao PV, Holm RH. Synthetic analogues of the active sites of iron-sulfur proteins. Chem. Rev. 2004;104:527–559. doi: 10.1021/cr020615+. [DOI] [PubMed] [Google Scholar]
- (4).Lill R. Function and biogenesis of iron-sulphur proteins. Nature. 2009;460:831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- (5).Rouault TA, Tong W-H. Iron-sulfur cluster biogenesis and human disease. Trends Genet. 2008;24:398–407. doi: 10.1016/j.tig.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Pierik AJ, Netz DJA, Lill R. Analysis of iron-sulfur protein maturation in eukaryotes. Nat. Protoc. 2009;4:753–766. doi: 10.1038/nprot.2009.39. [DOI] [PubMed] [Google Scholar]
- (7).Lill R, Mühlenhoff U. Maturation of Iron-Sulfur Proteins in Eukaryotes: Mechanisms, Connected Processes, and Diseases. Annu. Rev. Biochem. 2008;77:22–21. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- (8).Sheftel AD, Lill R. The power plant of the cell is also a smithy: The emerging role of mitochondria in cellular iron homeostasis. Ann. Med. 2009;41:82–99. doi: 10.1080/07853890802322229. [DOI] [PubMed] [Google Scholar]
- (9).Lill R, Mühlenhoff U. Iron-sulfur protein biogenesis in eukaryotes: Components and mechanisms. Annu. Rev. Cell Dev. Biol. 2006;22:457–486. doi: 10.1146/annurev.cellbio.22.010305.104538. [DOI] [PubMed] [Google Scholar]
- (10).Tsai CL, Barondeau DP. Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry. 2010;49:9132–9139. doi: 10.1021/bi1013062. [DOI] [PubMed] [Google Scholar]
- (11).Schmucker S, Martelli A, Colin F, Page A, Wattenhofer-Donzé M, Reutenauer L, Puccio H. Mammalian Frataxin: An Essential Function for Cellular Viability through an Interaction with a Preformed ISCU/NFS1/ISD11 Iron-Sulfur Assembly Complex. PLoS One. 2011;6:e16199. doi: 10.1371/journal.pone.0016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Zheng L, Cash VL, Flint DH, Dean DR. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J. Biol. Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- (13).Zheng L, White RH, Cash VL, Dean DR. Mechanism for the desulfurization of l-cysteine catalyzed by the nifS gene product. Biochemistry. 1994;33:4714–4720. doi: 10.1021/bi00181a031. [DOI] [PubMed] [Google Scholar]
- (14).Adam AC, Bornhovd C, Prokisch H, Neupert W, Hell K. The Nfs1 interacting protein Isd11 has an essential role in Fe/S cluster biogenesis in mitochondria. EMBO J. 2006;25:174–183. doi: 10.1038/sj.emboj.7600905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wiedemann N, Urzica E, Guiard B, Muller H, Lohaus C, Meyer HE, Ryan MT, Meisinger C, Mühlenhoff U, Lill R, Pfanner N. Essential role of Isd11 in mitochondrial iron-sulfur cluster synthesis on Isu scaffold proteins. EMBO J. 2006;25:184–195. doi: 10.1038/sj.emboj.7600906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Pandey A, Golla R, Yoon H, Dancis A, Pain D. Persulfide formation on mitochondrial cysteine desulfurase: Enzyme activation by a eukaryote-specific interacting protein and Fe-S cluster synthesis. Biochem. J. 2012;448:171–187. doi: 10.1042/BJ20120951. [DOI] [PubMed] [Google Scholar]
- (17).Smith AD, Agar J, Johnson KA, Frazzon J, Amster IJ, Dean DR, Johnson M. Sulfur transfer from IscS to IscU: The first step in iron-sulfur cluster biosynthesis. J. Am. Chem. Soc. 2001;123:11103–11104. doi: 10.1021/ja016757n. [DOI] [PubMed] [Google Scholar]
- (18).Bridwell-Rabb J, Fox NG, Tsai CL, Winn AM, Barondeau DP. Human frataxin activates Fe-S cluster biosynthesis by facilitating sulfur transfer chemistry. Biochemistry. 2014;53:4904–4913. doi: 10.1021/bi500532e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Shi Y, Ghosh M, Kovtunovych G, Crooks DR, Rouault TA. Both human ferredoxins 1 and 2 and ferredoxin reductase are important for iron-sulfur cluster biogenesis. Biochim. Biophys. Acta. 2012;1823:484–492. doi: 10.1016/j.bbamcr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Sheftel AD, Stehling O, Pierik AJ, Elsasser H-P, Mühlenhoff U, Webert H, Hobler A, Hannemann F, Bernhardt R, Lill R. Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 2010;107:11775–11780. doi: 10.1073/pnas.1004250107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Uzarska MA, Dutkiewicz R, Freibert SA, Lill R, Muehlenhoff U. The mitochondrial Hsp70 chaperone Ssq1 facilitates Fe/S cluster transfer from Isu1 to Grx5 by complex formation. Mol. Biol. Cell. 2013;24:1830–1841. doi: 10.1091/mbc.E12-09-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Vickery L, Cupp-Vickery J. Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation. Crit. Rev. Biochem. Mol. Biol. 2007;42:95–111. doi: 10.1080/10409230701322298. [DOI] [PubMed] [Google Scholar]
- (23).Maio N, Singh A, Uhrigshardt H, Saxena N, Tong W-H, Rouault TA. Cochaperone binding to LYR motifs confers specificity of iron sulfur cluster delivery. Cell Metab. 2014;19:445–457. doi: 10.1016/j.cmet.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Chandramouli K, Johnson M. HscA and HscB stimulate [2Fe-2S] cluster transfer from IscU to apoferredoxin in an ATP-dependent reaction. Biochemistry. 2006;45:11087–11095. doi: 10.1021/bi061237w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Bonomi F, Iametti S, Morleo A, Ta D, Vickery LE. Facilitated Transfer of IscU-[2Fe2S] Clusters by Chaperone-Mediated Ligand Exchange. Biochemistry. 2011;50:9641–9650. doi: 10.1021/bi201123z. [DOI] [PubMed] [Google Scholar]
- (26).Py B, Gerez C, Angelini S, Planel R, Vinella D, Loiseau L, Talla E, Brochier-Armanet C, Garcia-Serres R, Latour J-M, Ollagnier de Choudens S, Fontecave M, Barras F. Molecular organization, biochemical function, cellular role and evolution of NfuA, an atypical Fe-S carrier. Mol. Microbiol. 2012;86:155–171. doi: 10.1111/j.1365-2958.2012.08181.x. [DOI] [PubMed] [Google Scholar]
- (27).Tong W-H, Jameson GNL, Huynh BH, Rouault TA. Subcellular compartmentalization of human Nfu, an iron-sulfur cluster scaffold protein, and its ability to assemble a [4Fe-4S] cluster. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9762–9767. doi: 10.1073/pnas.1732541100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Bandyopadhyay S, Naik S, O’Carroll I, Huynh BH, Dean DR, Johnson M, Dos Santos P. A proposed role for the Azotobacter vinelandii NfuA protein as an intermediate iron-sulfur cluster carrier. J. Biol. Chem. 2008;283:14092–14099. doi: 10.1074/jbc.M709161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Mapolelo DT, Zhang B, Randeniya S, Albetel A-N, Li H, Couturier J, Outten CE, Rouhier N, Johnson MK. Monothiol glutaredoxins and A-type proteins: Partners in Fe-S cluster trafficking. Dalton Trans. 2013;42:3107–3115. doi: 10.1039/c2dt32263c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Shakamuri P, Zhang B, Johnson MK. Monothiol Glutaredoxins Function in Storing and Transporting [Fe2S2] Clusters Assembled on IscU Scaffold Proteins. J. Am. Chem. Soc. 2012;134:15213–15216. doi: 10.1021/ja306061x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Gupta V, Sendra M, Naik SG, Chahal HK, Huynh BH, Outten FW, Fontecave M, Ollagnier de Choudens S. Native Escherichia coli SufA, coexpressed with SufBCDSE, purifies as a [2Fe-2S] protein and acts as an Fe-S transporter to Fe-S target enzymes. J. Am. Chem. Soc. 2009;131:6149–6153. doi: 10.1021/ja807551e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Sheftel AD, Stehling O, Pierik AJ, Netz DJ, Kerscher S, Elsasser HP, Wittig I, Balk J, Brandt U, Lill R. Human ind1, an iron-sulfur cluster assembly factor for respiratory complex I. Mol. Cell. Biol. 2009;29:6059–6073. doi: 10.1128/MCB.00817-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Agar JN, Krebs C, Frazzon J, Huynh BH, Dean DR, Johnson MK. IscU as a scaffold for iron-sulfur cluster biosynthesis: Sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry. 2000;39:7856–7862. doi: 10.1021/bi000931n. [DOI] [PubMed] [Google Scholar]
- (34).Zhang B, Crack JC, Subramanian S, Green J, Thomson AJ, Le Brun NE, Johnson MK. Reversible cycling between cysteine persulfide-ligated [2Fe-2S] and cysteine-ligated [4Fe-4S] clusters in the FNR regulatory protein. Proc. Natl. Acad. Sci. U.S.A. 2012;109:15734–15739. doi: 10.1073/pnas.1208787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Marinoni EN, de Oliveira JS, Nicolet Y, Raulfs EC, Amara P, Dean DR, Fontecilla-Camps JC. IscS-IscU)2 complex structures provide insights into Fe2S2 biogenesis and transfer. Angew. Chem., Int. Ed. 2012;51:5439–5442. doi: 10.1002/anie.201201708. [DOI] [PubMed] [Google Scholar]
- (36).Colin F, Martelli A, Clemancey M, Latour JM, Gambarelli S, Zeppieri L, Birck C, Page A, Puccio H, Ollagnier de Choudens S. Mammalian Frataxin Controls Sulfur Production and Iron Entry during de Novo Fe4S4 Cluster Assembly. J. Am. Chem. Soc. 2013;135:733–740. doi: 10.1021/ja308736e. [DOI] [PubMed] [Google Scholar]
- (37).Xia B, Cheng H, Bandarian V, Reed GH, Markley JL. Human ferredoxin: Overproduction in Escherichia coli, reconstitution in vitro, and spectroscopic studies of iron-sulfur cluster ligand cysteine-to-serine mutants. Biochemistry. 1996;35:9488–9495. doi: 10.1021/bi960467f. [DOI] [PubMed] [Google Scholar]
- (38).Pagani S, Bonomi F, Cerletti P. Enzymic synthesis of the iron-sulfur cluster of spinach ferredoxin. Eur. J. Biochem. 1984;142:361–366. doi: 10.1111/j.1432-1033.1984.tb08295.x. [DOI] [PubMed] [Google Scholar]
- (39).Qi W, Li J, Chain CY, Pasquevich GA, Pasquevich AF, Cowan JA. Glutathione complexed Fe-S centers. J. Am. Chem. Soc. 2012;134:10745–10748. doi: 10.1021/ja302186j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Ryle MJ, Lanzilotta WN, Seefeldt LC, Scarrow RC, Jensen GM. Circular dichroism and X-ray spectroscopies of Azotobacter vinelandii nitrogenase iron protein. MgATP and MgADP induced protein conformational changes affecting the [4Fe-4S] cluster and characterization of a [2Fe-2S] form. J. Biol. Chem. 1996;271:1551–1557. doi: 10.1074/jbc.271.3.1551. [DOI] [PubMed] [Google Scholar]
- (41).Mansy SS, Wu G, Surerus KK, Cowan JA. Iron-sulfur cluster biosynthesis. Thermatoga maritima IscU is a structured iron-sulfur cluster assembly protein. J. Biol. Chem. 2002;277:21397–21404. doi: 10.1074/jbc.M201439200. [DOI] [PubMed] [Google Scholar]
- (42).Urbina HD, Silberg JJ, Hoff KG, Vickery L. Transfer of sulfur from IscS to IscU during Fe/S cluster assembly. J. Biol. Chem. 2001;276:44521–44526. doi: 10.1074/jbc.M106907200. [DOI] [PubMed] [Google Scholar]
- (43).Yoon T, Cowan JA. Iron-sulfur cluster biosynthesis. Characterization of frataxin as an iron donor for assembly of [2Fe-2S] clusters in ISU-type proteins. J. Am. Chem. Soc. 2003;125:6078–6084. doi: 10.1021/ja027967i. [DOI] [PubMed] [Google Scholar]
- (44).Prischi F, Konarev PV, Iannuzzi C, Pastore C, Adinolfi S, Martin SR, Svergun DI, Pastore A. Structural bases for the interaction of frataxin with the central components of iron-sulphur cluster assembly. Nat. Commun. 2010;1:95. doi: 10.1038/ncomms1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Shimomura Y, Kamikubo H, Nishi Y, Masako T, Kataoka M, Kobayashi Y, Fukuyama K, Takahashi Y. Characterization and crystallization of an IscU-type scaffold protein with bound [2Fe-2S] cluster from the hyperthermophile, Aquifex aeolicus. J. Biochem. 2007;142:577–586. doi: 10.1093/jb/mvm163. [DOI] [PubMed] [Google Scholar]
- (46).Wu G, Mansy SS, Wu S.-p., Surerus KK, Foster MW, Cowan JA. Characterization of an iron-sulfur cluster assembly protein (ISU1) from Schizosaccharomyces pombe. Biochemistry. 2002;41:5024–5032. doi: 10.1021/bi016073s. [DOI] [PubMed] [Google Scholar]
- (47).Que L, Holm RH, Mortenson LE. Extrusion of Fe2S2 and Fe4S4 Cores from Active-Sites of Ferredoxin Proteins. J. Am. Chem. Soc. 1975;97:463–464. doi: 10.1021/ja00835a064. [DOI] [PubMed] [Google Scholar]
- (48).Vranish JN, Russell WK, Yu LE, Cox RM, Russell DH, Barondeau DP. Fluorescent probes for tracking the transfer of iron-sulfur cluster and other metal cofactors in biosynthetic reaction pathways. J. Am. Chem. Soc. 2015;137:390–398. doi: 10.1021/ja510998s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Manicki M, Majewska J, Ciesielski S, Schilke B, Blenska A, Kominek J, Marszalek J, Craig EA, Dutkiewicz R. Overlapping binding sites of the frataxin homologue assembly factor and the heat shock protein 70 transfer factor on the Isu iron-sulfur cluster scaffold protein. J. Biol. Chem. 2014;289:30268–30278. doi: 10.1074/jbc.M114.596726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Majewska J, Ciesielski SJ, Schilke B, Kominek J, Blenska A, Delewski W, Song JY, Marszalek J, Craig EA, Dutkiewicz R. Binding of the chaperone Jac1 protein and cysteine desulfurase Nfs1 to the iron-sulfur cluster scaffold Isu protein is mutually exclusive. J. Biol. Chem. 2013;288:29134–29142. doi: 10.1074/jbc.M113.503524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Mapolelo DT, Zhang B, Naik SG, Huynh BH, Johnson MK. Spectroscopic and functional characterization of iron-sulfur cluster-bound forms of Azotobacter vinelandii (Nif)IscA. Biochemistry. 2012;51:8071–8084. doi: 10.1021/bi3006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Chandramouli K, Unciuleac M-C, Naik S, Dean DR, Huynh BH, Johnson M. Formation and properties of [4Fe-4S] clusters on the IscU scaffold protein. Biochemistry. 2007;46:6804–6811. doi: 10.1021/bi6026659. [DOI] [PubMed] [Google Scholar]
- (53).Webert H, Freibert SA, Gallo A, Heidenreich T, Linne U, Amlacher S, Hurt E, Muhlenhoff U, Banci L, Lill R. Functional reconstitution of mitochondrial Fe/S cluster synthesis on Isu1 reveals the involvement of ferredoxin. Nat. Commun. 2014;5:5013. doi: 10.1038/ncomms6013. [DOI] [PubMed] [Google Scholar]