In this study, bone marrow-derived mesenchymal stem cells (BMSCs) were indirectly cocultured with neonatal rat ventricular cardiomyocytes in vitro or intramyocardially transplanted into hypertrophic hearts in vivo. The results showed that isoproterenol-induced typical hypertrophic characteristics of cardiomyocytes were prevented by BMSCs in the coculture model in vitro and after BMSC transplantation in vivo, providing the first evidence for the treatment of myocardial hypertrophy using BMSCs.
Keywords: Mesenchymal stem cell, Cardiomyocyte, Crosstalk, Hypertrophy, Remodeling
Abstract
Bone marrow-derived mesenchymal stem cells (BMSCs) have emerged as a promising therapeutic strategy for cardiovascular disease. However, there is no evidence so far that BMSCs can heal pathological myocardial hypertrophy. In this study, BMSCs were indirectly cocultured with neonatal rat ventricular cardiomyocytes (NRVCs) in vitro or intramyocardially transplanted into hypertrophic hearts in vivo. The results showed that isoproterenol (ISO)-induced typical hypertrophic characteristics of cardiomyocytes were prevented by BMSCs in the coculture model in vitro and after BMSC transplantation in vivo. Furthermore, activation of the Ca2+/calcineurin/nuclear factor of activated T cells cytoplasmic 3 (NFATc3) hypertrophic pathway in NRVCs was abrogated in the presence of BMSCs both in vitro and in vivo. Interestingly, inhibition of vascular endothelial growth factor (VEGF) release from BMSCs, but not basic fibroblast growth factor and insulin-like growth factor 1, abolished the protective effects of BMSCs on cardiomyocyte hypertrophy. Consistently, VEGF administration attenuated ISO-induced enlargement of cellular size; the upregulation of atrial natriuretic peptide, brain natriuretic peptide, and β-myosin heavy chain expression; and the activation of Ca2+/calcineurin/NFATc3 hypertrophic pathways, and these pathways can be abrogated by blocking VEGFR-1 in cardiomyocytes, indicating that VEGF receptor 1 is involved in the antihypertrophic role of VEGF. We further found that the ample VEGF secretion contributing to the antihypertrophic effects of BMSCs originates from the crosstalk of BMSCs and cardiac cells but not BMSCs or cardiomyocytes alone. Interplay of mesenchymal stem cells with cardiomyocytes produced synergistic effects on VEGF release. In summary, crosstalk between mesenchymal stem cells and cardiomyocytes contributes to the inhibition of myocardial hypertrophy via inhibiting Ca2+/calcineurin/NFATc3 hypertrophic pathways in cardiac cells. These results provide the first evidence for the treatment of myocardial hypertrophy using BMSCs.
Significance
This study found that mesenchymal stem cells may crosstalk with cardiomyocytes, which causes a synergistic vascular endothelial growth factor (VEGF) release from both kinds of cells and then inhibits pathological cardiac remodeling following hypertrophic stimulation in cardiomyocytes in vitro and in vivo. Blockage of VEGF release from bone marrow-derived mesenchymal stem cells (BMSCs) abolishes the antihypertrophic actions of BMSCs in vitro and in vivo. On the contrary, VEGF administration attenuates hypertrophic signaling of calcineurin/ nuclear factor of activated T cell cytoplasmic 3 signal pathways. This study provides the first evidence for the treatment of myocardial hypertrophy using BMSCs.
Introduction
Regeneration of the myocardium in which irreversible cell loss occurs as a result of myocardial infarction and other forms of cardiac conditions has been a utopian goal of cardiologists [1, 2]. Bone marrow-derived mesenchymal stem cells (BMSCs) have the capacity to differentiate into cardiomyocytes and endothelial cells [3]. The therapeutic potential of BMSCs has also been implicated in certain heart diseases including myocardial infarction [4], diabetic cardiomyopathy [5, 6], dilated cardiomyopathy [7], etc. These factors made BMSCs one of the most attractive adult-derived cell populations for cardiovascular repair in heart diseases [8]. In addition to these injured cardiac conditions, whether stem cells can also be applied to treat hypertrophic growth of myocardium remained unexploited.
Cardiac hypertrophy, a stereotypic response of the heart to increased workload and thereby wall stress, is a thickening of heart walls caused by increased mass of myocardium to maintain cardiac output [9, 10]. It has traditionally been considered as an adaptive response of heart in the face of stress, and in the past decades, major research and therapeutic efforts have been made to impede the transition from hypertrophy to failure. However, accumulating evidence from studies in animal models and patients suggests that in most instances hypertrophy is a maladaptive process rather than a compensatory response to the change in mechanical load [9]. In response to pathological stimuli, prolonged hypertrophy is a major risk factor for the development of heart failure and cardiac sudden death, independent of the underlying causes of hypertrophy [9–11]. Accordingly, reversal of myocardial hypertrophy without adversely affecting contractile function has now been considered as an auspicious approach in the prevention and treatment of heart failure [12].
Here we show that coculture of BMSCs with cardiomyocytes is able to inhibit the hypertrophic growth and other deleterious changes of cardiomyocytes in response to isoproterenol (ISO) stimulation, and consistently intramyocardial injection of BMSCs is able to prevent adverse structural remodeling and restore contractile functions in hypertrophied hearts of rats. This antihypertrophy effect of BMSCs is ascribed to the inhibition of Ca2+/calcineurin/nuclear factor of activated T cells cytoplasmic 3 (NFATc3) signaling pathway by vascular endothelium growth factor (VEGF) that is amply secreted from both BMSCs and cardiac cells. The interplay between BMSCs and cardiomyocytes triggers and sustains the secretion of VEGF from these two cell types and in turn inhibits pathological myocardial hypertrophy.
Materials and Methods
Experimental Animals
Male SD rats weighing 230–280 g were purchased from the Experimental Animal Center of the Affiliated Second Hospital of Harbin Medical University (Harbin, China, http://www.hrbmush.edu.cn/). The rats were housed under conditions of constant temperature and controlled illumination (light on between 7 hours 30 minutes and 19 hours 30 minutes). Food and water were available ad libitum throughout the study. Animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Harbin Medical University (No. HMUIRB-2011-09). The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. Hearts for ventricular myocyte isolation were from neonatal rats. For BMSC transplantation and cardiac functional detection, the rats were anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneally) and maintained by administrating 2% isoflurane. For the experiments of BMSC isolation, morphological detection, real-time reverse-transcription polymerase chain reaction (PCR), and Western blot, rats were anesthetized with sodium pentobarbital (100 mg/kg), and confirmation of death was by exsanguination. The depth of anesthesia was monitored by toe pinch, respiratory rate, and heart rate.
Bone Marrow-Derived Mesenchymal Stem Cells
BMSCs were isolated and expanded from young male SD rats (weighing 100 ± 20 g) as previously described [13]. Briefly, bone marrow cells were collected from the femurs and tibias of SD rats and then plated in mesenchymal stem cells basal medium (StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) supplemented with mesenchymal stem cell stimulatory supplements (StemCell Technologies), and penicillin (100 U/ml)/streptomycin (100 U/ml) in culture flasks at 37°C in 5% CO2, 95% air in a humidified incubator. Three days later, nonadherent cells were washed out, and the adherent cells were expanded continuously. All the cells used in this study were then harvested when reaching 80% confluence by 0.25% trypsin (Sigma) at passage 3.
Neonatal Rat Ventricular Cardiomyocytes
The protocol for isolating and culturing neonatal rat ventricular cardiomyocytes (NRVCs) was as described previously [13]. Briefly, neonatal rat ventricles were cut into 1–2 mm3 cubes after the hearts were rapidly removed and then dissociated in 0.25% trypsin at 37°C. Heart tissues were trypsinized until the tissues disappeared, and cell suspensions were collected by centrifugation at 1,500 rpm for 145 seconds. The collected cells were then resuspended in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Logan, UT, http://www.hyclone.com) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, http://www.invitrogen.com) and penicillin (100 U/ml)/streptomycin (100 U/ml), and cultured at 37°C in 5% CO2, 95% air in a humidified incubator. After 90 minutes for fibroblast adherence, the cell suspension were plated into a 6-well plate at 3 × 105 cells per well. Three days later, NRVCs were cocultured with BMSCs.
Coculturing of BMSCs With NRVCs
To observe the influence of BMSCs on NRVCs, a coculture model of BMSCs and NRVCs was established in vitro as previously described with some modifications [14, 15]. Briefly, BMSCs and NRVCs were indirectly cocultured in a 1:10 ratio by using a semipermeable membrane of Transwell insert (pore size, 0.4 μm) (Corning Company), which separates two kinds of cells but allows the diffusion of secreted factors. Coculture was maintained with DMEM supplemented with 10% fetal bovine serum in humidified incubator at 37°C and 5% CO2. Monocultured NRVCs in the same medium were taken as control group.
Transfection of siRNAs
VEGF small interfering RNA (siRNA), insulin-like growth factor 1 (IGF-1) siRNA, basic fibroblast growth factor (bFGF) siRNA, and a nonsense siRNA (negative control) were purchased from Sangon Biotech (Shanghai, China, http://www.life-biotech.com/company/about_us.html), synthesized at Ambion (Austin, TX, http://www.ambion.com), and used in a final concentration of 300 pM. Transfection of BMSCs or NRVCs with VEGF, bFGF, and IGF-1 siRNAs were diluted with OptiMEM (Invitrogen, Carlsbad, CA, http://www.invitrogen.com), incubated with Xtreme transfection reagent (Roche, Indianapolis, IN, http://www.roche.com) in a six-well plate according to the manufacturer’s protocol.
Administration of Drugs
VEGF was injected into the heart via intramycoardial delivery way at five sites (upper, lower, left, right, and center, a dosage of 2 mg/kg for per injection site). To elucidate the subtypes of VEGF receptors (VEGFRs) that account for the beneficial actions of VEGF in ISO-induced cardiomyocyte hypertrophy, VEGFR-1 inhibitor (AMG-706, 2 nM), VEGFR-2 (VEGFR2-I, 70 nM), and VEGFR-3 blockers (MAZ-51, 10 μM) were applied to NRVCs before VEGF administration, respectively.
Measurement of Cell Surface Area
Cultured NRVCs in the slides were fixed with 4% paraformaldehyde for 0.5 hour. After rinsing, the sections were penetrated with 0.4% Triton X-100 for 1 hour and blocked in normal goat serum for 1 hour at room temperature. Then the sections were incubated with mouse anti-sarcomeric actinin antibody at 4°C overnight, washed, incubated with fluorescein isothiocyanate-conjugated goat anti-mouse antibody for 1 hour, washed again, and mounted in a fluorescence microscope (80i; Nikon, Tokyo, Japan, http://www.nikon.com). The surface areas of NRVCs were measured using Image-Pro Plus analysis software.
Cardiac Hypertrophy Model
The method to establish ISO-induced cardiomyocyte hypertrophy model was essentially the same as previous description with some modification [16]. Male SD rats were randomly divided into five groups, including the isoproterenol group (ISO at a concentration of 2.4 mg·kg−1·day−1 s.c. was administered for 10 days to induce cardiac hypertrophy; n = 20), ISO/BMSC group (BMSCs were intramyocardially transplanted into ventricular myocardium at a dose of 1 × 106 prior to ISO 2.4 mg·kg−1·day−1; n = 10), ISO/BMSCs+VEGF siRNA group (n = 10), ISO/BMSCs+VEGF siRNA control group (n = 10), and ISO+VEGF group (n = 10). After 10 days of treatment, after anesthesia, the hearts were removed for assessment. The hearts were sectioned across the ventricles for histological analyses.
BMSC Transplantation
BMSCs were transplanted into the hearts of SD rats before ISO injection. The heart was accessed through the fourth intercostal space after anesthesia. Intramyocardial injection of BMSCs (1 × 106 BMSCs into 50 μl of culture medium) with different treatment or medium alone (50 μl) was performed using a syringe with a 30-gauge needle 1 day before ISO injection. Five injection sites (upper, lower, left, right, and center; 0.2 × 106 cells in 10 μl for each site) in the left ventricle were chosen to deliver cells. Sham-operated animals were subjected to the same surgical procedure.
Echocardiography Measurements
Left ventricular (LV) function was assessed in anesthetized animals with two-dimensional guided M-mode and Doppler echocardiography with a 13-MHz linear probe (GE Medical Systems, Milwaukee, WI, http://www.ge.com) after 10 days of ISO injection. For anesthesia, rats were injected with isoflurane and received continuous inhaled anesthetic (2%) for the duration of the imaging session. The animals were placed in the supine or lateral position on a warming pad. Numeric images of the heart were obtained in both parasternal long-axis and short-axis views. Two-dimensional end-diastolic and end-systolic long-axis views of the LV were standardized as follows: inclusion of the apex, the posterior papillary muscle, the mitral valve, and the aortic root. End-diastolic and end-systolic areas were obtained by hand tracings of the LV endocardial contours, according to the American Society of Echocardiography leading-edge method.
ELISA
The culture media or cell samples were collected from BMSCs and NRVCs alone or coculture. The levels of VEGF, bFGF, and IGF-1 in the media or cell samples were measured by using the ELISA kits (purchased from, respectively, R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com and Wuhan Boster, Pleasanton, CA, http://www.bosterbio.com) according to the manufacturers’ instructions.
Hematoxylin & Eosin Staining
The left ventricle tissues of rat hearts near BMSC injection sites were obtained at the indicated time, fixed in 10% formalin containing phosphate-buffered saline, and embedded in paraffin. Serial 5-μm heart sections from each group were analyzed. Samples were stained with hematoxylin & eosin (H&E) trichrome.
Quantitative Real-Time PCR
Real-time quantitative reverse transcriptase PCR was used for the quantification of mRNA levels as previously described [17, 18]. Total RNA samples were extracted from cultured NRVCs using TRIzol reagent. After DNase I treatment, RNA was reverse-transcribed with reverse transcriptase (ReverTra Ace; Toyobo, Osaka, Japan, http://www.toyobo.co.jp/e). To detect the levels of atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and β-myosin heavy chain (β-MHC) mRNAs, quantitative real-time PCR was performed on ABI 7500 Fast Real Time PCR system (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). The results were standardized to control values from glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Measurement of [Ca2+]i
NRVCs were loaded with Fluo-3 and Fura Red (Molecular Probes) at 37°C for 45 minutes and then washed with Tyrode’s solution three times. After loading with Fluo-3 and Fura Red, the cultured NRVCs were transferred into a recording chamber and superfused with Tyrode’s solution. Fluorescent changes of cells loaded by Fluo-3 and Fura Red were detected using a flow cytometer (FACSCalibur; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) at 530 nm and 670 nm, respectively. Quantitative changes of intracellular Ca2+ level were inferred from the ratio of the Fluo-3/Fura Red fluorescence.
Western Blot
Total protein samples were extracted from the left ventricle of rats and cultured NRVCs with the procedures as previously described [19]. Nuclear protein samples were extracted using a Transfactor Extraction Kit (BD Biosciences, Clontech, Mountain View, CA, http://www.clontech.com) according to the manufacturers’ instructions. Protein concentration was determined using the BCA method as recommended by the manufacturer. After being boiled for 5 minutes, the collected protein samples were fractionated by SDS-PAGE (10%–15% polyacrylamide gels) and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA, http://www.millipore.com). The samples were blocked with milk powder for 2 hours at room temperature and then incubated with the primary antibodies ANP, BNP, β-MHC (Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com), calcineurin (Santa Cruz Biotechnology), and NFATc3 (Santa Cruz Biotechnology) at 4°C overnight. After washing, the membranes were incubated with a secondary rabbit polyclonal and purchased from Santa Cruz Biotechnology for 1 hour at room temperature. Western blot bands were quantified using Odyssey v1.2 software by measuring the band intensity for each group and normalizing to GAPDH or lamin B as an internal control.
Data Analysis
All values are expressed as means ± SEM. The data from experimental groups were compared by Student’s t test or analysis of variance followed by a post hoc analysis. A value of p < .05 was considered statistically significant.
Results
BMSCs Inhibited ISO-Induced Hypertrophy of Cardiomyocytes in Vitro
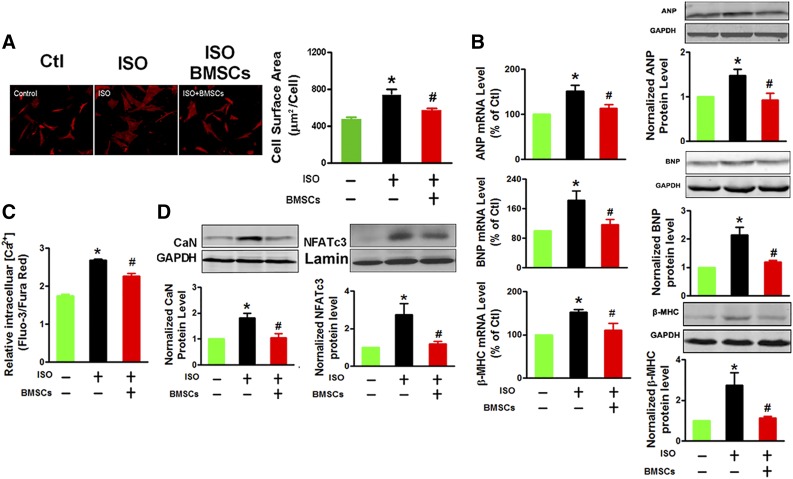
We first treated NRVCs with a β-adrenergic receptor agonist ISO (10 μM) for 24 hours in the culture media to induce cardiomyocyte hypertrophy (Fig. 1A). Typical phenotypes of hypertrophy were consistently observed (Fig. 1A, 1B). Strikingly, these hypertrophic responses were virtually absent in the NRVCs that had been cocultured with BMSCs for 24 hours. Cardiac hypertrophy is thought to be promoted by the persistent enhancement of intracellular Ca2+ concentration [Ca2+]i, which can activate a Ca2+/calmodulin-dependent protein phosphatase calcineurin (CaN) known as a specific hypertrophic activator [20, 21]. The key regulatory targets of CaN are the nuclear factor of activated T cells (NFATc) family of transcription factors, which when dephosphorylated by CaN can be translocated into the nucleus to activate transcription of hypertrophic target genes [22]. Furthermore, we observed a significant increase in [Ca2+]i of NRVCs exposed to ISO (Fig. 1C), but this ISO-induced Ca2+ overload was absent in NRVCs cocultured with BMSCs, consistent with the inhibition of cardiomyocyte hypertrophy. Similarly, the upregulation of CaN proteins seen in ISO-treated NRVCs was also attenuated after cocultured with BMSCs (Fig. 1D). As a downstream effector of the CaN-dependent hypertrophic signaling pathway, the expression of NFATc3 protein in the nucleus was downregulated in NRVCs cocultured with BMSCs (Fig. 1D). These data suggest that blockage of the Ca2+/CaN/NFATc3 pathway contributes to the antagonizing effects of BMSCs on ISO-induced cardiomyocyte hypertrophy.
Figure 1.
BMSCs inhibit ISO-induced cardiomyocyte hypertrophy. (A): BMSC coculturing suppresses the hypertrophic growth of neonatal rat ventricular cells (NRVCs) induced by β-adrenoceptor agonist ISO, as indicated by reduced cell surface area. The images represent immunostaining with α-actinin antibody (red signal; magnification ×200, at least 10 randomly selected fields in three separate experiments), and the bar charts are averaged cell surface area. (B): BMSC coculturing suppresses ISO-induced expression of the hypertrophic marker genes ANP, BNP, and β-MHC at both mRNA and protein levels in NRVCs, measured by quantitative polymerase chain reaction and immunoblotting analyses, respectively. (C): BMSC coculturing suppresses ISO-induced Ca2+ overload as indicated by the lowering of intracellular Ca2+ concentration ([Ca2+]i) in NRVCs. (D): BMSC coculturing suppresses ISO-induced upregulation of CaN (left) and NFATc3 (right) proteins in the nuclei of NRVCs. The data were obtained from three independent experiments. ∗, p < .05 versus Ctl; #, p < .05 versus ISO. Abbreviations: BMSC, bone marrow-derived mesenchymal stem cell; CaN, calcineurin; Ctl, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ISO, isoproterenol; β-MHC, β-myosin heavy chain; NFATc3, nuclear factor of activated T cells cytoplasmic 3.
BMSC Transplantation Prevented Cardiac Hypertrophy in Vivo
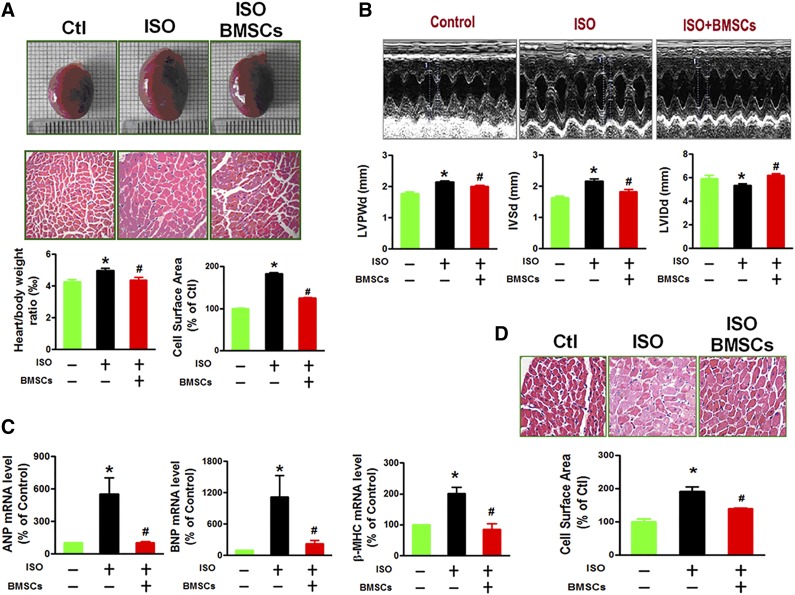
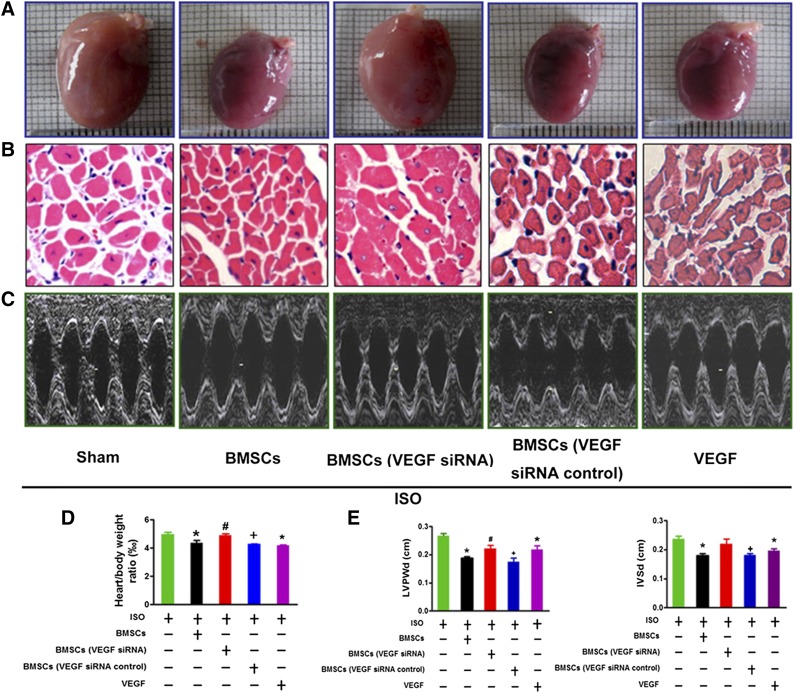
We then went on to examine the antihypertrophic effect of BMSCs under in vivo conditions with rats by transplanting BMSCs into left ventricular myocardium via intramyocardial delivery 1 day before ISO injection. The rats with ISO treatment for 10 days showed a significant augmentation of heart size, heart/body weight ratio, thickening of left ventricle walls, severe impairment of cardiac functions in rats with ISO treatment as revealed by echocardiographic examination, and enhanced expression of hypertrophic marker genes (Fig. 2A–2C). These adverse alterations were clearly ameliorated after BMSC transplantation. Most strikingly, BMSCs engrafted in the left ventricle were found also able to inhibit hypertrophic remodeling process occurring in the right ventricle (Fig. 2D). This finding suggests that the observed beneficial effects of BMSCs were likely mediated by secretion of certain antihypertrophic factors from BMSCs, cardiac cells, or both.
Figure 2.
BMSC transplantation attenuated ISO-induced cardiac structure remodeling in vivo. (A): BMSC transplantation prevents ISO-induced enlargement of heart size, increase of the heart/body weight ratios, and enlargement of cell size (at least 10 randomly selected fields in three separate experiments) as revealed by H&E staining of the left ventricular myocardium in rats. (B): BMSC transplantation ameliorates the abnormalities of cardiac structural and impairment of diastolic function in ISO-treated rats. Top: Representative echocardiographic images of showing thickening of left ventricle wall and interventricular septum and enlargement of left ventricular chamber at diastole. Bottom: Averaged data on selected echocardiographic parameters including LVPWd, IVSd, and LVIDd. (C): BMSC transplantation suppresses expression of hypertrophic marker genes ANP, BNP, and β-MHC in rats. The data were obtained from three independent experiments. (D): BMSC transplantation inhibits ISO-induced enlargement of cell size as revealed by H&E staining of the right ventricular myocardium (at least 10 randomly selected fields in three separate experiments). The data were obtained from five rats of each group. ∗, p < .05 versus Ctl; #, p < .05 versus ISO. Abbreviations: BMSC, bone marrow-derived mesenchymal stem cell; Ctl, control; ISO, isoproterenol; H&E, hematoxylin & eosin; IVSd, interventricular septal thickness at diastole; LVIDd, left ventricular internal dimension-diastole; LVPWd, left ventricular posterior wall dimension; β-MHC, β-myosin heavy chain.
VEGF Was Involved in the Antihypertrophic Actions of BMSCs
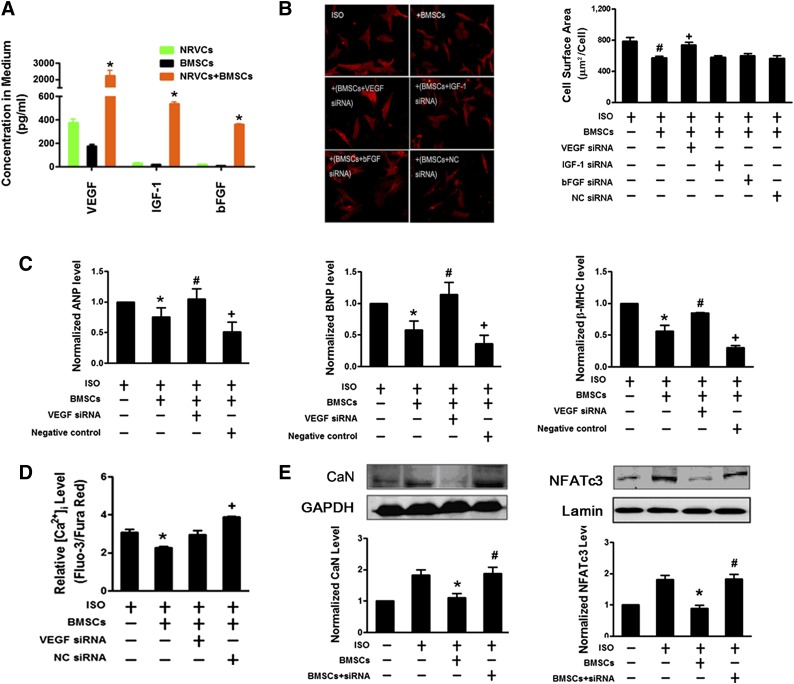
The above results established the efficacy of BMSCs in inhibit hypertrophic phenotypes both in vitro and in vivo. We then turned to decipher the underlying mechanisms for the protective effects. BMSCs have been known to play a central role in the repair of impaired hearts through paracrine mechanism [23, 24]. Several antiapoptotic and anti-inflammatory factors such as VEGF, IGF-1, and bFGF can be released by BMSCs after engraftment [7, 24, 25]. We first measured the level of secreted VEGF, IGF-1, and bFGF in the culture media of NRVCs and BMSCs. Notably, the concentrations of VEGF, IGF-1, and bFGF secreted into the media from the coculture of NRVCs and BMSCs were remarkably higher than those summed from both NRVCs and BMSCs origins (Fig. 3A), indicating that BMSCs and NRVCs mutually promote the release of these factors during coculturing. We then investigated whether increased VEGF, IGF-1, and bFGF secretion accounted for the antihypertrophic effects of BMSCs on ISO-treated NRVCs. Interestingly, the decrease of VEGF production by siRNA interference abrogated the protective effects of BMSCs on the enlargement of cellular size; the augment of ANP, BNP, and β-MHC expression; and the activation of Ca2+/calcineurin/NFATc3 hypertrophic pathway in NRVCs after ISO treatment (Fig. 3B–3E). Nevertheless, the depletion of IGF-1 and bFGF by their respective siRNAs failed to inhibit the antihypertrophic action of BMSCs. These observations suggest that the increase in VEGF plays a central role in the antihypertrophic effect of BMSCs.
Figure 3.
VEGF involves the reversal of cardiomyocyte hypertrophy by BMSCs. (A): Coculture of neonatal rat ventricular cardiomyocytes (NRVCs) with BMSCs increases the contents of VEGF, bFGF, and IGF-1 in the medium. ∗, p < .05 versus NRVC monoculture or BMSC monoculture. (B): Silencing of VEGF, but not bFGF and IGF-1, by siRNAs (300 pM) in BMSCs abolishes the amelioration of ISO-induced enlargement of cardiac cell size by BMSCs. BMSCs were pretreated with siRNAs by transfection and then cocultured with NRVCs. Left: Representative images of NRVCs (×200 magnification, at least 10 randomly selected fields in three separate experiments) with α-actinin staining. Right: Averaged cell surface area of NRVCs. The scrambled NC siRNA failed to produce the effects against BMSCs. siRNAs were transfected into BMSCs. (C): VEGF siRNA abolishes the inhibitory effects of BMSCs on the ISO-induced expression of hypertrophic marker genes ANP, BNP, and β-MHC mRNAs in NRVCs. (D): VEGF siRNA abolishes the inhibitory effects of BMSCs on the ISO-induced increase of [Ca2+]i in NRVCs. (E): VEGF siRNA abolishes the inhibitory effects of BMSCs on the ISO-induced upregulation and activation of CaN and nuclear NFATc3 proteins in NRVCs. The data were obtained from four independent experiments. ∗, p < .05 versus ISO; #, p < .05 versus BMSCs; +, p < .05 versus BMSCs transfected with VEGF siRNA. Abbreviations: ANP, atrial natriuretic peptide; bFGF, basic fibroblast growth factor; BMSC, bone marrow-derived mesenchymal stem cell; BNP, brain natriuretic peptide; CaN, calcineurin; Ctl, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IGF-1, insulin-like growth factor 1; ISO, isoproterenol; β-MHC, β-myosin heavy chain; NC, negative control; NFATc3, nuclear factor of activated T cells cytoplasmic 3; siRNA, small interfering RNA; VEGF, vascular endothelial growth factor.
Inhibition of VEGF Release Abrogated the Antihypertrophic Effects of BMSCs
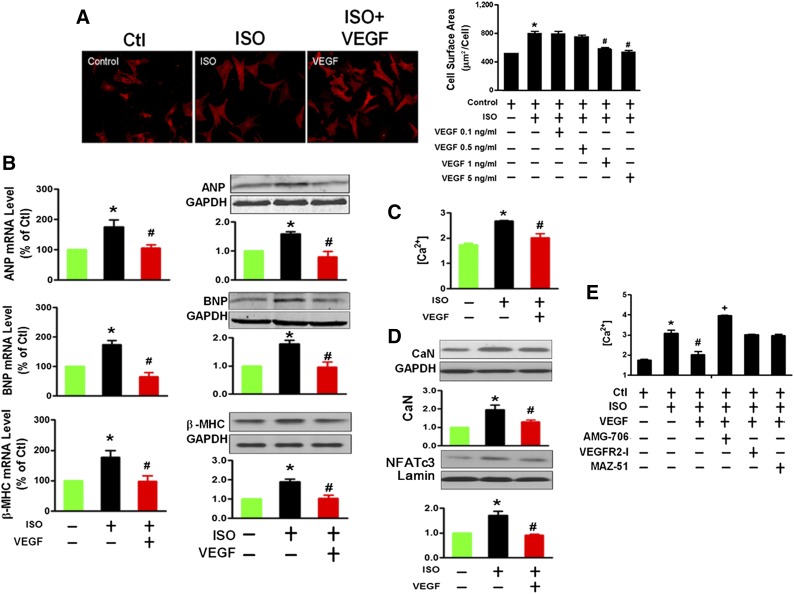
We further test the preventive role of VEGF in cardiomyocyte hypertrophy in vitro. NRVCs treated with VEGF 0.1 and 0.5 ng/ml for 24 hours did not affect ISO-induced cardiomyocyte hypertrophy. However, significant inhibition of cardiomyocyte hypertrophy was observed after VEGF 1.0 and 5.0 ng/ml treatment (Fig. 4A). These results are consistent with VEGF secretion experiments performed in BMSCs and NRVCs. The VEGF contents were 2.25 ± 0.55 ng/ml in coculture medium and 0.37 ± 0.06 ng/ml and 0.17± 0.03 ng/ml in BMSCs and NRVC monoculture media, respectively (as shown in Fig. 3A), which was less than 1.0 ng/ml and not sufficient to inhibit ISO-induced hypertrophy. In all subsequent experiments, we chose to use 5.0 ng/ml VEGF, which dramatically reduced ISO-induced hypertrophic changes as described above (Fig. 4B–4D). VEGF is known to stimulate cellular responses by binding to cell surface tyrosine kinase receptors (VEGFR-1, VEGFR-2, and VEGFR-3) [26]. We found that VEGFR-1 inhibitor obviously abrogated the beneficial effects of VEGF on intracellular Ca2+ overload, but VEGFR-2 and VEGFR-3 blockers failed (Fig. 4E). It strongly suggests that VEGF mediates the beneficial effects of BMSCs mainly through acting on VEGFR-1.
Figure 4.
VEGF prevents ISO-induced cardiomyocytes hypertrophy. (A): Effects of different concentrations of VEGF on enlargement of cell size in neonatal rat ventricular cardiomyocytes (NRVCs) treated with ISO (at least 10 randomly selected fields in three separate experiments). (B): VEGF (5 ng/ml) prevents the expression of the hypertrophic marker genes ANP, BNP, and β-MHC at both mRNA (left) and protein (right) levels in ISO-treated NRVCs. (C): VEGF prevents ISO-induced the increase of [Ca2+]i in NRVCs, examined with Fluo-3/Fura Red fluorescence detected using a flow cytometer. (D): VEGF attenuates the upregulation of CaN and nuclear NFATc3 proteins in ISO-treated NRVCs. ∗, p < .05 versus Ctl; #, p < .05 versus ISO, unpaired t test. (E): Inhibition of VEGFR-1 by its antagonist AMG-706 (2 nM), but not by VEGFR-2 (VEGFR2-I, 70 nM) and VEGFR-3 (MAZ-51, 10 μM) inhibitors, prevents the increase of [Ca2+]i in ISO-treated NRVCs. The data were obtained from three independent experiments. ∗, p < .05 versus ISO; #, p < .05 versus BMSCs; +, p < .05 versus VEGF. Abbreviations: ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; CaN, calcineurin; Ctl, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ISO, isoproterenol; β-MHC, β-myosin heavy chain; NFATc3, nuclear factor of activated T cells cytoplasmic 3 VEGF, vascular endothelial growth factor.
VEGF Mediated the Antihypertrophic Effects of BMSCs in Rats
We then investigated whether secretion of VEGF by BMSCs contributes to the prevention of hypertrophy in vivo. Our data showed an enormous increase in the myocardial content of VEGF with BMSC transplantation and abolishment of this increase when BMSCs had been pretransfected with VEGF siRNA (Fig. 5A). In parallel, both BMSC transplantation and VEGF administration by intramycoardial delivery prevented ISO-induced hypertrophic growth (Fig. 5B, 5D) and functional abnormality of the rat hearts (Fig. 5C, 5E). Moreover, the antihypertrophic effects of BMSCs were severely mitigated if pretreated with VEGF siRNA to silence VEGF expression (Fig. 5A–5E). The role of VEGF in mediating the antihypertrophic action of BMSCs was further evidenced by the fact that the BMSCs pretransfected with a negative control VEGF siRNA retained their ability to suppress hypertrophic remodeling. Consistently, intramyocardial injection of VEGF (10 mg/kg) produced the same effects as BMSCs (Fig. 5A–5E).
Figure 5.
VEGF mediates the reversal of cardiac hypertrophy by BMSCs. (A): BMSC transplantation or VEGF treatment reduces heart size in hypertrophic rats induced by ISO. The data were obtained from five rats from each group. (B): Typical examples of cross-sections of ventricular myocardium with H&E staining showing the normalization of enlarged cell surface area by BMSC transplantation or VEGF injection in ISO-treated rats (at least 10 randomly selected fields in three separate experiments). (C): Reprehensive echocardiography image from ISO-treated hearts with BMSCs, BMSC VEGF siRNA, or VEGF. (D, E): Reduction of the heart/body weight ratio and improvement of cardiac structure and diastolic function by BMSCs or VEGF in ISO-treated rats. The data were obtained from five rats from each group. Top: representative images of echocardiogram. Bottom: normalization of echocardiographic parameters LVPWd and IVSd by BMSCs or VEGF. ∗, p < .05 versus ISO; #, p < .05 versus ISO+BMSCs; +, p < .05 versus ISO+BMSCs+VEGF siRNA. Abbreviations: BMSC, bone marrow-derived mesenchymal stem cell; ISO, isoproterenol; H&E, hematoxylin & eosin; IVSd, interventricular septal thickness at diastole; LVPWd, left ventricular posterior wall dimension; siRNA, small interfering RNA; VEGF, vascular endothelial growth factor.
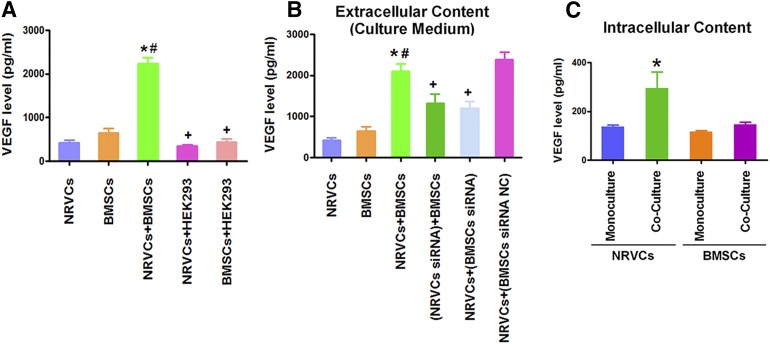
Crosstalk Between BMSCs and NRVCs Causes Synergistic Secretion of VEGF
We then wanted to understand what makes the positive synergistic increase in VEGF content in the coculture with NRVCs and BMSCs. To this end, we first clarified the source of VEGF: whether the VEGF measured from the culture medium was secreted from NRVCs or from BMSCs or both. Our data shown in Figure 6A indicate that NRVCs and BMSCs when cultured separately both could independently secrete VEGF, but its concentration is not sufficient to play an inhibitory role in cardiomyocyte hypertrophy as suggested in Figure 4A. Nevertheless, when cultured together, the amount of secreted VEGF was significantly greater than the sum from two separate cell sources and reached the concentration required for the inhibition of hypertrophic growth in NRVCs. Intriguingly, such a synergistic increase in VEGF concentration was not seen in the coculture of NRVCs with HEK293 cells nor in the coculture of BMSCs with HEK293 cells (Fig. 6A). These data indicate there is a specific crosstalk between NRVCs and BMSCs to amplify the VEGF secretion. To test this notion, we subsequently treated NRVCs with VEGF siRNA to deplete the cardiomyocyte VEGF and then cocultured these VEGF-free NRVCs with BMSCs. After 24 hours, we measured the VEGF level in the medium. This maneuver completely eliminated the increase of VEGF content (Fig. 6B). On the other hand, when the BMSCs pretreated with the VEGF siRNA were cocultured with nontreated NRVCs, a similar loss of the increase of VEGF content was observed. These results indicate that intracellular VEGF in both NRVCs and BMSCs is necessary for the synergistic increase of extracellular VEGF in the media. We finally made an effort to measure the intracellular levels of VEGF protein in NRVCs and BMSCs with or without coculturing. Our results demonstrated that the intracellular VEGF level was significantly elevated in NRVCs, but not in BMSCs, after coculturing (Fig. 6C). The data indicate that the interplay of BMSCs with NRVCs not only enhances the secretion of VEGF from BMSCs but also indirectly upregulates the intracellular VEGF level in NRVCs, which together leads to the observed elevation of extracellular VEGF content and in turn produces the antihypertrophic actions.
Figure 6.
BMSCs and NRVCs crosstalk to induce synergistic enhancement of VEGF secretion. (A): Comparisons of VEGF contents in the culture media of NRVCs or BMSCs alone or cocultures of these cells with human kidney cell line HEK293. Note that only NRVC/BMSC coculture boosts VEGF production. (B): Pretreatment of either NRVCs or BMSCs with VEGF siRNA, labeled NRVCs/siRNA or BMSCs/siRNA, eliminates the increases of VEGF contents in the medium of NRVCs/BMSC cocultures. (C): Intracellular VEGF contents with monoculture of NRVCs or BMSCs and cocultures of NRVCs and BMSCs. The data were obtained from four independent experiments. ∗, p < .05 versus NRVC monoculture; #, p < .05 versus BMSC monoculture; +, p < .05 versus NRVC/BMSC coculture. Abbreviations: BMSC, bone marrow-derived mesenchymal stem cell; NRVC, neonatal rat ventricular cardiomyocyte; VEGF, vascular endothelial growth factor.
Discussion
The main finding of the present study is that the interplay between BMSCs and NRVCs is capable of ameliorating the phenotypes of pathological cardiac hypertrophy by inhibiting the adverse remodeling processes in hypertrophic myocardium and ventricular cells. This is, to our knowledge, the first study to reveal the antihypertrophic efficacy of mesenchymal stem cells via interplaying with cardiomyocytes, although stem cells have been previously found to alleviate myocardial infarction and heart ischemia [7]. This finding may revise our current concept of stem cells: the crosstalk of stem cells with host cardiomyocytes beyond a one-sided action of stem cells on heart is not only capable of restoring the myocardial injuries by replacing dead cells in the degenerative pathological settings but also capable of inhibiting remodeling of myocardial hypertrophy. This study also helps to provide insight into stem cell pharmacology.
Plenty of studies demonstrate that BMSCs have a high capacity to differentiate into cardiomyocytes and endothelial cells [3]. Accordingly, BMSCs become a very attractive adult-derived cell population for cardiovascular repair and have displayed enormous therapeutic potential for heart diseases [7]. To date, accumulated evidence from preclinical and clinical studies has confirmed that the implantation of culture-expanded BMSCs conferred biological and functional protections in myocardium infarction [23, 27], diabetic cardiomyopathy [5], acute myocarditis [28], and dilated cardiomyopathy [7], although the concern about proarrhythmic potential was recently raised [29]. Akt [23], GSK-3β [30], Hif-1 [31], and Noth1 signaling [32] of BMSCs has been shown to be critical for cardiac repair in ischemic heart diseases. Nevertheless, the effects of transplanted BMSCs on pathological myocardial hypertrophy and its underling mechanisms were largely unknown.
Here, our data revealed that BMSCs exhibited significant inhibitory effects on ISO-induced hypertrophic growth of ventricular myocytes and the upregulation of hypertrophic marker ANP, BNP, and β-MHC both in vitro and in vivo. So, using BMSCs to inhibit hypertrophy might be a superior therapeutic strategy for preventing heart failure to any other interventions implemented at later stages. Consistently, recent evidence also supports that BMSCs implantation may improve regional cardiac remodeling including cardiomyocyte apoptosis and hypertrophy after cardiac infarction in ovine infarction model, although in vitro study suggests that BMSCs possibly played a potentiating role in the hypertrophy of hypoxia-treated cardiomyocytes [33, 34]. We also observed that replacing BMSCs, HEK293 cells cocultured with NRVCs did not exert the preventive effects on cardiomyocytes hypertrophy, suggesting that the antihypertrophic action is attributed to and the specific for BMSCs and not available for other types of cells such as HEK293. Strikingly, it was firstly revealed that the BMSCs engrafted in the left ventricular wall were able to prevent the hypertrophic response in the right ventricle, indicating a role of transmittable factors.
It is well documented that intracellular Ca2+/calmodulin-dependent signaling stimulates the expression of hypertrophic genes in cardiomyocytes through mediating calcineurin and NFATc3 [20, 21]. In the present study, Ca2+/calcineurin/NFATc3 hypertrophic pathway was also shown activated by ISO, whereas BMSC coculture or transplantation can block this hypertrophic pathway signal both in vitro and in vivo. Further study was necessary to clarify how BMSCs regulate hypertrophic pathway of both local and remote cardiomyocytes. Growing evidence demonstrates that paracrine effects make main contributions to the repair of heart injury by BMSC transplantation [23, 25]. A multitude of paracrine factors such as VEGF, bFGF, and IGF-1 are observed secreted into the microenvironment by BMSCs and then lead to cellular regeneration via neovascularization, anti-inflammation, antiapoptosis, antifibrosis, antiremodeling, and the activation of resident cardiac stem cells [13, 25, 35]. Consistent with these reports, our results unravel that the antihypertrophic effects of BMSCs critically depends on the synergistic secretion of VEGF, which inhibits the activation of the Ca2+/calcineurin/NFATc3 hypertrophic signaling pathway in cardiac cells, but not on IGF-1 and bFGF. VEGF has been widely reported on its beneficial impact on vascular growth and protection against myocardial ischemia. Consistently, our study also found that BMSCs with VEGF siRNA has no ability to increase the number of vessels in the heart, indicating that BMSCs promoted vascular growth (supplemental online Fig. 1). However, its influence on hypertrophy was not broadly reported. This study represents the first report that VEGF is able to inhibit the hypertrophic remodeling via blocking Ca2+-activated hypertrophy pathway. In agreement, it was reported that enhanced activation of VEGFR-1 signaling by VEGF contributes to copper-induced inhibition of cardiomyocyte hypertrophy. Previous studies demonstrated that VEGF can retard the transition from hypertrophy to heart failure [36], or VEGF-induced neovascularization may reverse cardiac fibrosis and functions before advanced disease stages [37]. These reports supported the antihypertrophic role of VEGF in cardiomyocytes, but in vitro reports show that VEGF contributed to ET-1-induced hypertrophy of neonatal rat ventricular myocytes [38]. This suggests that the different hypertrophic stimuli or models may be quite important in determining the influences of VEGF on hypertrophic growth.
The presence of transplanted BMSCs in ISO-treated hearts was identified by bromodeoxyuridine labeling, and the level of VEGF was confirmed increased in BMSC-treated hearts (supplemental online Fig. 2). More interesting, we found that the ample VEGFs not only originate from BMSCs but also from cocultured or host cardiac cells. Our study show that the coculturing of either NRVCs or BMSCs with HEK293 failed to enhance VEGF release, and silencing VEGF from either of these two cell origins renders a loss of the antihypertrophic action of BMSCs. These results support that the synergistic VEGF secretion that confers the antihypertrophic efficacy of BMSCs is owing to a specific crosstalk between cardiac cells and BMSCs. This finding further suggests that application of BMSCs should not be considered simply a “cell replacement” therapy; rather BMSCs serve to trigger activation of the signaling pathway through activating a gene expression program to boost production of VEGF in cardiac cells, which in turn facilitates VEGF secretion in BMSCs, leading to an antihypertrophic effect. Although the present study does not aim to elucidate how BMSCs and NRVCs interplay to amplify VEGF secretion, it is speculated that BMSCs might release a certain factor to stimulate VEGF secretion from both cardiomyocytes and BMSCs. This issue merits future detailed studies.
It should be clarified that this finding is limited to ISO-induced heart hypertrophy, and whether this finding will apply to other forms of hypertrophy is not clear. It has been reported that BMSCs promoted cardiac hypertrophy via hypoxia-induced paracrine mechanisms or promote more adaptive compensatory hypertrophy in the mice myocardial infarction model [34, 39]. However, a recent trial study showed that BMSC therapy has a positive effect on maladaptive hypertrophy after acute myocardial infarction measured by cardiac MRI cell [40]. The particular hypertrophic stimulus might play an important role in determining the impact of BMSCs. In addition, SDF-1 is another important cytokine factor released by BMSCs. The contribution of SDF-1 to antihyptrophic effects of BMSCs required further study (supplemental online Fig. 3).
Conclusion
We showed that crosstalk between BMSCs and cardiomyocytes caused substantial inhibition of molecular remodeling and restored cardiac functions in hypertrophic hearts, which is attributed to the secretion of VEGF from interplay of two kinds of cells and the consequent inhibition of the Ca2+/calcineurin/NFATc3 signaling pathway.
Supplementary Material
Acknowledgments
This work was supported by the Funds for Creative Research Groups of the National Natural Science Foundation of China (Grant 81121003), the National Natural Science Fund of China (Grant 81170096/81370245/30900601), and the Program for New Century Excellent Talents in Heilongjiang Provincial University (Grant 1252-NCET-013).
Author Contributions
B.C.: conception and design, provision of study materials, manuscript writing; X.T., X.L., X.W., J. Z., Y.W., F.Y., B.W., and Y. Liu: collection and assembly of data; Y.Z., C.X., and Z.P.: data analysis and interpretation; N.W.: administrative support; B.Y. and Y. Lu: financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perin EC, López J. Methods of stem cell delivery in cardiac diseases. Nat Clin Pract Cardiovasc Med. 2006;3(suppl 1):S110–S113. doi: 10.1038/ncpcardio0447. [DOI] [PubMed] [Google Scholar]
- 3.Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 4.Ripa RS, Haack-Sørensen M, Wang Y, et al. Bone marrow derived mesenchymal cell mobilization by granulocyte-colony stimulating factor after acute myocardial infarction: Results from the Stem Cells in Myocardial Infarction (STEMMI) trial. Circulation. 2007;116(suppl):I24–I30. doi: 10.1161/CIRCULATIONAHA.106.678649. [DOI] [PubMed] [Google Scholar]
- 5.Volarevic V, Arsenijevic N, Lukic ML, et al. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29:5–10. doi: 10.1002/stem.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang N, Li J, Luo R, et al. Bone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathy. Exp Clin Endocrinol Diabetes. 2008;116:104–111. doi: 10.1055/s-2007-985154. [DOI] [PubMed] [Google Scholar]
- 7.Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 8.Hahn JY, Cho HJ, Kang HJ, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Frey N, Olson EN. Cardiac hypertrophy: The good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 10.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang YJ. Cardiac hypertrophy: A risk factor for QT-prolongation and cardiac sudden death. Toxicol Pathol. 2006;34:58–66. doi: 10.1080/01926230500419421. [DOI] [PubMed] [Google Scholar]
- 12.Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of heart failure: A scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 13.Benzhi C, Limei Z, Ning W, et al. Bone marrow mesenchymal stem cells upregulate transient outward potassium currents in postnatal rat ventricular myocytes. J Mol Cell Cardiol. 2009;47:41–48. doi: 10.1016/j.yjmcc.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 15.Yamada Y, Yokoyama S, Wang XD, et al. Cardiac stem cells in brown adipose tissue express CD133 and induce bone marrow nonhematopoietic cells to differentiate into cardiomyocytes. Stem Cells. 2007;25:1326–1333. doi: 10.1634/stemcells.2006-0588. [DOI] [PubMed] [Google Scholar]
- 16.Ellison GM, Torella D, Karakikes I, et al. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J Biol Chem. 2007;282:11397–11409. doi: 10.1074/jbc.M607391200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong DL, Chen C, Huo R, et al. Reciprocal repression between microRNA-133 and calcineurin regulates cardiac hypertrophy: A novel mechanism for progressive cardiac hypertrophy. Hypertension. 2010;55:946–952. doi: 10.1161/HYPERTENSIONAHA.109.139519. [DOI] [PubMed] [Google Scholar]
- 18.Lin Z, Murtaza I, Wang K, et al. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci USA. 2009;106:12103–12108. doi: 10.1073/pnas.0811371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan H, Li X, Pan Z, et al. Tanshinone IIA protects against sudden cardiac death induced by lethal arrhythmias via repression of microRNA-1. Br J Pharmacol. 2009;158:1227–1235. doi: 10.1111/j.1476-5381.2009.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houser SR, Molkentin JD. Does contractile Ca2+ control calcineurin-NFAT signaling and pathological hypertrophy in cardiac myocytes? Sci Signal. 2008;1:pe31. doi: 10.1126/scisignal.125pe31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkins BJ, De Windt LJ, Bueno OF, et al. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol. 2002;22:7603–7613. doi: 10.1128/MCB.22.21.7603-7613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crabtree GR. Generic signals and specific outcomes: Signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 23.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 24.Uemura R, Xu M, Ahmad N, et al. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 25.Xu M, Uemura R, Dai Y, et al. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol. 2007;42:441–448. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rissanen TT, Markkanen JE, Gruchala M, et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;92:1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- 27.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohnishi S, Yanagawa B, Tanaka K, et al. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007;42:88–97. doi: 10.1016/j.yjmcc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Chang MG, Tung L, Sekar RB, et al. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation. 2006;113:1832–1841. doi: 10.1161/CIRCULATIONAHA.105.593038. [DOI] [PubMed] [Google Scholar]
- 30.Cho J, Zhai P, Maejima Y, et al. Myocardial injection with GSK-3β-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circ Res. 2011;108:478–489. doi: 10.1161/CIRCRESAHA.110.229658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai Y, Xu M, Wang Y, et al. HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol. 2007;42:1036–1044. doi: 10.1016/j.yjmcc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Hiroi Y, Ngoy S, et al. Notch1 in bone marrow-derived cells mediates cardiac repair after myocardial infarction. Circulation. 2011;123:866–876. doi: 10.1161/CIRCULATIONAHA.110.947531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Li T, Wei X, et al. Mesenchymal stem cell transplantation improves regional cardiac remodeling following ovine infarction. Stem Cells Translational Medicine. 2012;1:685–695. doi: 10.5966/sctm.2012-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu RX, Chen X, Chen JH, et al. Mesenchymal stem cells promote cardiomyocyte hypertrophy in vitro through hypoxia-induced paracrine mechanisms. Clin Exp Pharmacol Physiol. 2009;36:176–180. doi: 10.1111/j.1440-1681.2008.05041.x. [DOI] [PubMed] [Google Scholar]
- 35.Mignone JL, Murry CE. A repair “kit” for the infarcted heart. Cell Stem Cell. 2011;8:350–352. doi: 10.1016/j.stem.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izumiya Y, Shiojima I, Sato K, et al. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–893. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon O, Gilon D, He Z, et al. Vascular endothelial growth factor-induced neovascularization rescues cardiac function but not adverse remodeling at advanced ischemic heart disease. Arterioscler Thromb Vasc Biol. 2012;32:1642–1651. doi: 10.1161/ATVBAHA.112.248674. [DOI] [PubMed] [Google Scholar]
- 38.Shimojo N, Jesmin S, Zaedi S, et al. Contributory role of VEGF overexpression in endothelin-1-induced cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H474–H481. doi: 10.1152/ajpheart.00922.2006. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Malhotra D, Yeh CC, et al. Myocardial survival signaling in response to stem cell transplantation. J Am Coll Surg. 2009;208:607–613. doi: 10.1016/j.jamcollsurg.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rolf A, Assmus B, Schächinger V, et al. Maladaptive hypertrophy after acute myocardial infarction positive effect of bone marrow-derived stem cell therapy on regional remodeling measured by cardiac MRI. Clin Res Cardiol. 2011;100:983–992. doi: 10.1007/s00392-011-0330-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.