The effects of bone morphogenetic protein-2 (BMP-2) on SMAD1/5 signaling, proliferation, and differentiation in human adipose stem cells (hASCs) were analyzed. BMP-2 induced dose-dependent activation of SMAD signaling and decreased proliferation in all the cell lines studied. Furthermore, depending on the hASC donor line, BMP-2 also induced osteogenic and adipogenic differentiation in hASCs.
Keywords: Bone morphogenetic protein 2, Adipose stem cell, Cell signaling, SMAD-1/5, Bone tissue engineering, Differentiation
Abstract
Bone morphogenetic protein-2 (BMP-2) is a growth factor used to stimulate bone regeneration in clinical applications. However, there are contradicting reports on the functionality of BMP-2 in human adipose stem cells (hASCs), which are frequently used in tissue engineering. In this study, we analyzed the effects of BMP-2 on SMAD1/5 signaling, proliferation, and differentiation in hASCs. Our results indicated that BMP-2 induced dose-dependent (25–100 ng/ml) activation of SMAD signaling. Furthermore, the cell proliferation analysis revealed that BMP-2 (100 ng/ml) consistently decreased the proliferation in all the cell lines studied. However, the analysis of the differentiation potential revealed that BMP-2 (100 ng/ml) exhibited a donor-dependent dual role, inducing both osteogenic and adipogenic differentiation in hASCs. The quantitative alkaline phosphatase (qALP) activity and mineralization levels were clearly enhanced in particular donor cell lines by BMP-2 stimulus. On the contrary, in other cell lines, qALP and mineralization levels were diminished and the lipid formation was enhanced. The current study also suggests that hASCs have accelerated biochemical responsiveness to BMP-2 stimulus in human serum-supplemented culture medium compared with fetal bovine serum. The production origin of the BMP-2 growth factor is also important for its response: BMP-2 produced in mammalian cells enhanced signaling and differentiation responses compared with BMP-2 produced in Escherichia coli. These results explain the existing contradiction in the reported BMP-2 studies and indicate the variability in the functional end mechanism of BMP-2-stimulated hASCs.
Significance
This study examined how bone morphogenetic protein-2 (BMP-2) modulates the SMAD signaling mechanism and the proliferation and differentiation outcome of human adipose stem cells (hASCs) derived from several donors. The results indicate that BMP-2 triggers molecular SMAD signaling mechanisms in hASCs and regulates differentiation processes in human serum-culture conditions. Importantly, BMP-2 has dual activity, inducing osteogenic and adipogenic differentiation, subject to hASC donor line studied. These findings explain contradictory previous results and highlight the importance of further studies to understand how signaling pathways guide mesenchymal stem cell functions at the molecular level.
Introduction
The bone morphogenetic proteins (BMPs) are the best characterized cytokines proposed to drive osteogenic differentiation and are used in clinical applications to stimulate bone regeneration [1]. The BMPs, which belong to the transforming growth factor-β family, mediate their biological function through forming a complex with type I and II serine/threonine kinase receptors. These receptors, in turn, phosphorylate receptor-mediated SMADproteins, including SMAD1, 5, and 8. Activated SMAD proteins form complexes with SMAD4 protein and subsequently translocate to the nucleus, where they cooperate with other DNA-binding proteins to target specific genes such as distal-less homeobox (Dlx)-2/5 and osterix (Osx) for transcriptional regulation [2–4].
Among the BMPs, BMP-2 is best studied in the context of osteogenesis and has been indicated to have potential in bone formation. However, multiple studies have reported contradictory results of BMP-2 treatment in vitro and in vivo. The BMP-2 growth factor has been shown to induce osteogenesis [2, 5] and adipogenesis [6] in bone marrow-derived mesenchymal stem cell (BMSC) cultures in vitro as well as in live animal models [7, 8]. Also, viral BMP-2 transduction of human adipose stem cells (hASCs) has been shown to produce more bone precursor cells and calcified matrix than osteoblasts, indicating excellent response of hASCs to endogenous production of BMP-2 [9]. However, there are also opposing studies proposing that externally supplemented BMP-2 has only a very weak response, lack of response, or even a negative response in MSC osteogenesis [10–12]. There are indications that BMP-2 stimulus had no impact on its downstream SMAD-signaling mechanisms, or on expression of osteogenic genes or osteogenic responses such as mineralization in hASCs [11]. These contradicting results imply that functionality and osteogenic impact of BMP-2 on BMSCs and hASCs require further study to verify the benefit of BMP-2 use in clinical applications.
The function of BMP-2 in osteogenic differentiation of BMSCs and osteoblasts has been proposed to be highly dependent on cellular shape and molecular feedback loops [2, 13], such as cooperative mechanisms with Wnt/β-catenin, fibroblast growth factor (FGF) [14], and Notch signaling [15–17]. This indicates that the function of BMPs is intricately regulated by internal and external factors both positively and negatively, addressing complex molecular mechanisms functioning cooperatively in BMSC and ASC differentiation processes, as well. The complexity of the regulatory mechanisms also raises the question of whether there is variation in cellular growth factor responses of cell lines derived from different individuals. In this study, we investigated the effect of BMP-2 growth factor on signaling mechanisms and on the differentiation potential of the several hASC donor lines. Our results indicated that in all the cell lines studied, BMP-2 induced phosphorylation of SMAD1/5 signaling proteins, activation and translocation into cell nuclei. Furthermore, hASCs showed variable differentiation responses to BMP-2 treatment in osteogenic culture conditions, displaying osteogenic or adipogenic outcome depending on the donor cell line studied. BMP-2 responses in hASCs also showed clear dependence on culture conditions. BMP-2 induced higher SMAD activation in human serum (HS) conditions versus fetal bovine serum conditions (FBS). Finally, our results indicated that BMP-2 production origin plays an important role in its functionality since BMP-2 produced in mammalian cells induced stronger responses on hASCs when compared with growth factor produced in E. coli.
Materials and Methods
Ethics Statement
This study was conducted in accordance with the Ethics Committee of the Pirkanmaa Hospital District, Tampere, Finland (R03058). The hASCs used in this study were isolated from adipose tissue samples acquired from 10 female donors (mean age: 46 ± 25 years) undergoing surgery in the Department of Plastic Surgery, Tampere University Hospital. A written informed consent from all the donors was obtained for the use of the adipose tissue samples for research purposes.
Adipose Stem Cell Isolation and Culture
Human ASCs were isolated by mechanical and enzymatic procedure as described previously by Lindroos and coworkers [18]. Isolated hASCs were cultured in Dulbecco’s Modified Eagle Medium/Ham’s Nutrient Mixture F-12 1:1 mixture (Thermo Fisher Scientific Inc., Carlsbad, CA, https://www.thermofisher.com) supplemented with 5% HS (GE Healthcare, Buckinghamshire, U.K., http://www.gelifesciences.com), 1% l-glutamine (GlutaMAX; Thermo Fisher Scientific Inc.) and 1% antibiotics (100 U/ml penicillin and 0.1 mg/ml streptomycin; Thermo Fisher Scientific Inc.). This medium composition is referred to as basic medium (BM) in this article. After primary cell culture (passages 1–2), the surface marker expression of hASCs was analyzed by flow cytometry (fluorescence-activated cell sorting) (FACSAria; Becton, Dickinson and Company, Erembodegem, Belgium, http://www.bdbiosciences.com) (supplemental online data) [18]. The experiments with hASCs were carried out in passages 1–5.
For the quantitative alkaline phosphatase (qALP) activity, Alizarin red mineralization analyses, and Oil Red O analyses, 500 cells per well were plated on 24-well plates. In qALP and Oil Red O analyses, Nunc 24-well plates (Thermo Fisher Scientific Inc.) and in mineralization studies CellBIND (Corning Inc., Corning, NY, https://www.corning.com), 24-well plates were used. hASCs were cultured in 4 different culturing conditions: BM, BM supplemented with BMP-2, osteogenic medium (OM; supplemented with 10 mM β-glycerophosphate, 200 µM l-ascorbic acid 2-phosphate, and 5 nM dexamethasone), and OM supplemented with BMP-2. BMP-2 originated from either mammalian cultures (Chinese hamster ovary cells [CHO]; R&D Systems, Minneapolis, MN, https://www.rndsystems.com) or from Escherichia coli (Sigma-Aldrich, St. Louis, MO, https://www.sigmaaldrich.com). BMP-2 was used in concentration of 100 ng/ml unless otherwise mentioned. For the Western blot analysis, cells were cultured in 1% HS (GE Healthcare) and 1% FBS (Thermo Fisher Scientific Inc.).
Real-Time Polymerase Chain Reaction
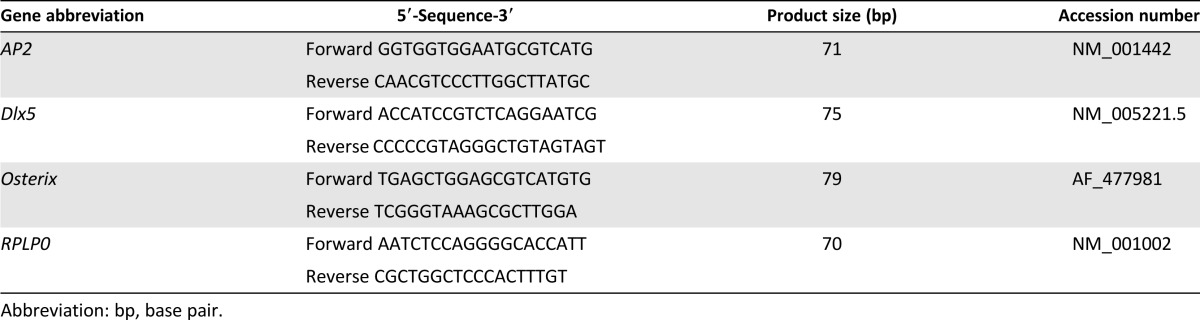
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of osteogenic and adipogenic marker genes was performed as described by Mesimäki and coworkers [1]. Briefly, 2,000 cells per well were plated on a 6-well plate (Thermo Fisher Scientific Inc.). CHO BMP-2 was used in RT-PCR experiments (R&D Systems). The total mRNA was isolated at the time points of days 7 and 14 using the NucleoSpin RNA II kit (Macherey-Nagel GmbH & Co., Düren, Germany, http://www.mn-net.com). The isolated mRNA was reverse transcribed to cDNA with the High-Capacity cDNA Reverse Transcriptase Kit (Thermo Fisher Scientific Inc.). The data were normalized to the expression of housekeeping gene RPLP0 (human acidic ribosomal phosphoprotein P0) and the relative expression of each gene was calculated using a mathematical model described previously [19]. The primer sequences (Oligomer Oy, Helsinki, Finland, http://www.oligomer.fi) and the accession numbers are presented in Table 1.
Table 1.
The sequences and accession numbers of the primers used in quantitative real-time polymerase chain reaction
Cell Number
The cell number of hASCs cultured in different conditions was analyzed at 14 and 19 days by CyQUANT Cell Proliferation Assay Kit (Thermo Fisher Scientific Inc.), according to the manufacturer’s protocol as described by Lindroos et al. and Tirkkonen et al. [18, 20].
Alkaline Phosphatase Activity, Mineralization, and Oil Red O-Lipid Formation
Analyses of the qALP activity, mineralization, and lipid formation were conducted as previously described [18, 20]. The activity of ALP was studied quantitatively at day 14, as described in the Sigma ALP procedure (Sigma-Aldrich). The qALP activity results were normalized with the cell number from the CyQUANT analysis.
The Alizarin red S staining of minerals was analyzed at days 14 and 19. Briefly, the cells were fixed with 70% ethanol for 1 hour (−20°C) followed by staining with 2% Alizarin red S solution (pH 4.1–4.3; Sigma-Aldrich) for 10 minutes at room temperature. Finally, for the quantitative analysis, the dye staining the calcium minerals was extracted from the samples with 100 mM cetylpyridinium chloride (Sigma-Aldrich). The intensity of the dye was analyzed with Victor 1420 multiplate reader (PerkinElmer Inc., Turku, Finland, http://www.perkinelmer.com) at 540 nm and the results were normalized with the cell number from the CyQUANT analysis [18, 20].
To assess the adipogenic differentiation of hASCs at 19 days, Oil Red O staining was conducted as previously described, with slight modifications [18, 20]. Cell nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI) for 5 minutes before the last washing steps. DAPI-stained nuclei and the formation of the large lipid droplets stained with the fluorescent Oil Red O were analyzed from the microscopy images by using the ImageJ program (U.S. National Institutes of Health, Bethesda, MD, http://imagej.nih.gov/ij/). The number of the lipid droplets was normalized with the number of the counted nuclei.
Immunocytochemical Staining
For the analysis of the subcellular localization of activated SMAD1/5, mesenchymal vimentin and phosphorylated SMAD1/5 were analyzed by immunocytochemical staining after 0 minutes, 30 minutes, and 2 hours of BMP-2 stimulation (E. coli). For the staining, 10,000 cells per well were plated on a 48-well plate (Thermo Fisher Scientific Inc.). Prior to BMP-2 induction, hASCs were starved for 24 hours in 1% HS medium. At each time point, cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) supplemented with 0.2% Triton X-100 for 15 minutes at room temperature. Blocking was done with 1% bovine serum albumin (Sigma-Aldrich) for 1 hour at 4°C. Subsequently, a double staining was conducted with the following primary antibodies: rabbit monoclonal anti-human-phosphorylated SMAD1/5 (pSMAD1/5; Cell Signaling Technology, Danvers, MA, http://www.cellsignal.com) and goat polyclonal anti-human vimentin (Merck Millipore, Billerica, MA, http://www.merckmillipore.com) overnight followed by secondary antibody staining with anti-goat Alexa 568 (Thermo Fisher Scientific Inc.) and anti-rabbit Alexa 488 (Thermo Fisher Scientific Inc.). Finally, samples were imaged using an Olympus microscope (IX51; Olympus Corp., Tokyo, Japan, http://www.olympus-global.com) equipped with a fluorescence unit and camera (DP30BW; Olympus Corp.).
Western Blotting and Immunodetection
For the analysis, 40,000 cells per well were plated on a 6-well plate (Thermo Fisher Scientific Inc.) and starved as described above for immunocytochemical staining, before treatment with BMP-2 from either CHO cells or E. coli. The medium was changed every third day. After a culture period of 24 hours, 3 and 7 days hASCs were lysed directly into 2× Laemmli sample buffer (20% glycerol, 6% SDS, 50 mM Tris pH 6.8, 10% β-mercaptoethanol) and samples were separated with SDS electrophoresis. After the electrophoretic separation, the proteins were transferred to polyvinylidene fluoride membrane (GE Healthcare, Waukesha, WI, http://www3.gehealthcare.com). After blocking, the target proteins were probed with the following primary antibodies: rabbit monoclonal anti-pSMAD1/5 (Cell Signaling Technology) and mouse monoclonal anti-β-actin (Santa Cruz Biotechnology, Dallas, TX, http://www.scbt.com) followed by horseradish peroxidase-conjugated secondary antibodies goat anti-mouse IgG (Santa Cruz Biotechnology) and anti-rabbit IgG (Cell Signaling Technology). Proteins were detected using enhanced chemiluminescence detection reagent (GE Healthcare). To analyze the basal levels of nonphosphorylated SMAD1, the pSMAD1/5 blots were stripped and blotted by rabbit monoclonal anti-SMAD1 antibody, followed by anti-rabbit IgG detection (supplemental online data). Semiquantitative normalization of pSMAD1/5 levels by SMAD1 basal levels was performed using ImageJ analysis software.
Statistical Analysis
Statistical analyses were performed with SPSS version 22 (IBM Corp., Armonk, NY, http://www.ibm.com). Alizarin red, qALP analysis, and CyQUANT measurements were performed with three to four hASC lines with three replicate samples of each. Oil Red O quantification was analyzed from three to four hASC lines with five replicative spots from each condition or treatment. Data are presented as mean ± SD. The pairwise comparisons between the effects of BMP-2 stimulus on ALP activity, mineralization, lipid formation, and cell proliferation were analyzed by using a nonparametric Mann-Whitney U test. The resulting p values were corrected with the Bonferroni multiple adjustment method based on the number of planned comparisons (supplemental online data; calculated p values are listed in supplemental online Tables 2–5). All the differences between and within the groups with adjusted p ≤ .05 were considered to be significant.
Results
BMP-2 Induces Donor Cell Line-Independent Activation of SMAD 1/5 Protein in hASCs
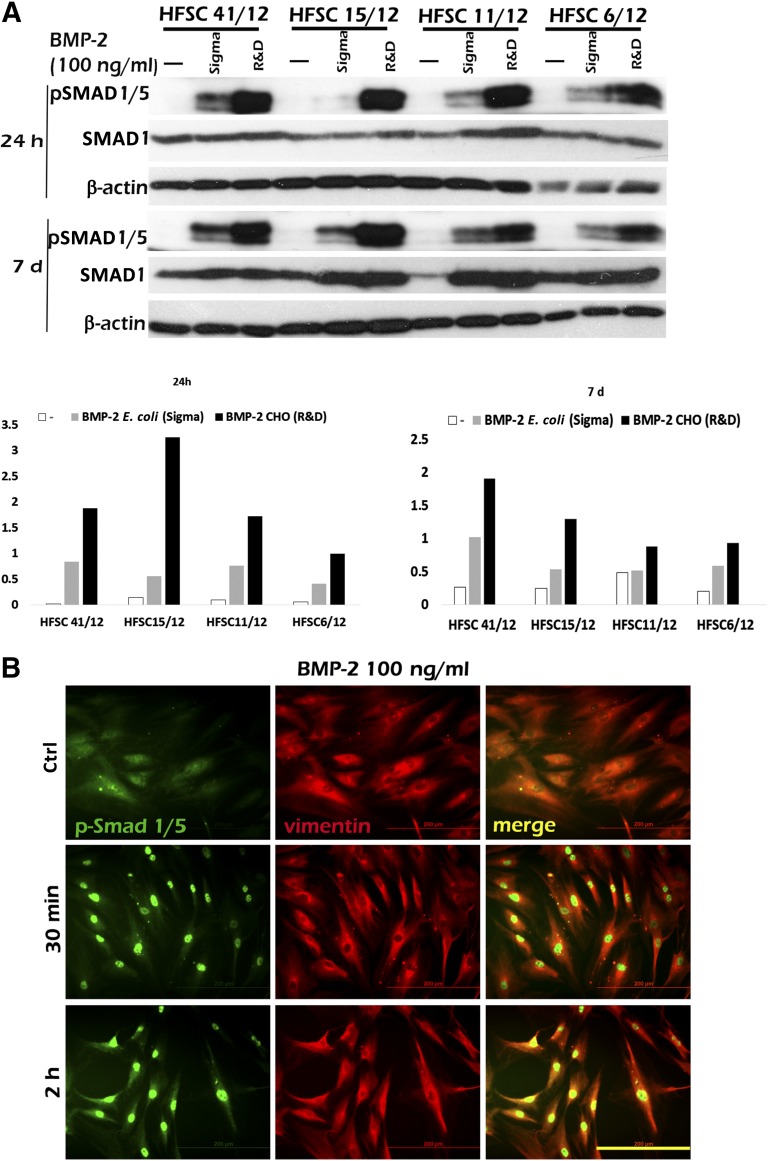
To analyze the biological functionality of BMP-2 in MSCs, we examined whether BMP-2 activates the internal SMAD pathway. For this, five different hASC lines (HFSC 41/12, 11/12, 15/12, 6/12, and 8/12) were analyzed after BMP-2 induction at time points 24 hours and 7 days by Western blotting of levels of phosphorylated SMAD 1/5, total SMAD1, and β-actin as an internal control. Quantitative results of the pSMAD1/5 levels revealed that SMAD1/5 was activated robustly by BMP-2 at both time points in all the hASC lines studied (Fig. 1A). Also, small hASC line-dependent variations of the pSMAD1/5 levels at BMP-2 stimulus were observed at both time points. However, minor levels of constitutively phosphorylated SMAD were apparent also in unstimulated control samples. Comparison of the effectiveness of BMP-2 produced in E. coli and in CHO revealed modest SMAD1/5 induction by BMP-2 produced in E. coli, but clearly enhanced induction by BMP-2 produced in CHO cells (Fig. 1A).
Figure 1.
BMP-2 induced phosphorylation of SMAD1/5 and translocation into nuclei. (A): Representative image of Western Blot analysis of SMAD1/5 phosphorylation, basal SMAD1, and β-actin levels in BMP-2 induction. BMP-2 was used in amount of 100 ng/ml, and BMP-2 from Sigma was produced in Escherichia coli, whereas BMP-2 from R&D was produced in CHO cells. Analysis time points were 24 hours and 7 days, and human adipose stem cell (hASC) lines used were HFSC 41/12, 15/12, 11/12, and 6/12. Phosphorylated SMAD levels were quantified by normalizing them with SMAD1 basal protein levels by using the ImageJ analysis tool. (B): Immunocytochemical analysis of translocation of p-SMAD1/5 was performed at 3 time points: 0 minutes (Ctrl), 30 minutes, and 2 hours. In the representative images, the hASC line used was HFSC 7/12; BMP-2 (E. coli) was used in a concentration of 100 ng/ml. Primary antibodies (p-SMAD1/5 [green] and vimentin [red]) and secondary antibodies (Alexa fluor 568 and 488) were used to detect the subcellular localization of p-SMAD1/5 and cytoplasmic vimentin proteins. Scale bar = 200 µm. Abbreviations: BMP-2, bone morphogenetic protein-2; CHO, Chinese hamster ovary; HFSC, human fat stem cell; R&D, R&D Systems; Sigma, Sigma-Aldrich.
We also studied the effect of culture conditions on SMAD activation. For this purpose, hASCs were cultured in HS or in FBS medium. In both media, pSMAD was activated dose dependently with BMP-2 stimulus. Surprisingly, BMP-2 produced in E. coli induced higher levels of pSMAD activation in the HS medium (Fig. 2A, 2B). Quantification of the intensity of the pSMAD bands and normalization to SMAD1 levels revealed that the difference between HS and FBS media was most apparent at 24 hours and at 7 days. Only higher doses of BMP-2 induced minor induction of pSMAD in the FBS medium (Fig. 2A, 2B). Similar findings were apparent also when SMAD activation of the osteoblast cell line and hBMSC line was analyzed in HS and FBS media in the presence of BMP-2: The SMAD was activated dose dependently by BMP-2 in the HS medium, whereas in the FBS medium, the overall levels of pSMAD were decreased at all time points (supplemental online Fig. 1; supplemental online data).
Figure 2.
BMP-2 induced SMAD activation in HS and FBS media. (A): Representative image of Western Blot analysis of BMP-2-induced SMAD1/5 phosphorylation, basal SMAD1, and β-actin levels in HS and FBS media. BMP-2 (Escherichia coli) was used in amount of 0, 25, 75, and 100 ng/ml. Analysis time points were 24 h, 3 days, and 7 days, and the hASC line used was human fat stem cell (HFSC) 8/12. (B): Semiquantitative analysis of phosphorylated SMAD1/5 levels normalized with SMAD1 levels. Quantification was performed with the ImageJ analysis program. Abbreviations: BMP-2, bone morphogenetic protein-2; FBS, fetal bovine serum; HS, human serum.
BMP-2 Activated SMAD Translocates to Nucleus in hASCs
In a first step, the functionality of recombinant human BMP-2 signaling was analyzed from the total cell lysates by Western blot. However, we wanted to study whether the phosphorylated SMAD retains its functionality and the signal is actually transferred to the nucleus of hASCs for the transcriptional processes. To further analyze the functionality of BMP-2, we performed analysis of the translocation of activated SMAD1/5 by immunocytochemical staining (human fat stem cell [HFSC] 7/12). Our results indicated that after 30 minutes of BMP-2 growth factor stimulus, pSMAD 1/5, stained with green, translocated into cell nuclei and remained there after 2 hours from the start of the stimulation. The red staining in Figure 1B indicates mesenchymal vimentin protein. Some of the pSMAD1/ 5 were also observed in unstimulated cells, indicating a minor level of basal SMAD activation (Fig. 1B). To gain a broader picture, we also performed the analysis of the SMAD translocation in BMSCs, 3 other hASC lines (HFSC 41/12, 15/12, and 25/12), and osteoblasts. These results indicate that SMAD1/5 is activated and translocated to nuclei in all the cell types studied by both BMP-2 growth factors (ie, those produced in E. coli and in the CHO cell line) (supplemental online Figs. 2, 3) and confirms that BMP-2 is a functional trigger for the SMAD-related molecular mechanisms in MSCs.
The Activity of the Early Osteogenic Marker ALP Is Differentially Enhanced by BMP-2 in hASCs Lines
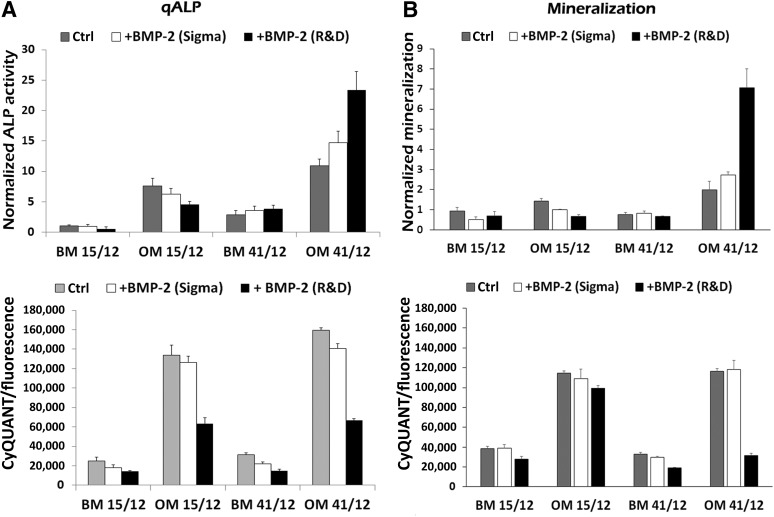
To analyze the functional outcome of BMP-2-SMAD signaling, we performed quantitative analysis of the early osteogenic marker, normalized alkaline phosphatase (ALP) enzymatic activity in BM and OM culture conditions in different hASC lines. Our results indicated that the BMP-2-induced response of ALP activity was highly variable between hASC lines. Based on our results, BMP-2 does not modulate qALP levels in BM; however, clear differences were apparent when cells were treated with BMP-2 in OM. From the studied hASC lines, we could clearly distinguish 2 types of responses to BMP-2 stimuli. Some cell lines showed enhancement of the qALP levels due to BMP-2 stimulus (Fig. 3A), whereas in others, qALP levels were diminished or retained at the same level by BMP-2 treatment (Fig. 3B). However, cell proliferation analysis by the CyQUANT method revealed that BMP-2 treatment decreases the proliferation efficiency in all the cell lines studied in both BM and OM (Fig. 3A, 3B). To further analyze this differential behavior, we used hASC lines expressing opposing responses to BMP-2 stimulus. hASCs were separated into 2 groups, group I (HFSC 41/12, 11/12, 9/12) and group II (HFSC 15/12, 6/12, and 7/12), based on whether the BMP-2 enhanced or decreased ALP activity, respectively. As indicated in Figure 3C and 3D, qALP results from cell lines in each group were combined, showing that BMP-2 enhanced ALP activity in group I and decreased activity in group II. Also, BMP-2 addition significantly decreased proliferation levels of hASCs in BM and OM in both groups (Fig. 3C).
Figure 3.
BMP-2 induction of ALP activity in human adipose stem cells. (A, B): qALP activity (three replicate samples per condition) normalized with fluorescent values from CyQUANT cell number analysis. Lower panels present fluorescence values from CyQUANT cell number analysis. hASC lines HFSC 41/12, 11/12, 19/11 represent group I (A) and hASC lines HFSC 6/12, 7/12, and 15/12 represent group II (B). (C): Results from group I were grouped together and given as normalized qALP values and fluorescence values from CyQUANT cell number analysis (three cell lines and three replicate samples per condition). (D): The same was done to group II. Results are expressed as mean ± SD. ALP results were standardized to the control condition. The BMP-2 (CHO) concentration was 100 ng/ml. Statistical analysis was performed with the Mann-Whitney U test and with Bonferroni correction. The level of significance was set at p < .05. Abbreviations: ALP, alkaline phosphatase; BM, basic culture medium; BMP-2, bone morphogenetic protein-2; HFSC, human fat stem cell; n.s., not significant; OM, osteogenic medium; qALP, quantitative alkaline phosphatase.
Mineralization of hASCs Is Differentially Promoted by BMP-2
To further analyze the osteogenic differentiation potential of BMP-2 (CHO), we performed Alizarin red mineralization analysis at time points of 14 and 19 days in BM and OM conditions. Alizarin red color formation due to mineralization of the samples was analyzed qualitatively (Fig. 4C) but also quantitatively (Fig. 4A, 4B, and 4D) by extraction of the color and measurement of the intensity of the extracted stain. Altogether, eight different hASC lines were analyzed and divided into groups I and II based on their response to BMP-2 in ALP analysis (HFSC41/12, 9/13, 11/12, and 19/11 in group I; HFSC 15/12, 44/12, 6/12, and 7/12 in group II). There was a significant difference between hASCs in their BMP-2-induced mineralization in OM. hASC lines in group I showed prominent enhancement of the mineralization at day 19. Also 3 of 4 cell lines showed a significant increase at day 14 (Fig. 4A, 4D).
Figure 4.
Impact of BMP-2 on mineralization of human adipose stem cells (hASCs). (A, B): Quantitative analysis of Alizarin red S mineralization assay normalized with fluorescence values from CyQUANT cell number analysis (three replicate samples per condition). Fluorescence values in the lower panels are from CyQUANT cell number analysis of hASC lines HFSC 41/12, 11/12, 19/11, and 9/13 representing group I (A), and hASC lines HFSC 6/12, 7/12, 44/12, and 15/12 representing group II (B). Results from group I were grouped together and represented as normalized Alizarin red S values; the lower panel presents fluorescence values from CyQUANT cell number analysis. (D): The same was done to group II (four cell lines and three replicates per condition). (C): Qualitative analysis of mineralization by Alizarin red S staining of HFSC lines 41/12 (group I) and 15/12 (group II). Images are from the whole 24-well area. Results are expressed as mean ± SD. Alizarin red S results were standardized to the control condition. The amount of BMP-2 (CHO) used was 100 ng/ml. Statistical analysis was performed with the Mann-Whitney U test and with Bonferroni correction. The level of significance was set at p < .05. Abbreviations: BM, basic culture medium; BMP-2, bone morphogenetic protein-2; ctrl, control; HFSC, human fat stem cell; OM, osteogenic medium; R&D, R&D Systems.
On the contrary, hASC lines in group II did not show any significant enhancement of mineralization in response to BMP-2 stimulus at any of the analyzed time points or in either medium. Actually, some hASC lines in group II had decreased mineralization levels in BMP-2-supplemented OM (Fig. 4B). Qualitative analysis of mineralization of hASC lines HFSC 41/12 from group I versus HFSC 15/12 from group II confirmed this finding, showing clearly enhanced mineralization in the hASC line from group I and decreased levels of mineralization in hASC of group II in BMP-2-supplemented OM (Fig. 4C). Finally, the mineralization results from the hASC lines in each group were combined, and showed that BMP-2 significantly increased mineralization in group I and decreased it in group II (Fig. 4D).
BMP-2 Growth Factor From Mammalian Origin Efficiently Modulates ALP Activity and Mineralization of hASCs
In order to study whether the production origin of the BMP-2 growth factor influences the response of hASCs, we analyzed the effect of BMP-2 from E. coli and mammalian CHO cells. We analyzed cell proliferation, qALP activity, and mineralization capacity of two donor cell lines, 15/12 (group II) and 41/12 (group I), stimulated with BMP-2 produced in E.coli and CHO. CyQUANT proliferation analysis revealed that with both BMPs, the proliferation of hASCs was decreased under all conditions. However, BMP-2 produced in CHO cells decreased proliferation more efficiently compared with BMP-2 from E. coli (Fig. 5A, 5B). Analysis of qALP and mineralization revealed that BMP-2 produced in CHO cells had a greater capacity to modulate the changes in OM in both cell lines. Also, the results between the cell lines were opposing, so the 15/12 cell line showed decreased qALP activity and mineralization, whereas cell line 41/12 clearly showed increased qALP activity and mineralization in OM supplemented with BMP-2 (Fig. 5A, 5B). Our results indicated that as in ALP activation and mineral formation, BMP-2 produced in CHO cells was the most efficient inducer of hASCs.
Figure 5.
Comparison of the effectiveness of BMP-2 produced in mammalian cells (Chinese hamster ovary [CHO]) and in Escherichia coli. (A, B): qALP activity (A) and Alizarin red S values (B) normalized with fluorescence values from CyQUANT cell number analysis. Lower panels present fluorescence values from CyQUANT cell number analysis. Presented human adipose stem cell lines are HFSC 41/12 (group I) and 15/12 (group II). Results are expressed as mean ± SD. ALP results were standardized to the control condition. The amount of BMP-2 (E. coli [Sigma] or CHO [R&D]) used was 100 ng/ml. Abbreviations: ALP, alkaline phosphatase; BM, basic culture medium; BMP-2, bone morphogenetic protein-2; ctrl, control; OM, osteogenic medium; qALP, quantitative alkaline phosphatase; R&D, R&D Systems; Sigma, Sigma-Aldrich.
BMP-2 Induces High Expression of Osteogenic and Adipogenic Markers and Formation of Lipid Vacuoles in hASCs
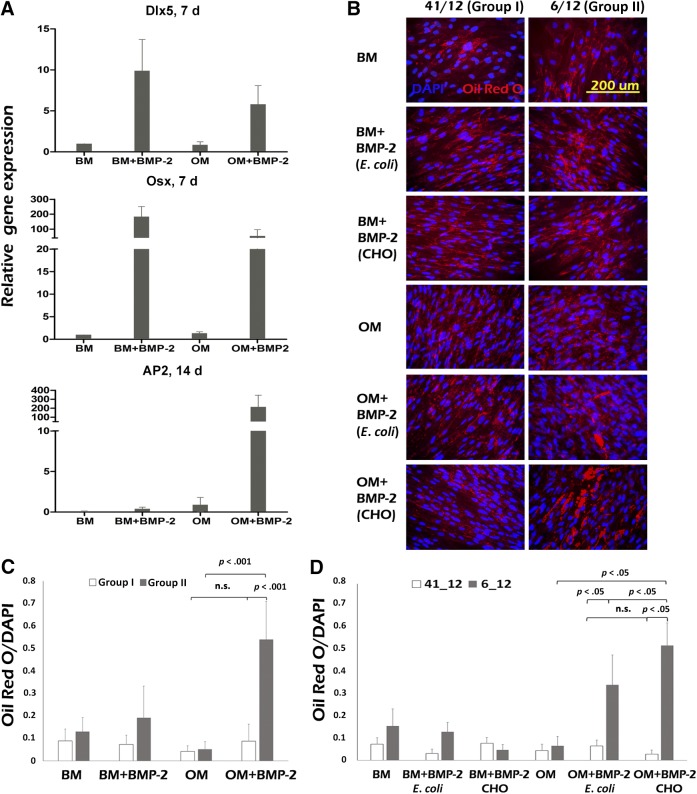
During the mineralization assays, we observed that some of the hASC lines produced large amounts of lipid vacuoles in the presence of BMP-2. Thus, we investigated further whether BMP-2 actually induces adipogenic differentiation in some of the hASC lines studied. We analyzed expression levels of the adipogenic marker adipocyte protein 2 (AP2), and the osteogenic markers osterix (Osx) and Dlx5 in hASC lines from group I and II. At day 7, BMP-2 (CHO) induced elevated expression of osteogenic markers osterix and Dlx5 and, at day 14, remarkably high levels of AP2. Expression levels of both osteogenic and adipogenic markers varied among hASC lines (Fig. 6A), but levels were not dependent on the group in which they were previously classified (data not shown). These results suggested that BMP-2 growth factor could induce osteogenic as well as adipogenic differentiation in hASCs and prompted us to further analyze the effect of BMP-2 stimulus on adipogenic differentiation in BM and OM. For this purpose, we performed Oil Red O staining of lipid vacuoles and semiquantitative analysis of lipid formation with ImageJ analysis at day 19.
Figure 6.
Expression of osteogenic/adipogenic marker genes and lipid formation in human adipose stem cells (hASCs). (A): Quantitative polymerase chain reaction analysis of relative mRNA expression of osteogenic markers Dlx5 and osterix (Osx) at the 7-day time point and adipogenic marker AP2 at the 14-day time point (3 hASC lines from group I and group II each were used). The amount of BMP-2 (CHO; R&D) used was 100 ng/ml. (B): Qualitative analysis of Oil Red O staining of lipid vacuoles (bright red) and nuclei of the cells (blue) at the 19-day time point. The amount of BMP-2 (CHO and Escherichia coli) used was 100 ng/ml. (C): Quantitative analysis of the Oil Red O lipid formation normalized with amount of nuclei in hASC lines in group I (n = 15) and group II (n = 20). The amount of BMP-2 (CHO) used was 100 ng/ml. (D): Quantitative analysis of the Oil Red O lipid formation normalized with the amount of nuclei in hASC lines 41/12 and 6/12 (n = 5). The amount of BMP-2 (E. coli and CHO) used was 100 ng/ml. Statistical analysis was performed with the Mann-Whitney U test and with Bonferroni correction. The level of significance was set at p < .05. Quantitative analysis was performed by the ImageJ analysis program. Abbreviations: AP2, adipocyte protein 2; BM, basic culture medium; BMP-2, bone morphogenetic protein-2; CHO, Chinese hamster ovary; Dlx, distal-less homeobox; OM= osteogenic medium; DAPI, 4',6-diamidino-2-phenylindole.
BM and OM induced modest lipid formation in all the hASC lines studied. However, BMP-2 treatment induced statistically significant lipid formation in hASC lines from group II, whereas in hASCs from group I, we did not observe any significant enhancement of the lipid formation (Fig. 6B, 6C).
We also wanted to analyze further the relevance of the BMP-2 production origin (ie, E. coli or CHO). Qualitative and semiquantitative Oil Red O analysis of the lipid formation of hASC lines 41/12 (group I) and 6/12 (group II) revealed that lipid formation was significantly enhanced with both types of BMP-2 in hASC line 6/12, although, CHO-produced BMP-2 induced higher levels of lipid formation compared with BMP-2 of E. coli origin. None of the BMP-2 types induced any significant lipid formation in hASC line 41/12 (Fig. 6B, 6D).
Discussion
Osteoinductive BMP-2 is the most prominent growth factor used in clinics in recent years [1, 21–23]. Unfortunately, recent publications describing BMP-2 efficacy on BMSCs and ASCs have been contradictory, and its role as an osteoinducer has been questioned [6, 11, 12, 20]. The current study aimed to further analyze the effect of BMP-2 growth factor in vitro on several hASC lines derived from different donors and to untangle the existing contradictions in previous results in our laboratory and among other laboratories.
Our results indicated that the functional mechanism of BMP-2-induced SMAD activation was present in all studied hASCs, hBMSCs, and osteoblasts, testifying to the molecular functionality of the BMP-2 growth factor and its receptor. Previous in vitro studies investigating the action of the BMP-2 in hASCs have suggested a lack of the BMP-2 effect or even a decreasing effect on the osteogenic differentiation of hASCs [11, 12, 20]. In these studies, ALP activity, mineralization, or gene expression of osteogenic markers such as Dlx5 and Osx were not affected by BMP-2 supplementation. Also BMP-2-related SMAD phosphorylation levels analyzed by Western blot and immunocytochemical staining were unaffected [11]. Our results with BMP-2 indicated that all of the studied hASC lines do respond to mammalian BMP-2, but the biological outcome in the differentiation process varies from one donor line to another. However, one common function for BMP-2 was observed in all hASCs studied: BMP-2 considerably decreased hASC proliferation rates. The same effect can be seen also in our previous work, where BMP-2 was studied in combination with biomaterials, bioactive glass, and β-tricalcium phosphate [12].
Our current results revealed that BMP-2 exerts dual action in differentiation processes of hASCs, and two types of donor cell lines could be distinguished based on their responses. High levels of BMP-2-induced mineralization were expected because of our results of SMAD activation at the molecular level. However, analysis of the expression of adipogenic marker gene AP2 revealed that in the presence of OM and BMP-2 stimulus, all hASC lines studied expressed remarkably high levels of this marker regardless of indicated group. Furthermore, some hASC lines clearly had a greater tendency toward adipogenic differentiation, as indicated by qualitative and quantitative Oil Red O lipid staining (Fig. 6). We also observed heterogeneity in the differentiation capacity within some donor cell lines, with simultaneous mineralization and lipid formation seen in some phase of the differentiation process. However, broader analysis of hASC lines would be required to confirm this heterogeneous behavior.
Our findings indicate that the functional outcome differs substantially among hASC lines; most likely, this is dependent on the other cooperative signaling mechanisms in addition to BMP-2. There are several reports and reviews describing molecular mechanisms of osteogenic and adipogenic differentiation of BMSCs and ASCs, indicating that critical molecular switches are usually part of Wnt, BMP, Notch, and FGF signaling cascades functioning cooperatively in regulation of these events [7, 24–26]. It is plausible that some hASC donor lines used in our studies have more adipocyte commitment and more functional adipogenic signaling pathways compared with others. Thus, hypothetically, these cells might be sensitized for BMP-2 functions toward adipogenic signaling mechanisms and, as a result, we observed enhanced lipid formation instead of mineralization upon BMP-2 stimulus. Park and coworkers reported that BMP-2 induces osteogenic and adipogenic differentiation of human alveolar bone-derived stromal cells [6]. In their study, dose-dependent adipogenic and osteogenic effects of BMP-2 were studied separately in adipogenic and osteogenic media. However, as a further distinction from their work, our study was performed in its entirety in basic and osteogenic culture conditions.
Cell surface marker analysis of the hASCs (supplemental online Table 1; supplemental online Fig. 4) revealed that average expression of the CD marker 34 was relatively high, whereas CD marker 73 expression was lower than stated in the International Society for Cellular Therapy minimal criteria for multipotent MSCs [27]. Based on previous publications by Patrikoski and coworkers [28] and Mitchell and coworkers [29], hASCs have changes in surface marker expression levels due to multiplied passages of the cells. However, in this study, hASCs in groups I and II had a similar CD marker expression pattern (supplemental online Fig. 4), suggesting that variation in expression of CD markers analyzed in this study was not related to differentiation outcome of BMP-2 stimulus. hASC lines in groups I and II had variation in donor age such that the average age in group I was 36 ± 15 years and, in group II, 56 ± 14 years (data not shown). Based on this finding, it is tempting to speculate that hASCs derived from younger donors would have higher potential to demonstrate BMP-2-induced osteogenesis than cells from older individuals.
Our results indicate that the functionality of BMP-2 is distinctly dependent on culture conditions. Western blot analysis of the phosphorylated SMAD1/5 indicated that the intracellular impact of BMP-2 in hASCs, hBMSCs, and osteoblasts was stronger in the HS medium than the FBS medium. The explanation for the different response to BMP-2 in HS and FBS media might be found in the differential expression of BMP receptors on the cell surface of hASCs. Most critical for the functionality of the BMP-2 growth factor is the responsive BMP receptor complex. BMPs exert their diverse biological effects through two types of transmembrane receptors, BMPR-I and BMPR-II, possessing the intrinsic serine/threonine kinase activity [30–32]. The binding of dimeric BMP ligands to heterotetrameric receptors activates their cytoplasmic kinase domain and further receptor-specific SMADs [33, 34]. However, the existence of the BMP-2 receptor on the hASC surface in the FBS medium has been indicated in the extensive studies by Zuk and coworkers [11, 35], suggesting that this might not be the definitive cause for the dysfunction of SMAD signaling mechanisms in hASCs. Furthermore, in this study, we tested only 1 brand of FBS; presumably, FBS from different manufacturers might have displayed a variable impact on BMP-2 functionality. Also, the bioactivity of the actual growth factor can vary in different culture conditions, since components of the FBS could actually inhibit the action of otherwise functional BMP-2 growth factor. Zuk and coworkers also disclosed an interesting possibility in their discussion referring to the differential miRNA expression of the hASCs as at the root of their BMP-2 responsiveness [11].
The conformation of the BMP-2 growth factor is also a very important issue for its biological functionality [36]. We compared the effect of human recombinant BMP-2 produced in E. coli and mammalian CHO cells on proliferation and osteogenic and adipogenic differentiation of hASCs. Our results clearly indicated that hASCs responded to mammalian-produced BMP-2 more efficiently. BMP-2 produced in CHO cells had a marked but donor-dependent impact on mineralization and lipid formation of hASCs. BMP-2 produced in E. coli also induced these processes, but the effect was clearly diminished compared with CHO-produced BMP-2. In all the hASC lines studied, both BMP-2 types also decreased proliferation of the donor cell line independently, although CHO-produced BMP-2 was more effective. This is probably due to more physiological conformation of the CHO-produced growth factor and that proteins produced in the mammalian system have been processed by cells with posttranslational glycosylation, unlike proteins produced in bacterial systems [36]. Therefore, the use of different types of growth factors might also partially explain variable results reported from several BMP-MSC studies. Variability in culture conditions, arrangements of responsive receptors, expression of growth factors by cells in certain conditions, status of the different populations, and internal cellular mechanisms might explain the fluctuating outcome of hASCs in BMP-2 stimulus and further studies are required to clarify molecular signaling and functional outcome in this context.
Conclusion
We examined how BMP-2 modulates signaling mechanisms, proliferation, and differentiation outcome of hASCs derived from several donors. Our results show that BMP-2 triggers molecular SMAD signaling mechanisms in hASCs and regulates differentiation processes in HS medium. The production origin of BMP-2 has an important role in its functionality on hASCs, since BMP-2 produced in mammalian cells induced higher responses than counterparts produced in E. coli. Based on our results, BMP-2 has two mechanisms of action, inducing osteogenic and adipogenic differentiation, depending on the hASC donor line. These findings partially explain contradictory previous results and highlight the importance of further studies to understand how signaling pathways guide MSC functions at the molecular level.
Supplementary Material
Acknowledgments
We thank Sari Kalliokoski and Anna-Maija Honkala for technical assistance. The work was supported by the Finnish Funding Agency for Technology and Innovation, the Jane and Aatos Erkko Foundation, and research funding from the Pirkanmaa Hospital District and Tampere Graduate Program in Biomedicine and Biotechnology.
Author Contributions
S.V.: conception and design, collection and assembly of the data, data analysis and interpretation, manuscript writing, final approval of the manuscript; M.O.: collection and assembly of the data, data analysis and interpretation, manuscript writing, final approval of the manuscript; R.A.: data analysis and interpretation, manuscript writing, final approval of the manuscript; M.J.: collection and assembly of the data, data analysis and interpretation, final approval of the manuscript; S.M.: data analysis and interpretation, manuscript writing, final approval of the manuscript, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Mesimäki K, Lindroos B, Törnwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Wang YK, Yu X, Cohen DM, et al. Bone morphogenetic protein-2-induced signaling and osteogenesis is regulated by cell shape, RhoA/ROCK, and cytoskeletal tension. Stem Cells Dev. 2012;21:1176–1186. doi: 10.1089/scd.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Guo J, Zhou Y, et al. The roles of bone morphogenetic proteins and their signaling in the osteogenesis of adipose-derived stem cells. Tissue Eng Part B Rev. 2014;20:84–92. doi: 10.1089/ten.teb.2013.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryoo HM, Lee MH, Kim YJ. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006;366:51–57. doi: 10.1016/j.gene.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Castro-Govea Y, Cervantes-Kardasch VH, Borrego-Soto G, et al. Human bone morphogenetic protein 2-transduced mesenchymal stem cells improve bone regeneration in a model of mandible distraction surgery. J Craniofac Surg. 2012;23:392–396. doi: 10.1097/SCS.0b013e318240fe9b. [DOI] [PubMed] [Google Scholar]
- 6.Park JC, Kim JC, Kim BK, et al. Dose- and time-dependent effects of recombinant human bone morphogenetic protein-2 on the osteogenic and adipogenic potentials of alveolar bone-derived stromal cells. J Periodontal Res. 2012;47:645–654. doi: 10.1111/j.1600-0765.2012.01477.x. [DOI] [PubMed] [Google Scholar]
- 7.James AW, Zara JN, Zhang X, et al. Perivascular stem cells: A prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Translational Medicine. 2012;1:510–519. doi: 10.5966/sctm.2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montgomery SR, Nargizyan T, Meliton V, et al. A novel osteogenic oxysterol compound for therapeutic development to promote bone growth: Activation of hedgehog signaling and osteogenesis through smoothened binding. J Bone Miner Res. 2014;29:1872–1885. doi: 10.1002/jbmr.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dragoo JL, Choi JY, Lieberman JR, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 10.Chou YF, Zuk PA, Chang TL, et al. Adipose-derived stem cells and BMP2: Part 1. BMP2-treated adipose-derived stem cells do not improve repair of segmental femoral defects. Connect Tissue Res. 2011;52:109–118. doi: 10.3109/03008207.2010.484514. [DOI] [PubMed] [Google Scholar]
- 11.Zuk P, Chou YF, Mussano F, et al. Adipose-derived stem cells and BMP2: Part 2. BMP2 may not influence the osteogenic fate of human adipose-derived stem cells. Connect Tissue Res. 2011;52:119–132. doi: 10.3109/03008207.2010.484515. [DOI] [PubMed] [Google Scholar]
- 12.Waselau M, Patrikoski M, Juntunen M, et al. Effects of bioactive glass S53P4 or beta-tricalcium phosphate and bone morphogenetic protein-2 and bone morphogenetic protein-7 on osteogenic differentiation of human adipose stem cells. J Tissue Eng. 2012;3:2041731412467789. doi: 10.1177/2041731412467789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan J, Park H, Lee MK, et al. Adipose-derived stem cells and BMP-2 delivery in chitosan-based 3D constructs to enhance bone regeneration in a rat mandibular defect model. Tissue Eng Part A. 2014;20:2169–2179. doi: 10.1089/ten.tea.2013.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maegawa N, Kawamura K, Hirose M, et al. Enhancement of osteoblastic differentiation of mesenchymal stromal cells cultured by selective combination of bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-2 (FGF-2) J Tissue Eng Regen Med. 2007;1:306–313. doi: 10.1002/term.41. [DOI] [PubMed] [Google Scholar]
- 15.Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112:3491–3501. doi: 10.1002/jcb.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viale-Bouroncle S, Gosau M, Morsczeck C. NOTCH1 signaling regulates the BMP2/DLX-3 directed osteogenic differentiation of dental follicle cells. Biochem Biophys Res Commun. 2014;443:500–504. doi: 10.1016/j.bbrc.2013.11.120. [DOI] [PubMed] [Google Scholar]
- 17.Viale-Bouroncle S, Klingelhoffer C, Ettl T, et al. A protein kinase A (PKA)/beta-catenin pathway sustains the BMP2/DLX3-induced osteogenic differentiation in dental follicle cells (DFCs) Cell Signal. 2015;27:598–605. doi: 10.1016/j.cellsig.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Lindroos B, Boucher S, Chase L, et al. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy. 2009;11:958–972. doi: 10.3109/14653240903233081. [DOI] [PubMed] [Google Scholar]
- 19.Pfaffl W. A new mathematical model for relative quantification in real-time RT-PCR. Nuclei Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tirkkonen L, Haimi S, Huttunen S, et al. Osteogenic medium is superior to growth factors in differentiation of human adipose stem cells towards bone-forming cells in 3D culture. Eur Cell Mater. 2013;25:144–158. doi: 10.22203/ecm.v025a10. [DOI] [PubMed] [Google Scholar]
- 21.Sándor GK, Tuovinen VJ, Wolff J, et al. Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: a case report and review of the clinical application of good manufacturing practice-level adipose stem cells for bone regeneration. J Oral Maxillofac Surg. 2013;71:938–950. doi: 10.1016/j.joms.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Sándor GK, Numminen J, Wolff J, et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Translational Medicine. 2014;3:530–540. doi: 10.5966/sctm.2013-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuckert KH, Jopp S, Osadnik M. The use of platelet rich plasma, bone morphogenetic protein-2 and different scaffolds in oral and maxillofacial surgery—literature review in comparison with own clinical experience. J Oral Maxillofac Res. 2011;2:e2. doi: 10.5037/jomr.2011.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatakun P, Núñez-Toldrà R, Díaz López EJ, et al. The effect of five proteins on stem cells used for osteoblast differentiation and proliferation: a current review of the literature. Cell Mol Life Sci. 2014;71:113–142. doi: 10.1007/s00018-013-1326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Titorencu I, Pruna V, Jinga VV, et al. Osteoblast ontogeny and implications for bone pathology: An overview. Cell Tissue Res. 2014;355:23–33. doi: 10.1007/s00441-013-1750-3. [DOI] [PubMed] [Google Scholar]
- 26.Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 27.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patrikoski M, Juntunen M, Boucher S, et al. Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy-compliant human adipose stem cells. Stem Cell Res Ther. 2013;4:27. doi: 10.1186/scrt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 30.Ehrlich M, Horbelt D, Marom B, et al. Homomeric and heteromeric complexes among TGF-β and BMP receptors and their roles in signaling. Cell Signal. 2011;23:1424–1432. doi: 10.1016/j.cellsig.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Ehrlich M, Gutman O, Knaus P, et al. Oligomeric interactions of TGF-β and BMP receptors. FEBS Lett. 2012;586:1885–1896. doi: 10.1016/j.febslet.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 32.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 33.Heldin CH, Moustakas A. Role of Smads in TGFβ signaling. Cell Tissue Res. 2012;347:21–36. doi: 10.1007/s00441-011-1190-x. [DOI] [PubMed] [Google Scholar]
- 34.Zieba A, Pardali K, Soderberg O, et al. Intercellular variation in signaling through the TGF-beta pathway and its relation to cell density and cell cycle phase. Mol Cell Proteomics. 2012;11:M111.013482. doi: 10.1074/mcp.M111.013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carreira AC, Lojudice FH, Halcsik E, et al. Bone morphogenetic proteins: Facts, challenges, and future perspectives. J Dent Res. 2014;93:335–345. doi: 10.1177/0022034513518561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.