Abstract
Background
HIV infection among persons who inject drugs (PWID) is a major international public health concern. Thus, the need to identify novel protective factors is of utmost importance. We therefore evaluated the impact of methadone maintenance therapy on HIV incidence among PWID in Vancouver, Canada.
Methods
Data were derived from a prospective cohort of PWID in Vancouver, Canada where methadone is widely available through family physician’s offices and dispensed by community pharmacies. We examined the role of methadone maintenance treatment on time to HIV incidence while adjusting for potential confounders.
Findings
Overall, 1639 HIV-negative individuals were recruited between May 1996 and May 2013 among whom there were 138 cases of HIV seroconversion during a median of 75·5 (interquartile range: 33·4 – 115·3) months of follow up. In multivariate Cox regression analyses, methadone maintenance therapy (adjusted relative hazard: 0·64 [95% confidence interval: 0·41 – 0·98]) remained independently associated with a reduced hazard of HIV infection after adjusting for socio-demographic characteristics and drug use patterns.
Interpretation
In this setting, where a low threshold program has made methadone widely available through primary care physicians, the use of methadone was independently associated with a reduced rate of HIV infection. These data reinforce the benefits of low threshold methadone on public health goals such as reducing the spread of HIV.
Funding
US National Institutes of Health, Canada Research Chair, Canadian Institutes of Health Research. The funder played no role in conducting the study, preparing the results, or the decision to submit for publication.
INTRODUCTION
HIV infection amongst people who inject drugs (PWID) is a major international public health concern. Injecting drugs is now responsible for greater than 30% of new HIV infections outside of sub-Saharan Africa, and it is estimated that three million PWID worldwide are HIV positive.1,2 In response to the pandemic, in 2011 the United Nations set a goal to reduce transmission of HIV amongst PWID by 50% by 2015.3 However, in June 2014, the United Nations Office on Drugs and Crime reported that HIV epidemics amongst PWID still remained a critical issue.4 The situation is particularly dire in SouthWest Asia and Eastern Europe where HIV incidence, primarily as a result of sharing of injection equipment among heroin injectors, remains among the highest in the world.4
In this context, there is a large body of evidence to support using methadone as a treatment for opioid use disorder and for improving outcomes related to HIV.5 Despite this evidence, barriers to methadone availability continue in many parts of the world, particularly in settings hardest hit by HIV transmission related to heroin use. In Russia, for example, where in some cities HIV prevalence among PWID is greater than 50%,6–8 a federal law prohibits the use of methadone for the treatment of opioid use disorder.9,10 Even in the United States, there is limited availability of methadone primarily due to programmatic restrictions that limit methadone availability to specialized clinics rather than through lower threshold strategies involving primary care physicians.11,12
In Canada, methadone prescribing and dispensation was largely deregulated in 1996.13 At that time, in British Columbia, there were 2,827 individuals enrolled in the methadone program and by the end of 2009, participant enrollment had increased to 11,033.14 Methadone is now widely available for the treatment of opioid use disorder and can be prescribed by primary care physicians and is dispensed daily for witnessed ingestion through community pharmacies rather than dedicated methadone programs.13,15 Once a period of stability is obtained, take-home dosing is available to patients. While methadone has been shown to reduce HIV risk in several settings,16–18 its benefits when provided through low threshold models has not been well studied. Therefore, the present study was undertaken to examine the effect of methadone maintenance therapy on HIV incidence in a Canadian setting with low threshold availability of methadone.
METHODS
Date sources
Our study was performed using data from the Vancouver Injection Drug Users Study (VIDUS), a longstanding prospective cohort of PWID in Vancouver, Canada, starting in 1996.19 Briefly, individuals were eligible to enroll in VIDUS if they had injected illicit drugs at least once in the previous month and resided in the Greater Vancouver region at enrollment. Participants responded to an interviewer-administered questionnaire and provided blood samples at enrollment and semi-annual follow-up visits. The cohort receives annual approval from the University of British Columbia/Providence Health Care Research Ethics Board.
For the purpose of our study, we defined “low threshold” methadone as methadone prescribed through primary care physicians’ offices and dispensed at community pharmacies rather than prescribed and dispensed at specialty clinics. We did not include various different program restrictions like take-home dosing, required counseling, or frequency of urine drug screening though details of the local guidelines are available.20
Statistical analysis
The present study was restricted to those PWID who were HIV negative at study recruitment and completed at least one follow-up interview between May 1996 and May 2013. Our primary outcome of interest was time to HIV seroconversion, defined as the time interval between recruitment into the cohort and estimated date of HIV seroconversion/infection. Date of HIV infection was estimated as the midpoint between the last HIV negative test and the first HIV positive test.21–23 HIV tests, initially reactive on enzyme-linked immunosorbent assay, were confirmed by Western Blot. Our primary explanatory variable of interest was use of methadone maintenance therapy (MMT) in the previous six months. Here, the methadone use variable was treated as a time-updated covariate based on data collected at each semi-annual follow up visit allowing for individuals to contribute follow up when both on or off of methadone. Individuals were defined as using methadone at each follow up if they had been prescribed and taken methadone at any time in each semi-annual period. We also considered secondary explanatory variables that might potentially confound the relationship between the primary explanatory variable and the outcome. These included gender (male vs. female); age (in years); ethnicity (Caucasian vs. other); incarceration defined as being in detention, prison or jail for overnight or longer (yes vs. no); sex work involvement defined as exchanging sex for money, food, drugs, shelter or other commodities (yes vs. no); at least daily injection heroin use (yes vs. no); at least daily injection cocaine use (yes vs. no); syringe borrowing (yes vs. no); required help injecting (yes vs. no); at least daily crack cocaine smoking (yes vs. no); and unprotected vaginal/anal sex (yes vs. no). Unless specified, all behavioural variables refer to activities taking place within the previous six months.
Initially, we calculated the incidence density rate of HIV infection using person-time methods. Then, baseline demographics and behaviours between baseline MMT users and non-MMT users were compared using Pearson’s chi-squared test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Subsequently, we stratified by methadone use in the previous six months at baseline and estimated the cumulative incidence probabilities of time to HIV infection using Kaplan-Meier methods. The cumulative incidence curves were compared via the log-rank test.
Next, we used bivariate and multivariate extended Cox regression analyses to assess the independent association between methadone use and time to HIV seroconversion. Here, potential confounders were first examined in the bivariate Cox regression analyses. To construct a multivariate model, we employed a conservative variable selection approach.24 Specifically, we first fit a full model with all potential confounders that were associated with time to HIV infection at p<0·05 in the bivariate analyses. Then, we used a stepwise approach to fit a series of reduced models, dropping secondary explanatory variables (i.e. non-methadone use) with less relative influence on the relationship between methadone use and time to HIV seroconversion. The final model was selected when the minimum relative change exceeded 5%. The remaining secondary variables were considered confounders of the association between methadone use and time to HIV seroconversion in the final multivariate analysis.24 To control for severity of opioid use disorder, we a priori elected to include daily heroin use in the multivariate analysis, though it was not necessary to force its inclusion in the final model as it met the above criteria. All statistical analyses were performed using the SAS software version 9·3 (SAS, Cary, NC, USA). All p-values are two sided.
Role of the funding source
The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Of the 1,879 individuals who were HIV negative at baseline, 1,639 (87%) had returned for at least one follow up visit to assess for HIV infection and were therefore eligible for the present study. In comparison to those HIV negative individuals who did not return for follow up, those included in the study sample were more likely to be older (median age: 36 years vs. 32 years, p<0·001) and less likely to engage in unprotected sex (37% vs. 45%, p=0·025) at baseline. There were no differences in methadone use at baseline.
Of the 1,639 eligible participants, 1,093 (67%) were male and the median age at baseline was 36 (interquartile range [IQR]: 28 – 42) years. During the study period, participants were followed up for a median of 75·5 (IQR: 33·4 – 115·3) months. Of the 1,639 participants, 138 cases of HIV seroconversion were reported during follow-up, resulting in an incidence density rate of 1·21 (95% confidence interval [CI]: 1·02 – 1·44) cases per 100 person-years. Stratified by methadone use and socio-demographic variables, the incidence density rates of HIV infection were as follows: 0·39 (95% CI: 0·19 – 0·78) cases per 100 person-years among participants on methadone at baseline, compared to 1·39 (95% CI: 1·16 – 1·67) cases per 100 person-years among those not on methadone at baseline; 0·98 (95% CI: 0·77 – 1·24) cases per 100 person-years among male participants, compared to 1·64 (95% CI: 1·27 – 2·11) cases per 100 person-years among female participants; and 0·98 (95% CI: 0·77 – 1·25) cases per 100 person-years among Caucasian participants, compared to 1·56 (95% CI: 1·22 – 2·00) cases per 100 person-years among non-Caucasian participants.
Among the study sample at baseline, 330 (20%) participants had been on MMT in the previous six months. Of the 330 participants, the median methadone dose reported at the baseline interview date was 80 (IQR: 55 – 100) mg. As shown in Table 1, at baseline, those participants taking methadone were more likely to be older (median age: 41 years vs. 35 years, p<0·0001); of Caucasian ethnicity (82% vs. 57%, p<0·0001); and smoke crack cocaine at least daily (31% vs. 22%, p=0·0007). Furthermore, at baseline, participants on MMT were less likely to be involved in sex work (18% vs. 24%, p=0·015); inject heroin at least daily (32% vs. 47%, p<0·0001); inject cocaine at least daily (16% vs. 34%, p<0·0001); borrow a used syringe (22% vs. 31%, p=0·003); require help injecting (29% vs. 38%, p=0·006); and engage in unprotected sex (27% vs. 40%, p<0·0001).
Table 1.
Baseline characteristics of VIDUS participants, stratified by participation in methadone maintenance therapy (MMT) in the previous six months, in Vancouver, Canada, 1996–2013.
| Characteristic | No MMT n = 1309 |
MMT n = 330 |
Odds Ratio (95% CI) | p-value |
|---|---|---|---|---|
| Gender | ||||
| Female | 429 (32·8) | 117 (35·5) | ||
| Male | 880 (67·2) | 213 (64·5) | 0·89 (0·69 – 1·14) | 0·356 |
| Age† | ||||
| Median (IQR) | 35 (26 – 41) | 41 (36 – 45) | 1·98 (1·73 – 2·27) | <0·0001 |
| Caucasian ethnicity | ||||
| Yes | 750 (57·3) | 271 (82·1) | ||
| No | 559 (42·7) | 59 (17·9) | 3·42 (2·53 – 4·63) | <0·0001 |
| Incarceration* | ||||
| Yes | 183 (14·0) | 38 (11·5) | ||
| No | 1126 (86·0) | 292 (88·5) | 0·80 (0·55 – 1·16) | 0·241 |
| Sex work involvement* | ||||
| Yes | 312 (23·8) | 58 (17·6) | ||
| No | 997 (76·2) | 272 (82·4) | 0·68 (0·50 – 0·93) | 0·015 |
| Heroin injection* | ||||
| <Daily | 693 (52·9) | 226 (68·5) | ||
| ≥Daily | 616 (47·1) | 104 (31·5) | 0·52 (0·40 – 0·67) | <0·0001 |
| Cocaine injection* | ||||
| <Daily | 863 (65·9) | 276 (83·6) | ||
| ≥Daily | 446 (34·1) | 54 (16·4) | 0·38 (0·28 – 0·52) | <0·0001 |
| Syringe borrowing* | ||||
| Yes | 399 (30·5) | 73 (22·1) | ||
| No | 910 (69·5) | 257 (77·9) | 0·65 (0·49 – 0·86) | 0·003 |
| Require help injecting* | ||||
| Yes | 491 (37·5) | 97 (29·4) | ||
| No | 818 (62·5) | 233 (70·6) | 0·69 (0·53 – 0·90) | 0·006 |
| Crack smoking* | ||||
| <Daily | 1021 (78·0) | 228 (69·1) | ||
| ≥Daily | 288 (22·0) | 102 (30·9) | 1·59 (1·21 – 2·07) | 0·0007 |
| Unprotected sex* | ||||
| Yes | 526 (40·2) | 88 (26·7) | ||
| No | 783 (59·8) | 242 (73·3) | 0·54 (0·41 – 0·71) | <0·0001 |
Note: VIDUS = Vancouver Injection Drug Users Study; CI = confidence interval; IQR = interquartile range.
Indicates behavior during the six-month period prior to the baseline interview.
Odds ratio based on 10-year increase.
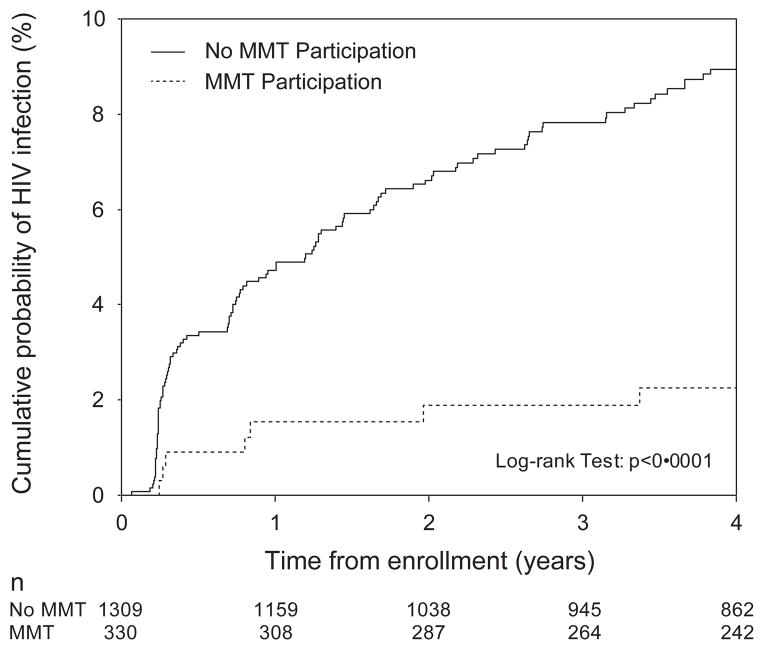
Participants who had been on MMT at baseline were followed up for a median of 74·4 (IQR: 46·5 – 83·7) months while those who were not on MMT at baseline had been followed up for a median of 76·2 (IQR: 30·2 – 128·3) months. As shown in Figure 1, after four years of follow up, the Kaplan-Meier cumulative HIV incidence of participants who used methadone at baseline was 2·3%, compared to the cumulative incidence of 8·9% among those not on methadone at baseline (log-rank p<0·0001). Among those ever on methadone during the study period, the median year of recruitment was 1999 (IQR: 1996 – 2006) whereas it was 1997 (IQR: 1996 – 2003) for those who never used methadone.
Figure 1.
Cumulative HIV incidence, stratified by participation in methadone maintenance therapy (MMT) in the last 6 months at baseline, in Vancouver, Canada, 1996–2013.
The bivariate and multivariate Cox regression results are shown in Table 2. In the bivariate analyses, participation in MMT was negatively associated with time to HIV infection (relative hazard [RH]: 0·50 [95% CI: 0·33 – 0·76]). In the multivariate analysis, adjusting for male gender, Caucasian ethnicity, daily cocaine injection and daily heroin injection, MMT remained independently associated with a lower hazard of HIV infection (adjusted relative hazard [ARH]: 0·64 [95% CI: 0·41 – 0·98]).
Table 2.
Bivariate and multivariate Cox regression analyses of the time to HIV infection among 1,639 PWID in Vancouver, Canada, 1996–2013.
| Unadjusted Relative Hazard (RH)
|
Adjusted Relative Hazard (RH)*
|
|||||
|---|---|---|---|---|---|---|
| Variable | RH | (95% CI) | p-value | RH | (95% CI) | p-value |
| Primary Variable of Interest: | ||||||
| Methadone maintenance therapy* | ||||||
| (Yes vs. No) | 0·50 | (0·33 – 0·76) | 0·0011 | 0·64 | (0·41 – 0·98) | 0·038 |
| Secondary Variables: | ||||||
| Age | ||||||
| (Per 10-year older) | 0·71 | (0·61 – 0·83) | <0·0001 | |||
| Caucasian ethnicity | ||||||
| (Yes vs. No) | 0·59 | (0·42 – 0·82) | 0·002 | 0·71 | (0·50 – 1·00) | 0·050 |
| Male gender | ||||||
| (Yes vs. No) | 0·57 | (0·41 – 0·80) | 0·0011 | 0·64 | (0·46 – 0·88) | 0·007 |
| Incarceration* | ||||||
| (Yes vs. No) | 1·64 | (1·14 – 2·34) | 0·007 | |||
| Sex work involvement* | ||||||
| (Yes vs. No) | 1·68 | (1·13 – 2·51) | 0·011 | |||
| Unprotected sex* | ||||||
| (Yes vs. No) | 1·21 | (0·85 – 1·74) | 0·292 | |||
| Syringe borrowing* | ||||||
| (Yes vs. No) | 1·99 | (1·38 – 2·87) | 0·0002 | |||
| Require help injecting* | ||||||
| (Yes vs. No) | 2·29 | (1·60 – 3·28) | <0·0001 | |||
| Crack smoking* | ||||||
| (≥Daily vs. <Daily) | 1·10 | (0·76 – 1·59) | 0·610 | |||
| Cocaine injection* | ||||||
| (≥Daily vs. <Daily) | 4·31 | (3·04 – 6·12) | <0·0001 | 3·76 | (2·60 – 5·45) | <0·0001 |
| Heroin injection* | ||||||
| (≥Daily vs. <Daily) | 1·90 | (1·35 – 2·69) | 0·0003 | 1·35 | (0·95 – 1·92) | 0·094 |
Note: PWID = persons who inject drugs; CI = confidence interval.
Behaviors refer to activities in the last six months. *Please see methods section for description of variable selection procedure.
When we examined if the cumulative number of semi-annual periods reporting methadone was associated with HIV incidence, and adjusted for the same confounders shown in Table 2, we found that longer duration of methadone exposure was marginally associated with reduced HIV infection (ARH: 0·92 [95% CI: 0·84 – 1·01] per additional semi-annual period reporting methadone, p=0·089).
DISCUSSION
In this setting, where methadone is available through primary care physicians and dispensed by community pharmacies, the use of methadone was independently associated with a reduced rate of HIV infection after adjusting for potential confounders including demographic and drug using characteristics.
The use of methadone has been associated with a range of benefits. These include decreased involvement in criminal activity,25 improved HIV treatment outcomes,5 retention in treatment 26 and increased quality of life.27 Despite this evidence, there has been longstanding debate around the safety and efficacy of methadone provision through low threshold settings where primary care physicians make methadone available. In France, the Methadone Induction in Primary Care for Opioid Dependence study randomized opioid use disorder participants to methadone induction at a primary care clinic or at a specialty clinic.28 They found that individuals induced onto methadone at primary care clinics were more likely to accept treatment, have greater satisfaction with no difference in treatment retention. The German ‘COBRA’ study compared individuals enrolled in large specialized centres to those enrolled in primary care settings and found significantly higher reduction in criminal behaviour and health risk behavior among those in primary care.29 Similarly, many other studies have reinforced that decreasing barriers to methadone by providing access through primary care physicians is safe, equally effective and increases access to an essential medical therapy.30–32 These findings are of particular importance in settings where HIV related to drug use remains high.
This study is limited by its observational design and reliance on self-reporting for several measures. Of note, our primary endpoint was based on objective laboratory evidence of HIV seroconversion. In addition, self-reported data has been previously used to control for potential confounding in observational studies involving PWID and was found to be valid.33 Ideally, our study would have not relied on methadone use by self-report. Unfortunately, urine methadone or other measures were not available in the present study and we note that self reported data are generally felt to be valid in this context.34 Since this is a non-randomized study, we must accept the possibility of residual confounding rather than a causal association between methadone maintenance and reduced HIV infection. While we sought to address this bias through multivariate adjustment of key demographic and behavioral predictors of HIV infection, there may be other unmeasured confounders. Unfortunately, past studies have demonstrated that our study was unable to undertake a thorough measurement of needle exchange use.35 To address this, we adjusted for syringe borrowing, which is known to be prevented through needle and syringe programs.36 We also note that our study was not recruited from within a methadone clinic where the rate of active intravenous drug use would be expected to be lower. Specifically, since the eligibility for the study was that participants had to be injecting drugs in the month prior to enrolment, and since recruitment was undertaken on the street, the rate of injecting and likely HIV incidence was higher than what might be anticipated in a clinic setting. A randomized controlled trial would be a better way to assess the impact of methadone maintenance on HIV. However, such trial in this instance would be unethical due to the known benefits of methadone.
In summary, in this setting, where methadone is available through primary care physicians and dispensed by community pharmacies, the use of methadone was independently associated with a reduced rate of HIV infection. While these data are limited by their observational nature, the findings reinforce the benefits of low threshold methadone on public health goals like reducing the spread of HIV.
Acknowledgments
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff. The study was supported by the US National Institutes of Health (R25DA037756 and VIDUS: R01DA011591) and the Canadian Institutes of Health Research through the Canadian Research Initiative on Substance Misuse (FMN-139148). This research was undertaken, in part, thanks to funding for a Tier 1 Canada Research Chair in Inner City Medicine, which supports Dr. Evan Wood. Dr. Kanna Hayashi is supported by the Canadian Institutes of Health Research. Dr. Julio Montaner is supported with grants paid to his institution by the British Columbia Ministry of Health and by the US National Institutes of Health (R01DA036307).
Footnotes
Contributors:
Keith Ahamad and Evan Wood designed and prepared the first draft of the manuscript. Paul Nguyen conducted the statistical analyses. All coauthors contributed to the drafting of the final manuscript.
Declaration of Interests:
JSGM has received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. VDL has received limited unrestricted funding, paid to her institution, from GlaxoSmithKline. KA has received limited unrestricted funding, paid to his institution, by Applied Strategic. All other authors declare no competing interests.
References
- 1.Joint UN Programme on HIV/AIDS. Report on the global AIDS epidemic. Geneva: 2013. [Google Scholar]
- 2.Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. The Lancet. 2008;372(9651):1733–45. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 3.Joint UN Programme on HIV/AIDS. [accessed November 3 2014];UNAIDS Strategy 2011–2015. 2011 http://www.unaids.org/en/aboutunaids/unaidsstrategygoalsby2015.
- 4.United Nations Office on Drugs and Crime. World Drug Report 2014. New York: United Nations publication; 2014. Sales No. E.14.XI.7. [Google Scholar]
- 5.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ: British Medical Journal. 2012:345. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhodes T, Platt L, Maximova S, et al. Prevalence of HIV, hepatitis C and syphilis among injecting drug users in Russia: a multi city study. Addiction. 2006;101(2):252–66. doi: 10.1111/j.1360-0443.2006.01317.x. [DOI] [PubMed] [Google Scholar]
- 7.Niccolai LM, Toussova OV, Verevochkin SV, Barbour R, Heimer R, Kozlov AP. High HIV prevalence, suboptimal HIV testing, and low knowledge of HIV-positive serostatus among injection drug users in St. Petersburg, Russia. AIDS and Behavior. 2010;14(4):932–41. doi: 10.1007/s10461-008-9469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes T, Lowndes C, Judd A, et al. Explosive spread and high prevalence of HIV infection among injecting drug users in Togliatti City, Russia. Aids. 2002;16(13):F25–F31. doi: 10.1097/00002030-200209060-00002. [DOI] [PubMed] [Google Scholar]
- 9.Elovich R, Drucker E. On drug treatment and social control: Russian narcology’s great leap backwards. Harm Reduction Journal. 2008;5(1):23. doi: 10.1186/1477-7517-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parfitt T. Vladimir Mendelevich: Fighting for drug substitution treatment. The Lancet. 2006;368(9532):279. doi: 10.1016/S0140-6736(06)69060-0. [DOI] [PubMed] [Google Scholar]
- 11.Peterson JA, Schwartz RP, Mitchell SG, et al. Why don’t out-of-treatment individuals enter methadone treatment programmes? International Journal of Drug Policy. 2010;21(1):36–42. doi: 10.1016/j.drugpo.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz RP, Jaffe JH, Highfield DA, Callaman JM, O’Grady KE. A randomized controlled trial of interim methadone maintenance: 10-month follow-up. Drug and alcohol dependence. 2007;86(1):30–6. doi: 10.1016/j.drugalcdep.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Fischer B. Prescriptions, power and politics: the turbulent history of methadone maintenance in Canada. Journal of public health policy. 2000:187–210. [PubMed] [Google Scholar]
- 14.Luce J, Strike C. A cross-Canada scan of methadone maintenance treatment policy developments. A report prepared for the Canadian Executive Council on Addictions. 2011 [Google Scholar]
- 15.Anderson JF, Warren LD. Client retention in the British Columbia methadone program, 1996–1999. Canadian Journal of Public Health/Revue Canadienne de Sante’e Publique. 2004:104–9. doi: 10.1007/BF03405776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metzger DS, Woody GE, McLellan AT, et al. Human immunodeficiency virus seroconversion among intravenous drug users in-and out-of-treatment: an 18-month prospective follow-up. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1993;6(9):1049–56. [PubMed] [Google Scholar]
- 17.Moss AR, Vranizan K, Gorter R, Bacchetti P, Watters J, Osmond D. HIV seroconversion in intravenous drug users in San Francisco, 1985–1990. Aids. 1994;8(2):223–32. doi: 10.1097/00002030-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Hartel DM, Schoenbaum EE. Methadone treatment protects against HIV infection: two decades of experience in the Bronx, New York City. Public health reports. 1998;113(Suppl 1):107. [PMC free article] [PubMed] [Google Scholar]
- 19.Strathdee SA, Patrick DM, Currie SL, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS. 1997;11(8):F59–65. doi: 10.1097/00002030-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 20.College of Physicians and Surgeons of British Columbia. [accessed June 4 2015];Methadone maintenance program: Clinical practice guideline. 2014 http://www.cpsbc.ca/files/pdf/MMP-Clinical-Practice-Guideline.pdf.
- 21.Macalino GE, Vlahov D, Sanford-Colby S, et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island prisons. American journal of public health. 2004;94(7):1218–23. doi: 10.2105/ajph.94.7.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strathdee SA, Patrick DM, Currie SL, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. Aids. 1997 doi: 10.1097/00002030-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Kral AH, Bluthenthal RN, Lorvick J, Gee L, Bacchetti P, Edlin BR. Sexual transmission of HIV-1 among injection drug users in San Francisco, USA: risk-factor analysis. The Lancet. 2001;357(9266):1397–401. doi: 10.1016/S0140-6736(00)04562-1. [DOI] [PubMed] [Google Scholar]
- 24.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. American journal of epidemiology. 1993;138(11):923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 25.Bell J, Hall W, Byth K. Changes in criminal activity after entering methadone maintenance. British journal of addiction. 1992;87(2):251–8. doi: 10.1111/j.1360-0443.1992.tb02699.x. [DOI] [PubMed] [Google Scholar]
- 26.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009:3. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maremmani I, Pani PP, Pacini M, Perugi G. Substance use and quality of life over 12 months among buprenorphine maintenance-treated and methadone maintenance-treated heroin-addicted patients. Journal of substance abuse treatment. 2007;33(1):91–8. doi: 10.1016/j.jsat.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Carrieri PM, Michel L, Lions C, et al. Methadone Induction in Primary Care for Opioid Dependence: A Pragmatic Randomized Trial (ANRS Methaville) PloS one. 2014;9(11):e112328. doi: 10.1371/journal.pone.0112328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittchen H-U, Apelt SM, Soyka M, et al. Feasibility and outcome of substitution treatment of heroin-dependent patients in specialized substitution centers and primary care facilities in Germany: A naturalistic study in 2694 patients. Drug and Alcohol Dependence. 2008;95(3):245–57. doi: 10.1016/j.drugalcdep.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Weinrich M, Stuart M. Provision of methadone treatment in primary care medical practices: review of the Scottish experience and implications for US policy. Jama. 2000;283(10):1343–8. doi: 10.1001/jama.283.10.1343. [DOI] [PubMed] [Google Scholar]
- 31.Fiellin DA, O’Connor PG, Chawarski M, Pakes JP, Pantalon MV, Schottenfeld RS. Methadone maintenance in primary care: a randomized controlled trial. Jama. 2001;286(14):1724–31. doi: 10.1001/jama.286.14.1724. [DOI] [PubMed] [Google Scholar]
- 32.Keen J, Oliver P, Rowse G, Mathers N. Does methadone maintenance treatment based on the new national guidelines work in a primary care setting? British Journal of General Practice. 2003;53(491):461–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Darke S. Self-report among injecting drug users: a review. Drug and alcohol dependence. 1998;51(3):253–63. doi: 10.1016/s0376-8716(98)00028-3. discussion 67–8. [DOI] [PubMed] [Google Scholar]
- 34.Darke S. Self-report among injecting drug users: a review. Drug and alcohol dependence. 1998;51(3):253–63. doi: 10.1016/s0376-8716(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 35.Wood E, Lloyd-Smith E, Li K, et al. Frequent needle exchange use and HIV incidence in Vancouver, Canada. The American journal of medicine. 2007;120(2):172–9. doi: 10.1016/j.amjmed.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Wodak A, Cooney A. Do needle syringe programs reduce HIV infection among injecting drug users: a comprehensive review of the international evidence. Substance use & misuse. 2006;41(6–7):777–813. doi: 10.1080/10826080600669579. [DOI] [PubMed] [Google Scholar]