Regenerative medicine products regulated by the US Food and Drug Administration (FDA) are classified on the basis of formal regulatory definitions for biologics, medical devices, and combination products, as well as human cells and tissues. FDA efforts to facilitate product development in this promising area for unmet needs include working with individual sponsors, interacting with the scientific and industry communities, participating in standards development, and developing policy and guidance.
Keywords: Regenerative medicine, Tissue engineering, U.S. Food and Drug Administration, Regulation
Abstract
Regenerative medicine (RM) is a popular term for a field of scientific and medical research. There is not one universally accepted definition of RM, but it is generally taken to mean the translation of multidisciplinary biology and engineering science into therapeutic approaches to regenerate, replace, or repair tissues and organs. RM products have the potential to provide treatments for a number of unmet needs but have substantial scientific and regulatory challenges that need to be addressed for this potential to be fully realized. FDA has established formal regulatory definitions for biologics, medical devices, and combination products, as well as human cells and tissues. Regenerative medicine products regulated by FDA are classified on the basis of these definitions, and the classification forms the basis for determining the regulatory requirements to each specific product. FDA regulations are generally written to allow the agency flexibility to accommodate new scientific questions raised by novel and evolving technologies. FDA efforts to facilitate product development in this novel and promising area include working with individual sponsors, interacting with the scientific and industry communities, participating in standards development, and developing policy and guidance.
Significance
Regenerative medicine is generally taken to mean the translation of multidisciplinary biology and engineering science into therapeutic approaches to regenerate, replace, or repair tissues and organs. This article provides an overview of the efforts of the U.S. Food and Drug Administration (FDA) to facilitate product development in the field commonly known was regenerative medicine. It provides an introduction to the processes by which FDA works with individual sponsors, interacts with the scientific and industry communities, participates in standards development, and develops formal FDA policy and guidance.
Introduction
Regenerative medicine (RM) is generally understood as a multidisciplinary approach to development of new products that are intended to repair, replace, or regenerate tissues and organs in patients. Challenges exist across the spectrum of RM product development. Manufacturing and product characterization for RM products may be complex. Seemingly minor manufacturing changes may have large and unpredicted effects on product characteristics. Preclinical animal models may not exist or be adequate to aid in the design of clinical studies; even if the models do exist, then factors such as size or immunology may limit interpretability of preclinical testing. Clinical testing also includes challenges; examples include distinguishing the clinical effect attributable to the investigational product from any effect attributable to potential variations among clinical care and surgical technique.
Major advances in medical science and technology over the years have led to novel products such as the regenerative medicine combination products that may not fit easily into the jurisdictional responsibility of any of the three U.S. Food and Drug Administration (FDA) medical product centers (i.e., Center for Drug Evaluation and Research; Center for Devices and Radiological Health; Center for Biologics Evaluation and Research [CBER]). FDA’s goal in this situation is to use available resources and expertise efficiently. Therefore, rather than generate an independent “review silo” with a sufficient quantity and diversity of review staff to accommodate regenerative medicine, FDA has relied on building a number of connections between the existing FDA review centers that possess relevant expertise. These connections span the regulatory process for specific products from presubmission jurisdictional and regulatory advice through the entire application review process to postmarketing monitoring. Information on how FDA allocates responsibilities for product oversight is available on the FDA website [1].
FDA Application Review Process
The scientific and regulatory issues raised by complex, novel RM products are many and subject to rapid change as technology progresses. To facilitate the development of this diverse group of products, FDA exercises flexibility in applying regulatory standards to ensure safe and effective products while working to avoid unnecessary regulatory burden. FDA uses a tiered regulatory approach when determining the quantity, quality, and type of manufacturing, and nonclinical and clinical data that would be required for an individual product development pathway. This approach allows for flexibility early in the investigational process when assessing potential risks associated with a specific product and in the design of adequate and well-controlled clinical studies. The regulatory review process emphasizes the scientific rationale and data to support product characterization and safety, preclinical studies designed to support use of specific products, and clinical trial design supported by product and preclinical data.
The first step for a sponsor of an investigational product is to determine the regulations that would be applicable to the product. In many cases, the regulatory route and lead center may be clear to the sponsor, and sponsors may contact the review group at FDA directly with specific questions about data requirements. However, in circumstances in which the regulatory route or lead center is not clear, sponsors may contact the FDA Office of Combination Products, which can provide formal or informal input into jurisdiction, or contact the jurisdiction officer at one of the medical product centers.
The Office of Cellular, Tissue, and Gene Therapies
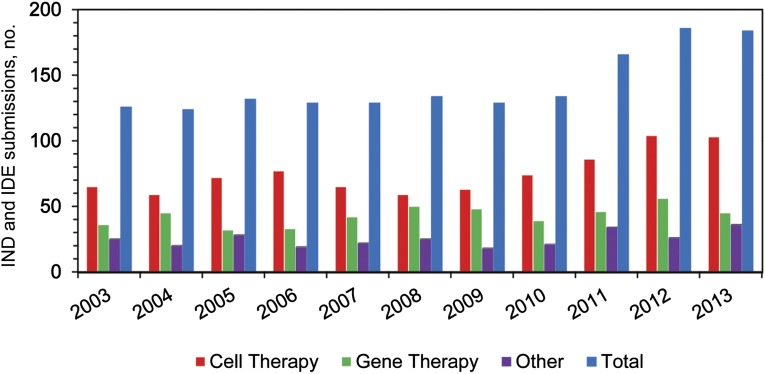
The Office of Cellular, Tissue, and Gene Therapies (OCTGT) in the CBER is responsible for the oversight of a variety of novel products, including stem cell and stem cell-derived products, somatic cell therapies, therapeutic vaccines and immunotherapy products, gene therapies, tissue and tissue-based products, and some related medical devices and combination products, including many cell-scaffold combination products. The increasing interest in these novel products is reflected in the number of investigational study applications that have been submitted to OCTGT, which have increased from approximately 125 per year during the years 2003–2010, to approximately 180 in 2012 and 2013 (Fig. 1).
Figure 1.
Numbers of new IND and IDE submissions to the Office of Cellular, Tissue, and Gene Therapies from 2003 to 2013. Abbreviations: IDE, investigational device exemption; IND, investigational new drug.
OCTGT is strongly committed to fostering a partnership with industry, patient advocates, academia, sponsors, and the public to promote and develop new therapies based on the latest advances in science and technology. OCTGT engages in pre-investigational new drug submission communications with sponsors as well as meetings to discuss later-stage development issues (Fig. 2). In addition to interactions with individual sponsors, OCTGT also develops regulatory policy, issues both product-specific and cross-cutting guidance, conducts mission-related research, and performs stakeholder outreach through workshops, external presentations, webinars, and roundtable meetings.
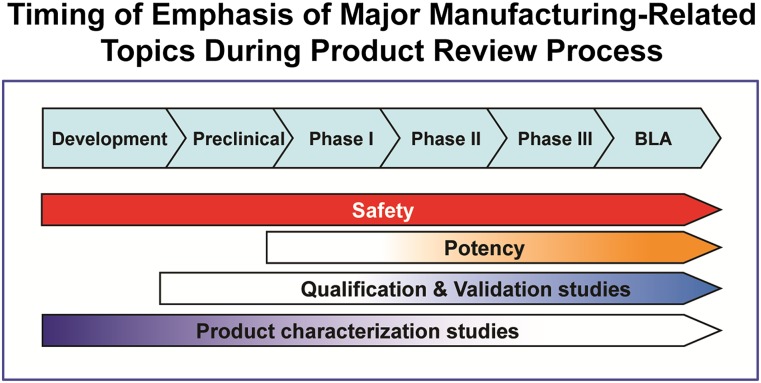
Figure 2.
Timing of emphasis of major manufacturing-related topics during the product review process. The figure illustrates the typical relative importance of major manufacturing-related topics over the time course of typical product development for regenerative medicine products. Note that safety is a significant emphasis throughout product development, as clinical safety of product can be affected by manufacturing issues anywhere throughout development and postlicensure. In contrast to safety, product characterization studies, qualification and validation studies and development of a potency assay should typically build on each other so that an early emphasis on identification and measurement of product characterization attributes enables qualification and validation of a subset of these attributes and eventual development and validation of potency assay by the time of licensure. Agency prospective input to manufacturers also typically follows these patterns. Abbreviation: BLA, Biologics License Application.
Laboratory-based researchers/reviewers in OCTGT conduct regulatory science-related research in RM. For example, one ongoing, multi-investigator project addresses the ability of frequently used standards and technology to adequately characterize mesenchymal stromal cells (MSCs) for potential use as investigational products in clinical trials. The goal of this research is to use in vivo and in vitro assays to identify critical attributes that may correlate with safety and efficacy of MSC products.
Engagement With Federal Agencies, Regulated Industry, International Regulators, and the Research Community
FDA works closely with industry and academia on product development issues. In addition to meeting with individual sponsors, FDA organizes or participates in workshops, holds FDA advisory committee meetings, and participates in standards activities, scientific/professional meetings, and other educational outreach efforts.
Of particular note is FDA participation, along with a number of other federal agencies, including the National Institutes of Health (NIH), National Institute of Standards and Technology (NIST), Department of Defense, and National Science Foundation, in the Multi-Agency Tissue Engineering Science (MATES) Interagency Working Group. Since its inception in 2000, the goals of MATES have been the following: facilitate communication across departments/agencies by regular information exchanges, enhance cooperation through cosponsorship of scientific meetings and workshops, facilitate development of standards, and monitor technology by undertaking cooperative assessments of the status of the field. In 2007, under the auspices of the Office of Science Technology Policy, MATES published a strategic plan for federal government investments in tissue science and engineering [2].
An example of industry/academia outreach is OCTGT’s participation in yearly roundtable meetings with the California Institute of Regenerative Medicine. These meetings provide a mechanism to discuss development challenges that our stakeholders have identified for the stem cell and regenerative medicine fields. At these meetings, OCTGT works to ensure that the RM research community is aware of the regulatory requirements for clinical translation of these products.
As a reflection of the international character of the research and development in RM, FDA has discussions with other international regulators to gain a broad understanding of the regulations and principles for oversight of these products. The goals of these discussions are regulatory convergence of requirements and scientific approaches. Two key examples of this are the FDA, European Medicines Agency (EMA), Health Canada Advanced Therapy Medicinal Product cluster that meets under the European Commission/EMA-FDA Bilateral, and the Cell Therapy Working Group and Gene Therapy Working Group that meet under the auspices of the International Pharmaceutical Regulators Forum [3, 4].
Guidance Documents Specific for OCTGT-Regulated RM Product Development
To assist researchers and product developers in understanding how FDA applies regulatory requirements, FDA publishes guidance documents that provide the agency’s current thinking and recommendations on how to address regulatory requirements. Guidance recommendations are nonbinding, and sponsors have the choice of meeting the regulatory requirements by using an alternative approach. Guidance recommendations frequently provide an initial approach that is adaptable to a specific product.
OCTGT has an active guidance development program, and has published a number of final and draft guidance documents related to products in OCTGT [5] (supplemental online Table 1). An updated list of guidances planned for the future is published biannually in FDA’s Guidance Agenda [6] (supplemental online Table 2).
As mentioned previously, the novelty of RM products frequently necessitates novel interpretations of regulations. When new interpretations become sufficiently frequent, OCTGT communicates new policy by publishing new guidances. For example, OCTGT developed guidance for the application of the long-standing requirements related to potency [21 Code of Regulations (CFR) 600.3(s)] and potency testing (21 CFR 610.10) of cellular and gene therapy products [7]. Such guidances provide recommendations and frameworks that can facilitate development of strategies to meet regulatory requirements.
FDA’s Role in Accelerating Innovation
In addition to efforts focused on RM, there are a number of agency cross-cutting initiatives that could be used to accelerate the development of RM products. For example, FDA has four regulatory programs that are intended to facilitate and expedite development of new drugs to address unmet medical need in the treatment of serious or life-threatening conditions. The four programs are: Fast Track, Breakthrough Therapy, Accelerated Approval, and Priority Review. As a consequence of the FDA Safety and Innovation Act (FDASIA) that was enacted in July 2012, FDA issued the guidance, “Expedited Programs for Serious Conditions-Drugs and Biologics,” which compiled explanations of, and recommendations for, the four programs in a single document [8]. These initiatives are important for OCTGT products, as evidenced by the eight breakthrough designations granted by OCTGT between the passage of breakthrough program in FDASIA in July 2012 and May 2015.
Equally important in accelerating innovation are FDA’s leveraging efforts with other groups to solve difficult challenges whose solutions extend beyond the scope of a regulatory fix. FDA’s long-term interactions with the NIH’s Office of Science Policy and its Recombinant DNA Advisory Committee (RAC) (a Federal Advisory Committee Act committee that provides recommendations to NIH on basic and clinical research in the area of recombinant DNA research), which include symposia planning and meeting participation, is an ongoing example of this leveraging. Specifically, over the last 5 years the RAC has held three symposia (2010, 2013, 2015), to explore the scientific challenges posed by the development of the T-cell immunotherapy field as the field has developed over that time period. The field of T-cell immunotherapy includes gene-modified T-cell receptors that recognize a tumor antigen in the context of HLAs, as well as chimeric antigen receptors (CAR T-cells), that recognize cell-surface tumor antigens in an MHC independent fashion. Experimental agents in this field pose a number of different challenges, including dosing and toxicity issues and need for costimulation, as well as issues related to the design of the products. These scientific symposia provided valuable opportunities for discussion and generation of consensus understanding of these challenges and potential solutions in a neutral venue that can inform NIH and FDA activities related to this promising field.
Similarly, in the field of mitochondrial research, the publication of scientific articles describing nonclinical studies of mitochondrial manipulation technologies that were being developed with the goal of prevention of transmission of mitochondrial disease from affected women to their children raised both scientific and ethical questions that were ripe for public discussion prior to initiation of clinical trials. FDA convened a meeting of the Cellular, Tissue and Gene Therapies Advisory Committee (CTGTAC) to examine the general scientific issues and clinical trial design issues raised by developments in the field. The CTGTAC is a Federal Advisory Committee Act committee that provides recommendations to FDA on scientific and clinical issues related to these product areas. Because the ethical and social policy issues that could be raised by these technologies were beyond the scope of the CTGTAC, the FDA subsequently commissioned the Institute of Medicine to examine the ethical and social policy questions and produce a consensus study report of its assessment of these issues (as of July 1, 2015 the Institute of Medicine was officially renamed the National Academy of Medicine, as a constituent part of the newly formed National Academies of Sciences, Engineering, and Medicine).
FDA Role in Enhancing Regulatory Science
Similarly, as a consequence of Title XI of FDASIA, Section 1124, FDA issued a plan in July 2013 for enhancing regulatory science, entitled “Strategy and Implementation Plan for Advancing Regulatory Science for Medical Products” [9]. The plan outlines FDA’s priorities to ensure that advances in regulatory science for medical products are adopted, and includes a description of the kind of metrics that will be tracked to show progress. The regulatory priority areas in the plan are consistent with those previously identified in the 2011 Agency Strategic Plan for Regulatory Science [10]. One of the eight priority areas in this strategic plan is to ensure FDA readiness to evaluate innovative emerging technologies. Regenerative medicine, including cell- and tissue-based products, falls under the emerging technology umbrella. In July 2013, FDA published an article titled “Continuing to Strengthen FDA’s science approach to emerging technologies” that describes how FDA is facilitating the area of emerging technologies consistent with the strategic plan [11].
Standards development could be particularly beneficial to RM by facilitating test methodology. FDA staff, including staff from OCTGT, participate in standards development activities with several Standard Development Organizations (SDOs), whose participants include federal agencies (FDA, NIST, NIH, and so forth), regulated industry, and academic investigators. One SDO activity in which FDA and NIST have been particularly active is Division IV of ASTM International Committee F04, which is focused on development of detailed (“vertical”) standards for tissue engineered medical products. Another SDO activity in which FDA and NIST are heavily involved is the International Standards Organization (ISO) technical committee, ISO TC276, a newly established ISO emphasizing RM product development.
In response to stakeholders’ interest in standards for RM products, OCTGT held a workshop titled “Synergizing Efforts in Standards Development for Cellular Therapies and Regenerative Medicine Products” on March 31, 2014, at FDA headquarters. The purpose of the workshop was to bring together a broad range of stakeholders interested in the clinical development of cellular therapies and regenerative medicine products to inform them on (a) the role that federal agencies play in standards development; (b) the different types of standards that can be useful in RM; and (c) the organizations that are developing standards for such products. In addition, the workshop provided an opportunity for discussion of areas ripe for current and future standards development, and mechanisms to support standards development through collaboration.
Conclusion
The translation of a promising research finding into a medical product is a challenge, even in traditional fields of medical research. Translation of basic research findings in a still-emerging field such as regenerative medicine is even more challenging for many reasons, such as the novelty of product manufacturing and composition, preclinical testing models, clinical applications, and regulatory expectations. All stakeholders (government, academia, and industry) in RM have opportunities for innovation to address these challenges. FDA’s efforts to address these challenges are consistent with the agency’s public health mission. This mission is manifest in FDA’s efforts to assure safety and effectiveness of existing products while simultaneously facilitating innovation of new products. As a result, FDA is actively involved in tailoring application of existing regulations to specific RM products, developing formal guidance on regulatory requirements for RM products, participating in formal and informal outreach, participating in consensus standards development, and conducting regulatory science research that can be used broadly in the RM field.
Supplementary Material
Acknowledgments
We thank W. Bryan, K. Benton, and P. Marks for editorial comments and T. Finn for assistance with the figures.
Author Contributions
C.M.W.: conception and design, manuscript writing, final approval of manuscript; R.D.M. and S.L.S.: conception and design, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.US Food and Drug Administration. Combination products. Available at http://www.fda.gov/CombinationProducts/default.htm. Accessed May 7, 2015.
- 2.Multi-Agency Tissue Engineering Science (MATES) Interagency Working Group. Available at http://tissueengineering.gov/welcome-s.htm. Accessed June 12, 2014.
- 3.European Commission/European Medicines Agency - US Food and Drug Administration bilateral: update. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2014/04/news_detail_002078.jsp&mid=WC0b01ac058004d5c1. Accessed October 2, 2015.
- 4.International Pharmaceutical Regulators Forum. Purpose. Goals. Available at http://www.i-p-r-f.org/en/. Accessed November 12, 2014.
- 5.US Food and Drug Administration. Cellular and gene therapy guidances. Available at http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/default.htm. Accessed May 7, 2015.
- 6.U.S. Food and Drug Administration. 2015 Guidance agenda. Available at http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/UCM431409.pdf. Accessed May 7, 2015.
- 7.U.S. Food and Drug Administration. Guidance for industry: potency tests for cellular and gene therapy products. Available at http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM243392.pdf. Accessed May 7, 2015.
- 8.U.S. Food and Drug Administration. Guidance for industry: expedited programs for serious conditions- drugs and biologics. Available at http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm358301.pdf. Accessed January 5, 2015.
- 9.U.S. Food and Drug Administration. Strategy and implementation plan for advancing regulatory science for medical products. Available at http://www.fda.gov/downloads/RegulatoryInformation/Legislation/SignificantAmendmentstotheFDCAct/FDASIA/UCM359956.pdf. Accessed January 5, 2015.
- 10.U.S. Food and Drug Administration. Advancing regulatory science at FDA. A strategic plan (August 2011). Available at http://www.fda.gov/ScienceResearch/SpecialTopics/RegulatoryScience/ucm267719.htm. Accessed January 5, 2015.
- 11.Anatol R, Bauer S, Epstein S, et al. Continuing to strengthen FDA’s science approach to emerging technologies. Nanomedicine (Lond) 2013;9:594–599. doi: 10.1016/j.nano.2013.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.