Human adipose-derived stem/stromal cell spheroids enriched in undifferentiated cells were efficiently prepared by using three-dimensional floating culture in non-cross-linked hyaluronic acid gel. The results suggest the superior plasticity and growth factor-secreting potential of the spheroids and clearly indicate their therapeutic power for promoting tissue regeneration. As an easy local injectable, spheroids are expected to be a powerful tool to treat various stem cell-depleted conditions/organs through nonintravenous administration.
Keywords: Three-dimensional culture, Non-cross-linked hyaluronic acid, Adipose-derived stem/stromal cells, Spheroids, Ischemia-reperfusion injury, Muse cells, Floating culture, Wound healing, Angiogenesis, Vascular endothelial cells
Abstract
Three-dimensional culture of mesenchymal stem/stromal cells for spheroid formation is known to enhance their therapeutic potential for regenerative medicine. Spheroids were prepared by culturing human adipose-derived stem/stromal cells (hASCs) in a non-cross-linked hyaluronic acid (HA) gel and compared with dissociated hASCs and hASC spheroids prepared using a nonadherent dish. Preliminary experiments indicated that a 4% HA gel was the most appropriate for forming hASC spheroids with a relatively consistent size (20–50 µm) within 48 hours. Prepared spheroids were positive for pluripotency markers (NANOG, OCT3/4, and SOX-2), and 40% of the cells were SSEA-3-positive, a marker of the multilineage differentiating stress enduring or Muse cell. In contrast with dissociated ASCs, increased secretion of cytokines such as hepatocyte growth factor was detected in ASC spheroids cultured under hypoxia. On microarray ASC spheroids showed upregulation of some pluripotency markers and downregulation of genes related to the mitotic cell cycle. After ischemia-reperfusion injury to the fat pad in SCID mice, local injection of hASC spheroids promoted tissue repair and reduced the final atrophy (1.6%) compared with that of dissociated hASCs (14.3%) or phosphate-buffered saline (20.3%). Part of the administered hASCs differentiated into vascular endothelial cells. ASC spheroids prepared in a HA gel contain undifferentiated cells with therapeutic potential to promote angiogenesis and tissue regeneration after damage.
Significance
This study shows the therapeutic value of human adipose-derived stem cell spheroids prepared in hyarulonic acid gel. The spheroids have various benefits as an injectable cellular product and show therapeutic potential to the stem cell-depleted conditions such as diabetic chronic skin ulcer.
Introduction
Human adipose-derived stem/stromal cells (hASCs) represent an abundant source of multipotent adult mesenchymal stem cells, which are easily obtained from subcutaneous adipose tissue via liposuction [1]. They can differentiate into not only adipocytes but also multiple lineages including osteogenic, chondrogenic [2], hepatogenic [3], myogenic [4], neurogenic [5], and angiogenic cell types [6, 7], although their differentiation potential appears to be generally limited. Recently, it has come to attention that human mesenchymal stem cells from the bone marrow, skin dermis, and adipose tissue contain a small fraction of privileged stem cells with greater plasticity, homing capacity, and higher stress resistance [8–10]. The selected cells, which are also called multilineage differentiating stress enduring (Muse) cells, are reported to divide symmetrically only when cultured under floating conditions and to form cell clusters. This result may also suggest that monolayer culture expansion gives rise only to cells with lower plasticity, although adherent monolayer culture is widely used to expand human bone marrow mesenchymal stem cells and ASCs. Indeed, the expression of pluripotency makers or the differentiation potential of adult stem cells is known to gradually decrease over time during monolayer culture [11, 12].
Currently, the application of three-dimensional (3D) cell culture techniques for spheroid formation is receiving great interest, because stem cell spheroids tend to show higher therapeutic potential. Many studies have suggested that spheroid formation can boost functionality of stem cell components and avoid unfavorable migration when injected into local tissues. Several culture methods have been used to induce spheroid formation, including methods using a nonadherent specific dish [13–15], a spinner flask [16, 17], hanging drops [18, 19], and hydrogels [20, 21]. These methods facilitate cell-cell contacts and cell-matrix adhesion with the secreted extracellular matrix (ECM) [22]. By forming spheroids with native cell morphology, the cells show significant differences in pluripotent capacity compared with their counterparts cultured in a monolayer [23–25]. However, some issues for spheroid preparation remain to be optimized or resolved. If the size of spheroids is too large, central necrosis may develop, because the core of large spheroids will experience limited nutrient diffusion and severe hypoxia in vivo, particularly after injection into ischemic tissue [26, 27]. Additionally, inadvertent infusion of large spheroids into vessels can result in vascular thrombosis and aggravating an ischemic condition. Specific microplates for spheroid formation are commercially available, but the cost can be a concern depending on the number of spheroids to be prepared.
The aim of this study was to develop a simple and efficient method of spheroid preparation, which would provide a large amount of ASC spheroids with an appropriate and uniform size in a short time interval, for application in clinical ASC-based regenerative therapies. Because hyaluronic acid (HA) is a safe and histocompatible matrix [28] and is used for many clinical and research applications, such as an injectable carrier of stem cells and an injectable drug for cosmetic volumization or treating osteoarthritis, we used non-cross-linked HA gel for 3D culture preparation of hASC spheroids. In this study, we optimized the preparation method and investigated the biological properties and therapeutic potential of hASC spheroids prepared in a HA gel compared with monolayer-cultured hASCs.
Materials and Methods
Cell Isolation and Culture
Liposuction aspirates were obtained from healthy female donors (body mass index: 18–22) undergoing liposuction of the abdomen or thighs. Prior to the procedure, each patient provided her informed consent using an institutional review board-approved protocol. Human ASCs were isolated from the aspirated fat as described previously [29]. Briefly, the aspirated fat was washed with phosphate-buffered saline (PBS) and digested on a shaker at 37°C in PBS containing 0.075% collagenase for 30 minutes. Mature adipocytes and connective tissue were separated from cell pellets by centrifugation (760g, 5 minutes). The pellets were rinsed and resuspended after being filtered through 100-μm mesh. The suspension was disseminated at a density of 1.0 × 106 nucleated cells per 100-mm dish and cultured at 37°C in a humid 5% CO2 atmosphere. The culture medium was Dulbecco’s modified Eagle’s medium (DMEM; Nissui, Tokyo, Japan, http://www.nissui-pharm.co.jp) supplemented with 10% fetal bovine serum (FBS). Primary cells were cultured for 7 days and were defined as passage 0. The medium was replaced three times per week. Cells were passaged every week with 0.25% trypsin, 2 mM EDTA for 5 minutes at 37°C. Cultured hASCs at passages 1 and 2 were used in the following experiments. Detailed characterization of the cultured hASCs used in this study was previously published [29].
3D Floating Culture in a HA Gel
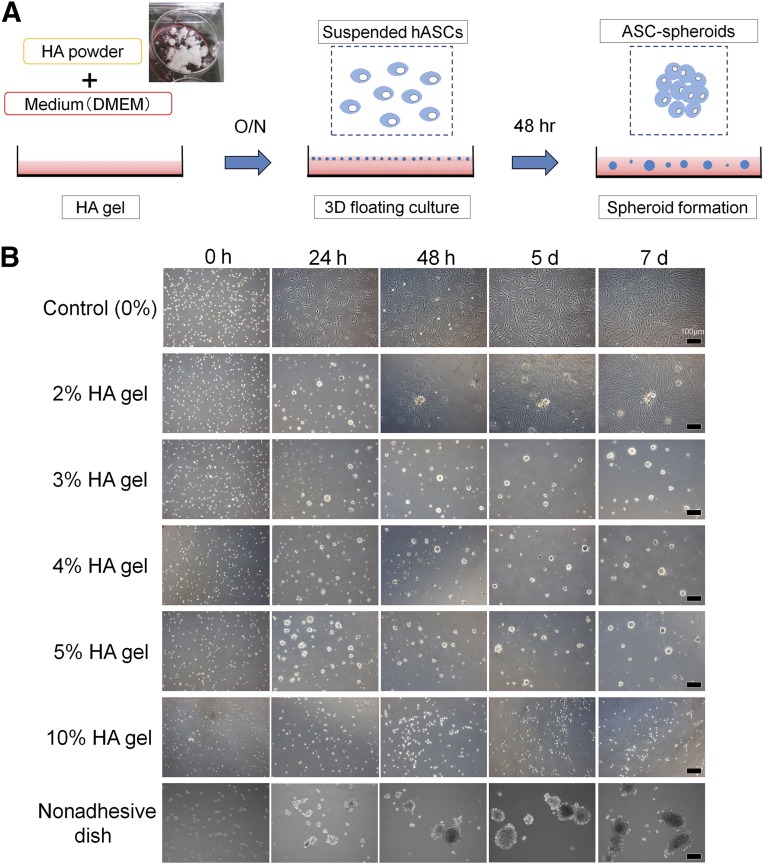
HA gel was prepared by mixing HA powder and DMEM overnight on a plastic dish (Fig. 1A). The HA powder was a sterilized (pharmaceutical grade) product and non-cross-linked with an average molecular weight of 1,000 kDa (Kikkoman Biochemifa, Tokyo, Japan, http://biochemifa.kikkoman.co.jp). Suspended hASCs (in culture medium with the same volume as HA gel) were disseminated in the HA gel (1.0 × 104 cells per cm2) and incubated (37°C, 5% CO2) for 48 hours. Consequently, hASC spheroids were formed in the HA gel. For example, a 4% HA gel culture was prepared from 5% HA gel and a quarter volume of added cell suspension. To harvest the spheroids, medium was added to dilute HA gel, followed by incubation for 3 hours (37°C, 5% CO2). The diluted supernatant containing hASC spheroids was collected in a silicon tube. ASC spheroids were obtained as a pellet from HA gel by three times dilution of HA gel with PBS and centrifugation (760g, 5 minutes).
Figure 1.
Three-dimensional floating culture in HA gel. (A): First step (left). HA gel is prepared by mixing sterilized HA powder and culture medium (DMEM) overnight. Middle: Second step. Human ASCs (1.0 × 104 cells per cm2) are disseminated on the HA gel. Right: Final step. After incubation for 48 hours (37°C, 5% CO2), ASCs formed spheroids in the gel. For retrieval, culture medium is added to dilute HA gel and incubated for 3 hours. The diluted supernatant containing ASC spheroids is transferred into a silicon tube, and ASC spheroids are collected as a pellet by centrifugation (760g, 5 minutes). (B): Three-dimensional culture in HA gels (0%, 2%, 3%, 4%, 5%, or 10%) or on a nonadhesive dish. Human ASCs were seeded in the HA gels of various (0%–10%) concentrations. In 2%–3% HA gel ASC formed spheroids, but some of them sank and proliferated on the bottom of the dish like an explant culture. In contrast, in 4%–5% HA gel formation of floating spheroids was completed at 48 hours without further substantial change in size afterward. Spheroids were not formed in 10% HA gel. On a nonadhesive dish ASC spheroids continued growing until day 7. Scale bars = 100 μm. Abbreviations: 3D, three-dimensional; ASC, adipose-derived stem/stromal cell; d, day(s); DMEM, Dulbecco’s modified Eagle’s medium; h or hr, hour(s); HA, hyaluronic acid; hASCs, human adipose-derived stem/stromal cells; O/N, overnight.
As a preliminary experiment, various concentrations of HA gel (final concentrations: 0%, 2%, 3%, 4%, 5%, and 10%) were used. Suspended hASCs (1.0 × 104 cells per cm2) were disseminated in each HA gel and observed for 7 days. Furthermore, for comparison, hASCs were also cultured on a nonadhesive dish (catalog no. 657970; Greiner Japan, Tokyo, Japan, http://www.greinerbioone.com) for spheroid formation.
Microarray Analysis
Samples were prepared as follows. Monolayer-cultured hASCs were harvested with 0.25% trypsin, 2 mM EDTA for 5 minutes at 37°C and were dissolved with Isogen (Wako, Osaka, Japan, http://www.wako-chem.co.jp) as a control sample. ASC spheroids were harvested after 48 hours of culture in 4% HA gel and were dissolved with Isogen as well. Total RNA was purified using the RNeasy mini kit (Qiagen Inc., Valencia, CA, http://www.qiagen.com) according to the manufacturer’s recommendations. Fluorescently labeled cRNA was synthesized using a Quick Amp Labeling Kit (Agilent Technologies, Santa Clara, CA, http://www.agilent.com). Labeled cRNAs were hybridized with the SurePrint G3 human GE microarray 8 × 60K (G4851A; Agilent Technologies). Microarrays were scanned using a G2505C microarray scanner, and the results were analyzed using Feature extraction software (Agilent Technologies). Finally, we analyzed gene expression using GeneSpring GX 12.5 software (Agilent Technologies).
ELISA
Human ASC spheroids and monolayer-cultured hASCs were seeded at 1.0 × 105 cells in 60-mm dishes and cultured in DMEM with FBS for 24 hours (37°C, 5% CO2). Subsequently, the medium was exchanged for DMEM without FBS, and each dish was incubated under hypoxic (1% O2) or normoxic (6% O2) conditions. After 48 hours, the culture media were collected and filtered (0.22-μm filter, Millex-GV filter; Millipore, Billerica, MA, http://www.millipore.com). Enzyme-linked immunosorbent assay (ELISA) kits (Ray Biotech, Inc., Norcross, GA, http://www.raybiotech.com) for hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), platelet-derived growth factor (PDGF-BB), and basic fibroblast growth factor (bFGF) were used. The absorbance was spectrophotometrically measured at 450 nm using an infinite microplate reader (M1000; Tecan Group, Männedorf, Switzerland, http://www.tecan.com).
Immunostaining of Spheroids for Stem Cell Markers
Prepared hASC spheroids were embedded in OCT compound (Sakura Finetek, Tokyo, Japan, http://www.sakura-finetek.com). Cryopreserved samples were cut at 8-μm thickness and immunostained after fixation with 4% paraformaldehyde. The following primary antibodies were used for immunohistochemistry: rabbit anti-NANOG antibody (GeneTex, Irvine, CA, http://www.genetex.com), rabbit anti-OCT3/4 antibody (GeneTex), rabbit anti-SOX2 antibody (GeneTex), rat anti-SSEA-3 antibody (Millipore), and mouse anti-CD90 antibody (Biolegend, San Diego, CA, http://www.biolegend.com). These primary antibodies were detected with Alexa Fluor 568-conjugated anti-rabbit IgG (Molecular Probes, Carlsbad, CA, http://probes.invitrogen.com), fluorescein isothiocyanate-conjugated anti-rat IgM (Jackson ImmunoResearch, West Grove, PA, http://www.jacksonimmuno.com), and Alexa Fluor 488-conjugated anti-mouse IgG (Molecular Probes). Samples were incubated with blocking solution containing 5% FBS and 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) in PBS at room temperature for 60 minutes. Samples were then incubated with primary antibodies overnight at 4°C. After three washes with PBS, samples were incubated with secondary antibodies for 60 minutes at room temperature. Nuclei were stained with Hoechst 33342 (Dojindo, Kumamoto, Japan, http://www.dojindo.com). All images were taken with fluorescent microscopy (Keyence, Osaka, Japan, http://www.keyence.com) using the same laser intensity and detection sensitivity.
Mouse Model for Ischemia-Reperfusion Injury to Adipose Tissue
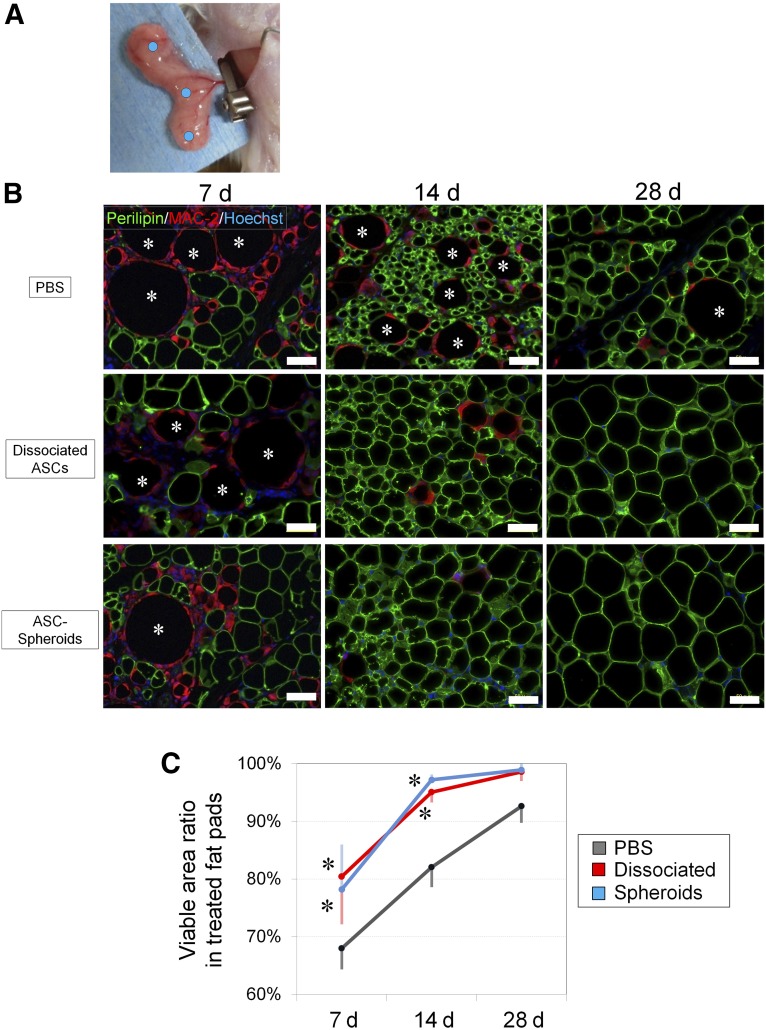
Ten- to twelve-week-old male severe combined immunodeficient (SCID) mice (C.B-17/lcr-scid/scidJcl) were purchased from CLEA Japan, Inc. (Tokyo, Japan, http://www.clea-japan.com). All animal experiments were performed with approval from the Institutional Animal Care and Use Committee of the University of Tokyo. After SCID mice were fasted for 24 hours, they were anesthetized with inhaled isoflurane, and a 1.5-cm skin incision was made in the inguinal region. Under a surgical microscope, the subcutaneous inguinal fat pad was elevated with the main nutrient vessels arising from the femoral vessels intact. The nutrient vessels were clamped with a vessel microclip for 4 hours to induce temporary ischemia. This injury and repair model is reliable to see the wound-healing process as previously published [30]. Immediately after the vessels were released to allow reperfusion, 200 μl of treatment solution was injected with a 30-gauge needle into the fat pad at three points. The treatment solution was PBS (n = 6), monolayer-cultured hASCs (n = 6), or hASC spheroids (n = 6). The dissociated hASCs were obtained by conventional monolayer culture (5.0 × 105 cells per 10-cm dish) for 48 hours. By contrast, hASC spheroids were obtained by 3D floating culture (5.0 × 105 cells per 10-cm dish) in a 4% HA gel for 48 hours. The cultured hASCs obtained from a single subject were used in the animal experiment. The adipose tissue samples were harvested carefully under the surgical microscope at various intervals (at 7, 14, and 28 days) after ischemia-reperfusion injury, weighed, and examined by immunohistochemistry. Therapeutic effects were evaluated with a “tissue repair score,” which was calculated by multiplying the survival area ratio and the relative weight of the fat pad. The survival area ratio is the percentage of perilipin-positive area in the histological cross-sections of the tissue, and the relative weight of the fat pad is (weight of fat pad)/(weight of body).
Immunostaining of Adipose Tissue
Harvested adipose tissue samples were zinc-fixed (Zinc Fixative; BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) and paraffin-embedded. The samples were sectioned at 5 μm and subjected to the following staining procedures. The following primary antibodies were used for immunohistochemistry: guinea pig anti-perilipin antibody (Progen Biotechnik, Heidelberg, Germany, http://www.progen.de), rat anti-MAC-2 antibody (Cedar Lane Laboratories, Burlington, Canada, http://www.cedarlanelabs.com), anti-58K human Golgi protein antibody (Abcam, Cambridge, U.K., http://www.abcam.com). Isotypic antibodies were used as a negative control for each immunostaining. Alexa Fluor 488- or 568-conjugated secondary antibodies (Molecular Probes) were used for visualization. Vessels (vascular endothelial cells) were stained with Alexa Fluor 594-conjugated isolectin GS-IB4 (Molecular Probes), and nuclei were stained with Hoechst 33342 (Dojindo). All images were captured with fluorescent microscopy (Keyence, Osaka, Japan) using the same laser intensity and detection sensitivity. The area composed of perilipin-negative (dead adipocytes) or perilipin-positive (viable adipocytes) cells was evaluated by image analysis software (Photoshop CS6; Adobe Systems, San Jose, CA, http://www.adobe.com).
Statistical Analysis
The results were expressed as the means ± SD. Comparisons between the two groups were performed with Welch’s t test. Comparisons of multiple groups were done by Tukey’s tests. A value of p < .05 was considered statistically significant.
Results
Appropriate Concentration of HA Gel for 3D Floating Culture
Suspended hASCs were disseminated in each well of a 6-well dish (1.0 × 104 cells per cm2) filled with various concentrations of HA gel (Fig. 1B). In 2%–3% HA gels, some ASCs and ASC spheroids sunk to the bottom of the dish and then proliferated on the dish. In contrast, in 4%–5% HA gel hASCs did not sink; spheroid formation was completed within 48 hours, and the spheroid size did not change substantially afterward. No spheroid was formed in the 10% HA gel. Based on the above results, 3D culture in 4% HA gel for 48 hours was used for hASC spheroid preparation in the following experiments. On the nonadhesive dish, hASCs formed spheroids but continued growing until 7 days resulting in spheroids of very variable size.
Morphological Difference in Spheroids Prepared by HA Gel and on a Nonadhesive Dish
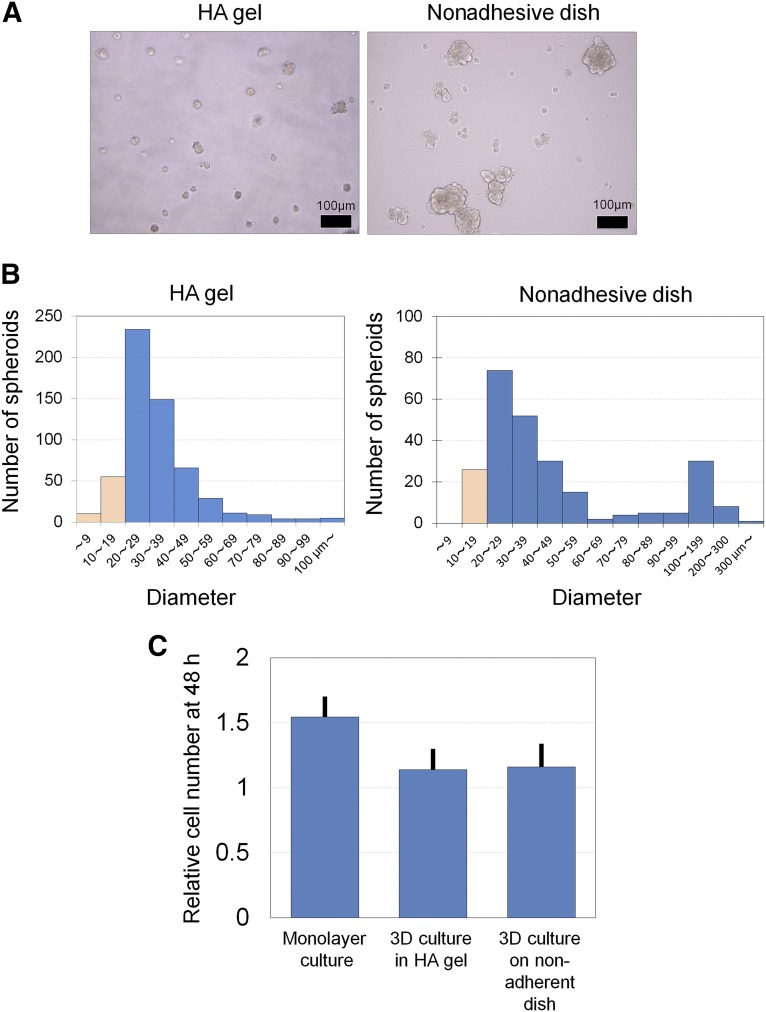
Human ASC spheroids were prepared using either 3D culture in a HA gel or nonadhesive dish culture for 48 hours (Fig. 2A). The histograms of spheroid diameter showed that hASC spheroids formed in a HA gel presented a unimodal distribution with relatively small (approximately 30 μm) size (Fig. 2B). On the other hand, hASC spheroids formed on a nonadhesive dish showed a bimodal distribution (with peaks at 20–60 and 100–200 μm). Cell proliferation was relatively suppressed in spheroid formation by 3D culture in the HA gel or on a nonadhesive dish compared with monolayer culture (Fig. 2C).
Figure 2.
Differences between spheroids prepared in a HA gel and on a nonadherent dish. (A): Microscopic images of adipose-derived stem/stromal cell (ASC) spheroids that were prepared by 48-hour 3D culture in 4% HA gel or on a nonadherent dish. Scale bars = 100 μm. (B): Representative histograms of ASC spheroid diameter prepared in 4% HA gel or on a nonadherent dish. Cell aggregates with a diameter >20 μm were regarded as spheroids (blue). In the HA gel the spheroid diameter showed a unimodal distribution and was relatively small. By contrast, the diameter showed bimodal distribution on the nonadhesive dish, and a substantial number of ASC spheroids were >100 μm in diameter. Abbreviations: 3D, three-dimensional; h, hour(s); HA, hyaluronic acid.
Microarray (Monolayer Culture vs. 3D Floating Culture in HA Gel)
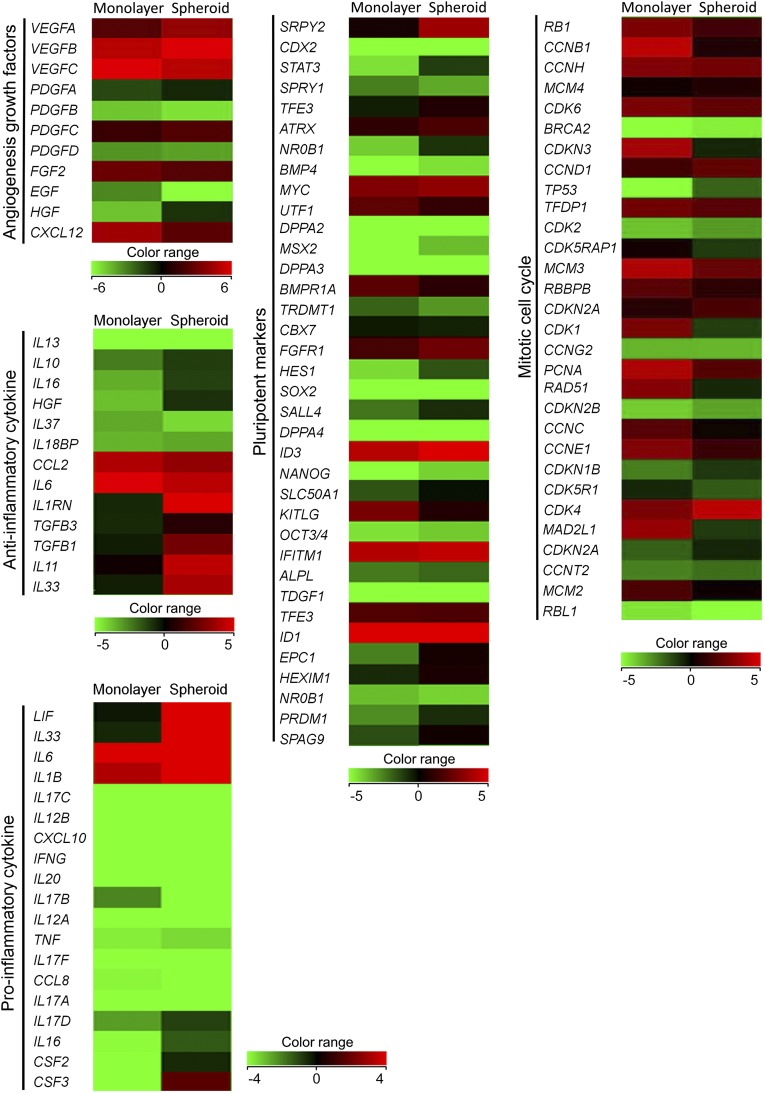
Microarray analyses were performed to analyze differences in gene expression between monolayer-cultured hASCs and hASC-spheroids prepared by 3D culture in the HA gel. Spheroid formation promoted gene expression of some angiogenesis-related growth factors (VEGFA, VEGFB, HGF, PDGFA, PDGFC), anti-inflammatory factors (IL1RN, IL11, etc.) and pluripotent stem cell markers (NANOG, OCT3/4, STAT3, etc.) compared to monolayer culture (Fig. 3). In addition, gene expression related to the mitotic cell cycle was generally decreased by spheroid formation in the HA gel.
Figure 3.
Microarray analysis of human adipose-derived stem/stromal cells (ASCs) prepared by monolayer culture versus ASC spheroids by three-dimensional culture in hyaluronic acid gel. ASC spheroids showed increased gene expression of some angiogenic growth factors (VEGFA, VEGFB, HGF, PDGFA, PDGFC), anti-inflammatory factors (IL1RN, IL11, etc.), and pluripotent markers (NANOG, OCT3/4, STAT3, etc.) compared with monolayer-cultured ASCs. In contrast, gene expression related to the mitotic cell cycle decreased thoroughly in ASC spheroids.
ELISA for Angiogenic Growth Factors
ELISA results indicated that secretion of VEGF, HGF, and EGF, but not PDGF-BB, generally increased under hypoxic conditions in nonspheroid and spheroid hASCs (supplemental online Fig. 1). Spheroid hASCs showed greater secretion of HGF than nonspheroid hASCs under both normoxic and hypoxic conditions. PDGF-BB secretion did not differ between the groups. In addition, bFGF secretion was at an undetectable level in all groups (data not shown).
Stem Cell Marker Expression of hASC Spheroids
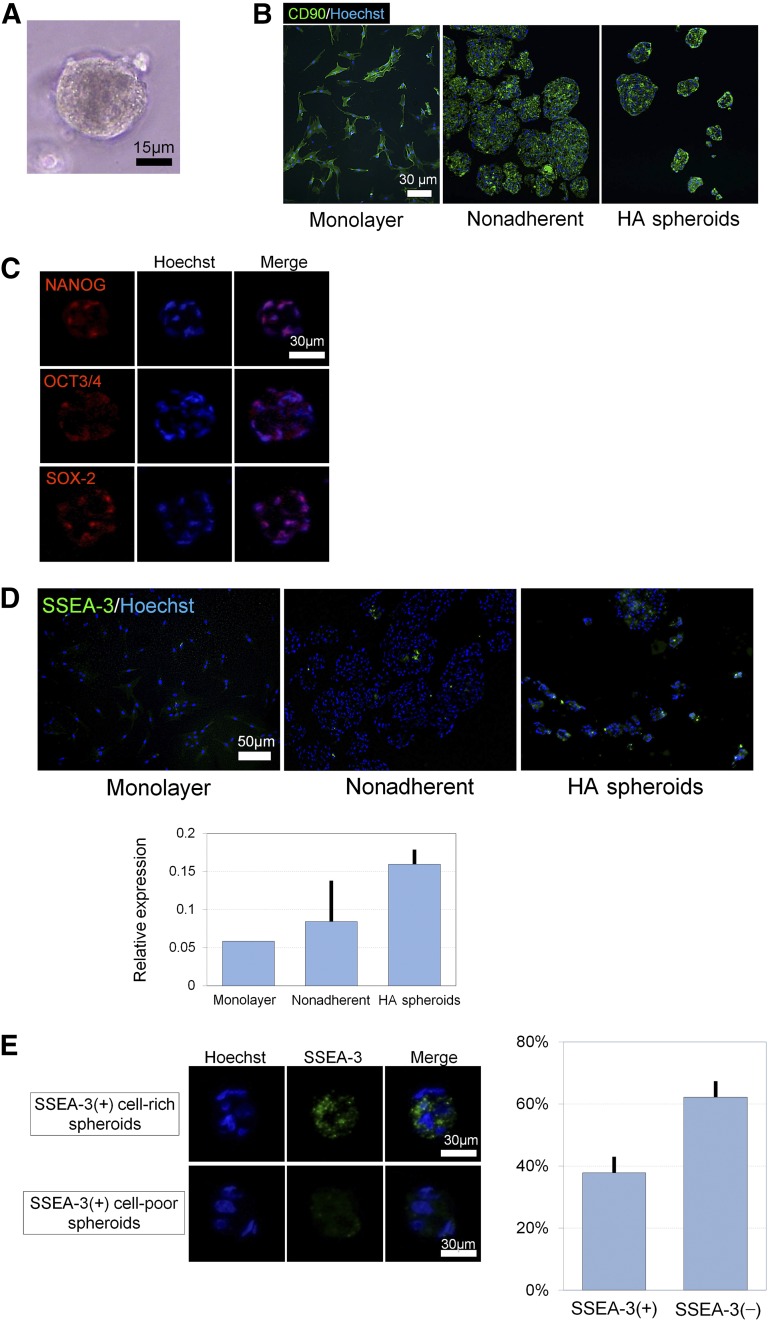
Spheroids of hASCs prepared in the HA gel were orbicular, and their surface looked smooth (Fig. 4A). Immunocyto-/histochemistry indicated that all hASCs expressed CD90 in monolayer culture, nonadherent spheroid culture, and HA-gel spheroid culture (Fig. 4B). It was also indicated that ASC spheroids expressed various stem cell markers such as NANOG, OCT3/4, and SOX-2 (Fig. 4C). SSEA-3, which is a specific marker of Muse cells, was expressed only ~1% of hASCs in monolayer culture, but it was more highly expressed spheroid culture, especially in hASC spheroids prepared in HA gel (Fig. 4D). Approximately 40% of the spheroids were rich in SSEA-3-positive hASCs (Fig. 4E).
Figure 4.
Immunostaining of adipose-derived stem/stromal cell (ASC) spheroids prepared in HA gel. (A): Microscopically, spheroids were consistently orbicular in shape and had a smooth surface. Scale bar = 15 μm. (B): Immunocyto-/histochemistry for CD90. CD90 was expressed by almost all cells in monolayer, nonadherent, and HA spheroids. Scale bar = 30 μm. (C): Representative immunostaining of cryosections. ASC spheroids expressed pluripotent stem cell markers such as NANOG, OCT3/4, and SOX-2. Scale bar = 30 μm. (D): Immunocyto-/histochemistry for SSEA-3. SSEA-3 was most highly expressed by HA spheroids compared with monolayer-cultured ASCs and nonadherent culture spheroids. Scale bar = 50 μm. (E): Some of the ASC spheroids were positive for SSEA-3, a pluripotent stem cell (muse cell) marker. SSEA-3(+)-rich spheroids constituted approximately 40% of ASC spheroids. Scale bars = 30 μm. Abbreviation: HA, hyaluronic acid.
Wound Healing Process After Ischemia-Reperfusion Injury of Inguinal Fat Pad and Therapeutic Effects of Monolayer-Cultured or Spheroid ASCs
The inguinal fat pads of SCID mice underwent ischemia-reperfusion injury (Fig. 5A) and were harvested 7, 14, or 28 days after the injury. After the injury, numerous adipocytes were killed and became oil droplets (perilipin-negative round areas) of various sizes (depending on the number of dead adipocytes) that were later surrounded by infiltrated MAC-2-positive macrophages for phagocytosis (crown-like structure) by day 7 (degeneration phase; Fig. 5B). Thereafter, the injury activated tissue-resident stem/progenitor cells such as mouse ASCs, which then started to differentiate into adipocytes (perilipin-positive small adipocytes) by day 14. The regeneration process including adipogenesis and angiogenesis was clearly seen on day 14 in all samples (regeneration phase). Tissue degeneration looked similar among all treated groups on day 7, but tissue regeneration happened more quickly and consistently in hASC-treated or hASC spheroid-treated samples compared with PBS-treated controls on day 14. Indeed, immunohistology after 28 days indicated that the tissue was well-filled with intact size adipocytes, and wound healing looked complete in samples treated with hASCs or hASC spheroids, although PBS-treated samples still showed the typical features of ongoing wound healing such as lipid droplets and small immature adipocytes. Viable adipocyte area (perilipin-positive area), as well as the total area, was measured, and the calculated qualitative data indicated a significant difference in the tissue viability and wound healing process among the groups (Fig. 5C).
Figure 5.
Histological evaluation of cell-based treatments for ischemia-reperfusion injury of inguinal fat pad in SCID mice. (A): The main nutrient vessels arising from the femoral vessels were clamped with a microclip for 4 hours and then allowed to perfuse. Mice models were divided into three groups, and each group was treated with an injection of PBS, monolayer-cultured ASCs, or ASC spheroids at three points (blue dots) of the fat pad. (B): Immunohistochemistry for perilipin (viable adipocytes) and MAC-2 (macrophages) revealed that both monolayer-cultured ASCs and ASC spheroids improved the adipose tissue repair and regeneration after ischemia-reperfusion injury. Many dead adipocytes (perilipin-negative) surrounded by infiltrated M1 macrophages were detected at 7 days, indicating substantial tissue damage by the injury. At 14 days, adipose regeneration (perilipin-positive small adipocytes) was seen, and the tissue repair was almost completed by 28 days in samples treated with monolayer-cultured or spheroid ASCs. Asterisks show dead adipocytes. Scale bars = 50 μm. (C): At 7 and 14 days, samples treated with monolayer-cultured or spheroid ASCs showed an increased viable area ratio compared with PBS-treated samples, suggesting that the injury repair process was accelerated by the cell treatments. ∗, p < .05 compared with control (PBS). Abbreviations: ASC, adipose-derived stem/stromal cell; d, days; PBS, phosphate-buffered saline.
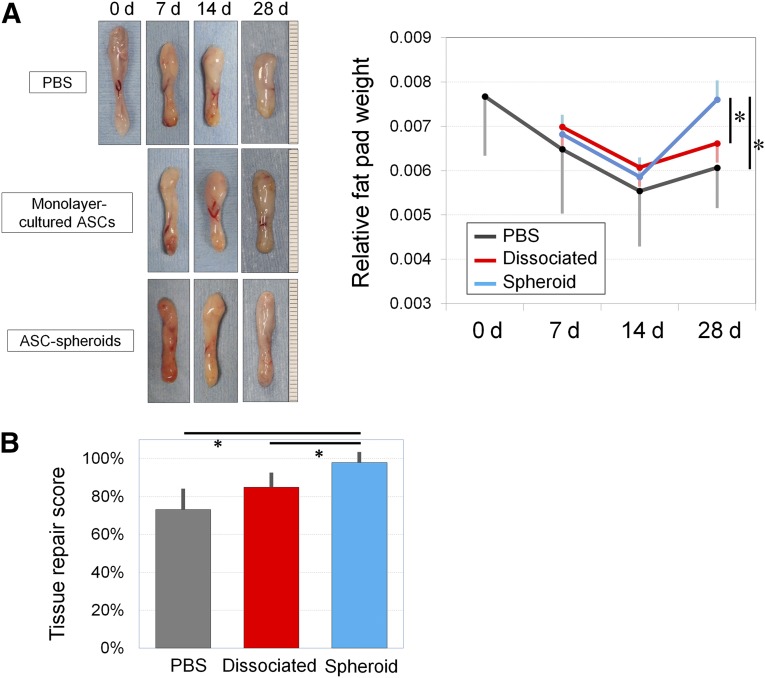
Quantitative evaluation of wound healing from the injury was also performed. The gross weight of the fat pad and the whole body was measured (Fig. 6A). PBS-treated samples gradually atrophied over time and were smallest among the treatment groups on day 7, 14, and 28. The relative weight of hASC spheroid-treated samples recovered quickly after day 14 and was significantly larger compared with the other groups on day 28. Overall tissue repair was scored by multiplying the survival area ratio and the relative weight of the fat pad, and there was the least fat atrophy (2.0% ± 0.6%) in samples treated with ASC spheroids compared with the control (26.8% ± 11.0%) and ASC-treated groups (15.1% ± 7.7%; Fig. 6B).
Figure 6.
Treatment with human ASCs for ischemia-reperfusion injury. (A): Representative samples of treated inguinal fat pads harvested at 7, 14, and 28 days. PBS-treated samples showed injury-induced atrophy on day 28. The relative sample weight (sample weight/body weight) decreased after injury and reached its nadir on day 14 in each group, followed by weight recovery caused by adipose regeneration. The sample weights on day 28 were significantly greater in the spheroid-treated group compared with the other groups. ∗, p < .05. (B): Tissue recovery score was calculated by multiplying the relative weight and the viable area ratio of samples to show the gross recovery of damaged adipose tissue (100% means complete recovery to the level before injury). Nearly full recovery was seen in spheroid-treated samples, which was significantly superior to the other two groups. ∗, p < .05. Abbreviations: ASC, adipose-derived stem/stromal cell; d, days; PBS, phosphate-buffered saline.
Immunohistochemical Evaluation of Angiogenesis and Incorporation of Administered hASC Spheroids
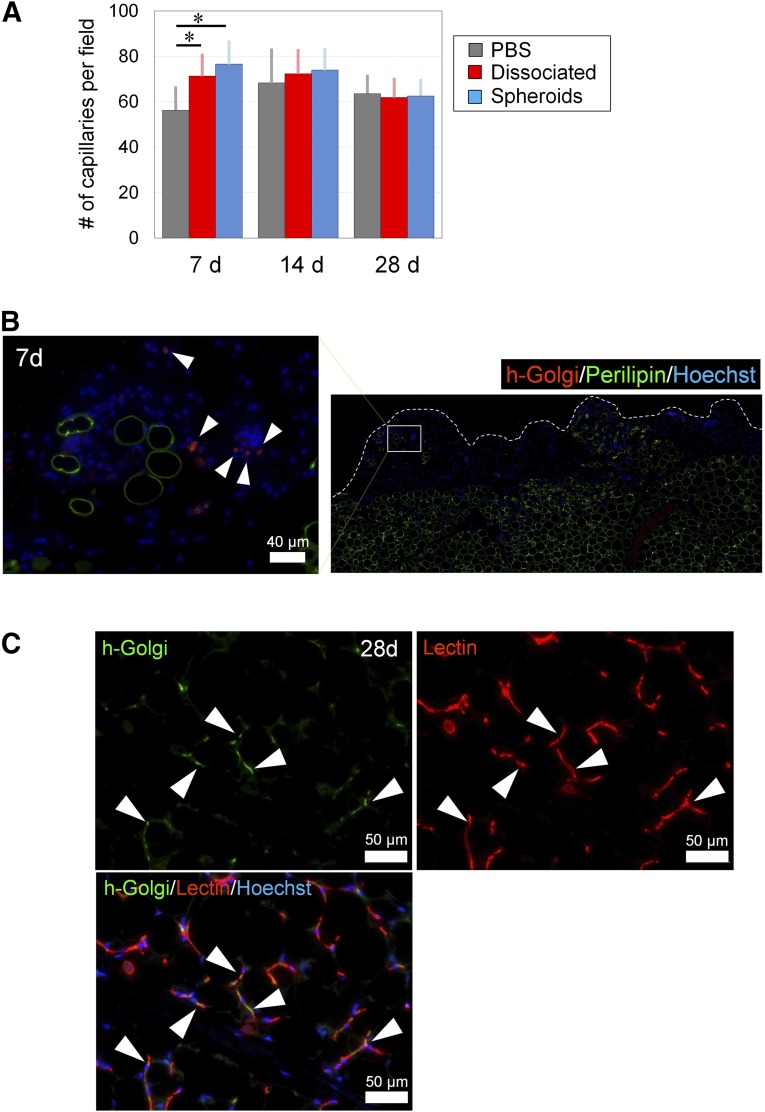
Vascular endothelial cells were counted using lectin-stained sections of samples treated with hASC spheroids. Although the ASC spheroids group displayed the highest capillary density on day 7, the change was no longer significant on day 28 when angiogenesis/adipogenesis was almost completed (Fig. 7A). On day 7, human Golgi-positive cells (administered cells derived from hASC spheroids) were detected mainly in the degenerated area in the periphery of the injured inguinal fat pad in samples both treated with hASCs and hASC spheroids (Fig. 7B), suggesting that the injected hASCs migrated and homed selectively to the damaged area and were involved in the repair process. On day 28, immunostaining indicated that a number of hASC spheroid-derived cells (human Golgi-positive cells) were incorporated into the repaired tissue (Fig. 7C). Substantial parts of hASCs were lectin-positive and located between adipocytes, suggesting that they differentiated into vascular endothelial cells and contributed to the reconstructed capillary vessel network.
Figure 7.
Histological assessment of vasculature and contribution of administered adipose-derived stem/stromal cells (ASCs). (A): The number of capillaries were counted using microsections stained with isolectin. Although the ASC spheroid group showed the highest capillary density at 7 days, this change was no longer significant at 4 weeks. (B): Immunostained section of spheroid-treated sample at 1 week. The human Golgi-positive cells (injected ASCs) were detected mainly in the degenerated adipose area, which showed less perilipin expression. Bar = 40 μm. (C): Immunostained section of spheroid-treated sample at 4 weeks. Some of the h-Golgi(+) cells (arrowheads) expressed isolectin, indicating that they were incorporated into the repaired adipose tissue as vascular endothelial cells. Scale bars = 50 μm. ∗, p < .05. Abbreviations: d, days; h-Golgi(+), human Golgi-positive; PBS, phosphate-buffered saline.
Discussion
HA is a glycosaminoglycan disaccharide composed of alternately repeating units of d-glucuronic acid and N-acetylglucosamine. HA is naturally found in the ECM of many human tissues/organs including the skin, joints, and eyes. In particular, the skin tissue contains the highest amount, which is approximately 50% of the total HA in the body [31]. Therefore, various preparations of HA are widely used as pharmaceutical and cosmetic products targeting the skin and subcutaneous tissue. In this study, we used the non-cross-linked HA powder (pharmaceutical grade), which was able to resolve rapidly with the culture medium. With this advantage, formed cell spheroids were isolated easily only by dilution of the gel and subsequent centrifugation without using an enzyme digestion. In the 3D floating culture in HA gel, hASC spheroids could be prepared over a comparative time interval to nonadherent culture system (as short as 48 hours) with relatively uniform size (approximately 30 μm in diameter) compared with 3D culture with a nonadhesive culture dish. For local injection of cell spheroids, a certain size such as 20 μm is required to avoid unfavorable local diffusion or migration into the circulation [32], but spheroids larger than 150 μm can induce central necrosis of spheroids/aggregation [33].
Cell spheroids/aggregates can be formed by various 3D culture methods including hanging drop, nonadherent culture flask, various biodegradable coatings, and scaffolds or gels of ECM components or synthetic materials [34, 35]. Furthermore, previous studies have suggested that spheroids produce growth factors to a greater degree than controls in vitro or in vivo and show therapeutic potential and that cell spheroids/aggregates may show pluripotent potential (perhaps because of cell selection or dedifferentiation). Our microarray data suggest that gene expression of some angiogenesis-related growth factors (such as VEGFA, VEGFB, HGF, PDGFA, and PDGFC) and pluripotent markers (such as NANOG, OCT3/4, and STAT3) are upregulated and that cell mitosis-related genes are down-regulated in HA-gel spheroid culture compared with monolayer culture. Increased secretion of cytokines such as HGF was also confirmed in ASC spheroids cultured under hypoxia. Immunohistology showed that ASC spheroids contained cells expressing some pluripotency markers such as NANOG, OCT3/4, and SOX-2. Additionally, approximately 40% of spheroids contained SSEA-3-positive cells, suggesting the efficient enrichment of muse cells by the 3D floating culture process. Because expanding the SSEA-3-positive cells by monolayer adherent culture was difficult (approximately 1%–2%; data not shown) [8], a 3D floating culture in HA gel may selectively expand the SSEA-3-positive population, which we recently showed has greater therapeutic capacity for a refractory diabetic skin ulcer even without forming spheroids [36]. Indeed, numerous single cells were also observed at 48 hours in the HA gel, and thus only a specific ASC population may have expanded and/or aggregated under this condition.
HA gel culture has several specific characteristics. Although mesenchymal cells generally express the cell surface receptor for HA (CD44), HA appears not to alter cellular biological properties and functions compared with other major ECM components such as collagen, fibronectin, and laminin. Because spheroid formation did not occur when the HA gel was stiff at high concentrations, its mechanical properties may be key for the appropriate formation of spheroids. The results indicate that it is possible to efficiently obtain within 3 days a large number of ASC spheroids with a relatively uniform diameter using common cultureware and an incubator. HA is already clinically used as an injectable pharmaceutical in many formulations, and thus its safety and negligible immunoreaction have been established. Therefore, ASC spheroids prepared in a HA gel can be easily injected without extraction (along with the HA gel as a safe cell carrier). The 4% non-cross-linked HA gel (0.1 ml) was absorbed in several days in the subcutis of mice (data not shown), and the degradation duration can be adjusted by modification of the molecular weight and cross-linking ratio of the HA products.
In the animal study, we used an ischemia-reperfusion injury to the fat pad in immunodeficient mice. Four hours of ischemia and subsequent reperfusion induced substantial damage to the adipose tissue, resulting in tissue atrophy (30% tissue loss) after recovery at 28 days. Acute tissue damage was greater in the periphery of the fat pad, leading to local tissue necrosis at 7 days, but damage was minimized in the spheroid-treated group, as indicated by an increased angiogenesis (increasing number of lectin-positive vascular endothelial cells). Regenerative changes, including adipogenesis (perilipin-positive small adipocytes between dead adipocytes), were observed at 7–14 days to a differential extent among the treatment groups. Samples treated with spheroid ASCs and monolayer-cultured ASCs clearly showed accelerated tissue repair at 14 days, when many unscavenged adipocytes remained, and newly grown small preadipocytes were seen in PBS-treated samples. Tissue repair was almost completed by 28 days, and spheroid-treated samples showed remarkable regeneration after 14 days. Cells finally recovered up to nearly an intact level (2.0% atrophy in viable adipose volume), which was significantly better than samples treated with monolayer-cultured ASCs showing 15.1% atrophy. Thus, although even monolayer-cultured ASCs indicated a therapeutic value in this model, ASC spheroids prepared in the HA gel clearly showed a superior capacity to enhance tissue regeneration and rescue the damaged tissue from irreversible atrophy.
Histological tracing of administered cells partly uncovered the fate of the administered spheroids. On day 7, injected human spheroids had already dissociated; they migrated and homed predominantly to the damaged peripheral region, suggesting that they were attracted by soluble factors derived from damaged tissue and were already involved in the tissue repair process. The sections of day 28 fat pads indicated that a number of injected spheroid ASCs were incorporated into the regenerated adipose tissue. Although it remains unknown whether they became adipocytes, ASCs definitively differentiated into vascular endothelial cells and contributed to the newly formed vascular network. ELISA assay showed upregulated HGF levels in HA spheroids compared with monolayer culture and elevated expression of EGF under hypoxic condition compared with normoxic condition in both monolayer culture and HA spheroids. EGF may have contributed to promoting healing in both monolayer and HA spheroid treatments, and HGF may further have helped neo-vascularization in HA spheroid-treated models. We also observed a substantial number of ASCs that were neither adipocytes nor endothelial cells and may be incorporated as perivascular ASCs for future remodeling of the tissue.
Conclusion
Human ASC spheroids enriched in undifferentiated cells were efficiently prepared by using 3D floating culture in non-cross-linked HA gel. The results suggest the superior plasticity and growth factor-secreting potential of the spheroids and clearly indicate their therapeutic power for promoting tissue regeneration. Unlike dissociated cells, their high engraftment efficiency and differentiation into vascular endothelial cells suggest the privileged therapeutic capacity of the spheroids. HA is a degradable ECM material, and its safety is well-established, indicating its practical value as a material for 3D culture and as an injectable cell carrier. Spheroid formation has various advantages other than enhancement of cell functionality; this process can avoid unfavorable migration of locally injected cells, leading to more consistent incorporation and therapeutic effects of the administered cells. As an easy local injectable, spheroids are expected to be a powerful tool to treat various stem cell-depleted conditions/organs through nonintravenous administration.
Supplementary Material
Acknowledgments
This study was supported by the Japanese Ministry of Education, Culture, Sports, Science, and Technology Grant-in-Aid for Scientific Research B3-24390398 and Grant-in-Aid for Challenging Exploratory Research 25670749.
Author Contributions
K.M.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; J.F., H.I., and H.T.: collection and/or assembly of data, data analysis and interpretation; K.D.: collection and/or assembly of data; S.K.: collection and/or assembly of data; K. Kinoshita, K. Kanayama, H.K., and T.M.: collection and/or assembly of data; I.H. and A.K.: data analysis and interpretation; H.N.: data analysis and interpretation, administrative support; K.Y.: conception and design, data analysis and interpretation, financial support, administrative support, final approval of manuscript, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malafaya PP, Pedro AJ, Peterbauer A, et al. Chitosan particles agglomerated scaffolds for cartilage and osteochondral tissue engineering approaches with adipose tissue derived stem cells. J Mater Sci Mater Med. 2005;16:1077–1085. doi: 10.1007/s10856-005-4709-4. [DOI] [PubMed] [Google Scholar]
- 3.Seo MJ, Suh SY, Bae YC, et al. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258–264. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 4.Bai X, Alt E. Myocardial regeneration potential of adipose tissue-derived stem cells. Biochem Biophys Res Commun. 2010;401:321–326. doi: 10.1016/j.bbrc.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Safford KM, Safford SD, Gimble JM, et al. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol. 2004;187:319–328. doi: 10.1016/j.expneurol.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y, Sun Z, Liao L, et al. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 8.Kuroda Y, Kitada M, Wakao S, et al. Unique multipotent cells in adult human mesenchymal cell populations. doi: 10.1073/pnas.0911647107. [published correction appears in: Proc Natl Acad Sci USA 2014;111:8312]. Proc Natl Acad Sci USA 2010;107:8639–8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakao S, Kitada M, Kuroda Y, et al. Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci USA. 2011;108:9875–9880. doi: 10.1073/pnas.1100816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitada M, Wakao S, Dezawa M. Muse cells and induced pluripotent stem cell: Implication of the elite model. Cell Mol Life Sci. 2012;69:3739–3750. doi: 10.1007/s00018-012-0994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baer PC, Griesche N, Luttmann W, et al. Human adipose-derived mesenchymal stem cells in vitro: Evaluation of an optimal expansion medium preserving stemness. Cytotherapy. 2010;12:96–106. doi: 10.3109/14653240903377045. [DOI] [PubMed] [Google Scholar]
- 12.Park E, Patel AN. Changes in the expression pattern of mesenchymal and pluripotent markers in human adipose-derived stem cells. Cell Biol Int. 2010;34:979–984. doi: 10.1042/CBI20100124. [DOI] [PubMed] [Google Scholar]
- 13.Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Itaka K, Ohba S, et al. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials. 2009;30:2705–2715. doi: 10.1016/j.biomaterials.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Park IS, Rhie JW, Kim SH. A novel three-dimensional adipose-derived stem cell cluster for vascular regeneration in ischemic tissue. Cytotherapy. 2014;16:508–522. doi: 10.1016/j.jcyt.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735–749. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- 17.Bhang SH, Cho SW, La WG, et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–2747. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Bartosh TJ, Ylöstalo JH, Mohammadipoor A, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amos PJ, Kapur SK, Stapor PC, et al. Human adipose-derived stromal cells accelerate diabetic wound healing: Impact of cell formulation and delivery. Tissue Eng Part A. 2010;16:1595–1606. doi: 10.1089/ten.tea.2009.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rustad KC, Wong VW, Sorkin M, et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials. 2012;33:80–90. doi: 10.1016/j.biomaterials.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WY, Chang YH, Yeh YC, et al. The use of injectable spherically symmetric cell aggregates self-assembled in a thermo-responsive hydrogel for enhanced cell transplantation. Biomaterials. 2009;30:5505–5513. doi: 10.1016/j.biomaterials.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Cheng NC, Wang S, Young TH. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. 2012;33:1748–1758. doi: 10.1016/j.biomaterials.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 23.Bartosh TJ, Wang Z, Rosales AA, et al. 3D-model of adult cardiac stem cells promotes cardiac differentiation and resistance to oxidative stress. J Cell Biochem. 2008;105:612–623. doi: 10.1002/jcb.21862. [DOI] [PubMed] [Google Scholar]
- 24.Lin SJ, Jee SH, Hsiao WC, et al. Enhanced cell survival of melanocyte spheroids in serum starvation condition. Biomaterials. 2006;27:1462–1469. doi: 10.1016/j.biomaterials.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 25.González-Cruz RD, Fonseca VC, Darling EM. Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. Proc Natl Acad Sci USA. 2012;109:E1523–E1529. doi: 10.1073/pnas.1120349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang CC, Chen CH, Hwang SM, et al. Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells. 2009;27:724–732. doi: 10.1634/stemcells.2008-0944. [DOI] [PubMed] [Google Scholar]
- 27.Cheng NC, Chen SY, Li JR, et al. Short-term spheroid formation enhances the regenerative capacity of adipose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Translational Medicine. 2013;2:584–594. doi: 10.5966/sctm.2013-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almond A. Cell Mol Life Sci. 2007;64:1591–1596. doi: 10.1007/s00018-007-7032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 30.Suga H, Eto H, Shigeura T, et al. IFATS collection: Fibroblast growth factor-2-induced hepatocyte growth factor secretion by adipose-derived stromal cells inhibits postinjury fibrogenesis through a c-Jun N-terminal kinase-dependent mechanism. Stem Cells. 2009;27:238–249. doi: 10.1634/stemcells.2008-0261. [DOI] [PubMed] [Google Scholar]
- 31.Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(suppl 1):302–312. doi: 10.1111/j.1524-4725.2008.01046.x. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimura K, Aoi N, Suga H, et al. Ectopic fibrogenesis induced by transplantation of adipose-derived progenitor cell suspension immediately after lipoinjection. Transplantation. 2008;85:1868–1869. doi: 10.1097/TP.0b013e3181775136. [DOI] [PubMed] [Google Scholar]
- 33.Anada T, Fukuda J, Sai Y, et al. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials. 2012;33:8430–8441. doi: 10.1016/j.biomaterials.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 34.Yeh HY, Liu BH, Hsu SH. The calcium-dependent regulation of spheroid formation and cardiomyogenic differentiation for MSCs on chitosan membranes. Biomaterials. 2012;33:8943–8954. doi: 10.1016/j.biomaterials.2012.08.069. [DOI] [PubMed] [Google Scholar]
- 35.Liu BH, Yeh HY, Lin YC, et al. Spheroid formation and enhanced cardiomyogenic potential of adipose derived stem cells grown on chitosan. Biores Open Access. 2013;2:28–39. doi: 10.1089/biores.2012.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinoshita K, Kuno S, Ishimine H, et al. Therapeutic potential of adipose-derived SSEA-3-positive muse cells for treating diabetic skin ulcers. Stem Cells Translational Medicine. 2015;4:146–155. doi: 10.5966/sctm.2014-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.