Abstract
The information on the epidemiology and control of coccidian parasites of broilers in Kashmir valley is based on the reports available from other regions of the world. With this background, the present study was conducted to investigate the seasonal prevalence of the disease in the temperate agro-climatic conditions of Kashmir valley. A standard protocol for sampling was followed according to which five birds per 10,000 is sufficient to diagnose coccidiosis. Microscopic examination (under 10× and 40× objective lens) was used to reveal the presence of coccidial oocysts. Different species of genus Eimeria were identified on the basis of their predilection site, morphology and size. Coccidiosis was most prevalent in autumn 45.12 ± 2.55 (September 47.5 %, October 42.42 % and November 45.46 %) followed by summer 30.84 ± 6.86, spring 23.81 ± 2.81 and winter 20.29 ± 6.40. In summer, prevalence of disease was low but afterwards prevalence of disease rose up from August to October. In spite of high relative humidity in winter, disease showed low prevalence rate. Over all prevalence for the whole year was 29.87 %. Among species Eimeria tenella was the most dominant parasite showing highest prevalence of 18.13 %. Variation in incidence of coccidiosis with respect to seasons showed a strong correlation and data was found to be statistically significant with P < 0.05. The results obtained would be quite useful to devise appropriate and effective control strategies and prophylactic programs for coccidiosis in poultry unique to this climatic zone and other parts of the world with similar climatic and poultry production systems.
Keywords: Coccidiosis, Poultry, Seasonal impact
Introduction
Coccidiosis is the commonest and economically most important diseases of poultry world-wide (Shirley et al. 2005). It has been documented that coccidiosis is the most consistently reported health problem in poultry (Biggs 1982; Rose 1987; Williams 1999). In all parts of the world where confinement rearing is practiced, coccidiosis represents a major disease problem demanding the attention of poultry producers, feed manufactures, and poultry disease experts (Reid 1978). Poultry industry in US suffers in excess of one to two billion US dollars in annual losses relating to coccidial infection, treatment, and prevention (Danforth and Augustine 1989; Talebi and Mulcahy 1995; Yun et al. 2000). In India, estimation has revealed that commercial broiler industry is a major sufferer due to coccidiosis wherein 95.61 per cent of the total economic loss occurs due to the disease (Bera et al. 2010).
Coccidial parasites are highly species specific, and acquired immunity can be achieved once the coccidia complete their life cycle. However, birds can harbor the disease and be carriers after infection, increasing the likelihood of spreading coccidiosis (Williams 1998).There are seven valid species of chicken coccidia, E. acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. praecox, and E. tenella (Shirley 1986) each species developing in a particular location within the chick digestive tract. In coming 40 years, as the world becomes ever more dependent upon poultry, coccidia will still require control and researchers will need to provide the farmers with a larger armoury of control strategies (Shirley and Lillehoj 2012).
The use and management of suitable bedding material is a crucial part of bird welfare in intensive production systems. Poultry litter becomes wet when the rate of water addition (urine/faeces/spillage) exceeds the rate of removal (evaporation) (Collett 2007). Litter cushions the bird from the hard floor, provides insulation and has the capacity to both absorb and facilitate evaporation of faecal, urinary and spilled water (Collett 2012). Poor management practices such as damp litter promote oocyst sporulation, contaminated feeders and drinkers, poor ventilation facilities and high stocking densities can aggravate the clinical infection (Khan et al. 2006).
Materials and methods
Collection of samples
The 375 gut samples in the study were randomly collected from Ganderbal, Srinagar, Baramullah, Kupwara, Budgam and Pulwama districts of Kashmir valley. Information collected at the time of sampling included farmer’s name, address, farm location, flock age, flock size and use of coccidiostats in the feed for that flock and previous coccidiosis infection within the last year in the farm. Samples were taken from flocks 20–35 days of age. Sample size was taken as per Mattiellio (Mattiello 1990), according to which a sample of five birds per 10,000 is sufficient to diagnose coccidiosis. Guts were collected sterile plastic bags, labeled, and transport to Parasitology laboratory, Department of Zoology, University of Kashmir.
Initial examination and processing
All the guts were cut into different portions (duodenum, jejunum, ilium, large intestine and caeca) with the help of sharp veterinary grade scissors (Fig. 1), and the gut contents of respective portions were examined microscopically (under 10× and 40× objective lens) to reveal the presence of coccidial oocysts as described earlier (Soulsby 1982). Mucosae of the intestines were also examined for gross lesions. A series of scrapings of the duodenum and jejunum were obtained for diagnosis of the subclinical coccidiosis according to the method described by Mattiello (1990). A sample was considered to be negative if three slides from the same sample were observed with no oocysts (Fig. 2). Different species of genus Eimeria were identified on the basis of their predilection site, morphology and size (Anonymous 1984).
Fig. 1.
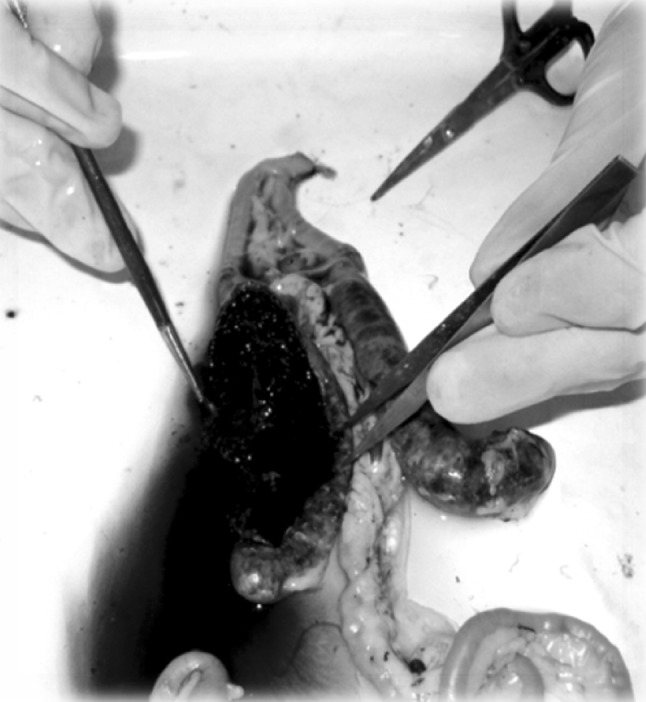
Internal bleeding within the caecum
Fig. 2.
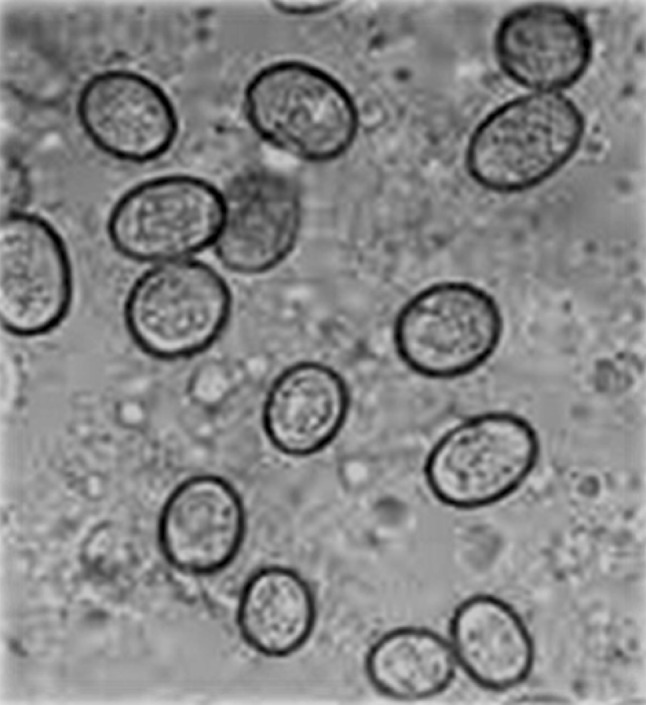
Eimerian oocysts
Stastical analysis
The whole data was fed into Microsoft Excel 2010, a computer program (SPSS 16.0 for windows) and Primer software was used for data analysis. The data was represented as mean of replicates followed by standard deviation i.e. Mean ± standard deviation (SD). The mean value in each group was analyzed and compared with others by Student’s t test. P Values less than 0.05 were considered statistically significant.
Result
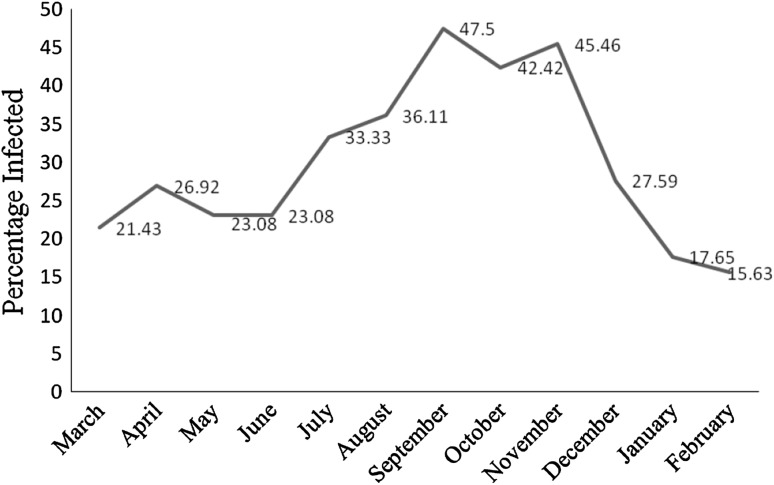
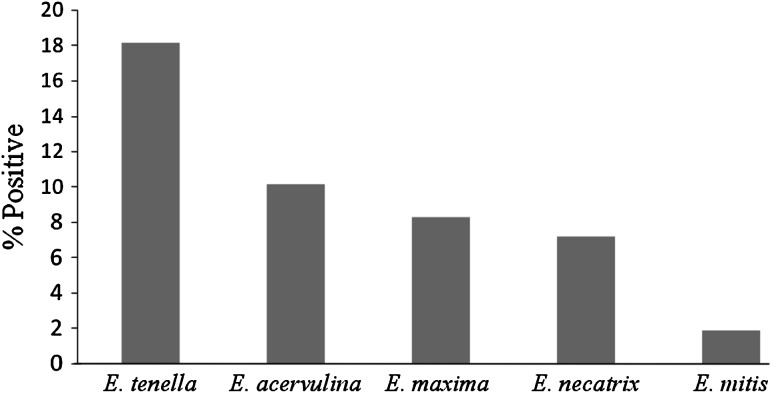
A total of 375 gut samples of poultry birds (broilers) were collected from different poultry farms of Kashmir valley. On examining 375 gut samples 112 were found to harbor the Eimeria parasite. In the present study, data showed that coccidiosis was more prevalent in autumn (September 47.5 %, October 42.42 % and November 45.46 %) followed by summer, spring and winter (Table 1; Fig. 3). The most dominating among all the species was E. tenella showing the highest prevalence (18.13 %), followed by E. acervulina (10.13 %), E. maxima (8.26 %), E. necatrix (7.2 %) and E. mitis (1.86 %) (Table 2; Fig. 4).
Table 1.
Prevalence pattern of the coccidian parasites in broiler chicks across poultry farms in the Kashmir Valley
| Month | Season | Examined | Infected | % Infected | Mean ± SD |
|---|---|---|---|---|---|
| March | Spring | 28 | 6 | 21.43 | 23.81 ± 2.81 |
| April | 26 | 7 | 26.92 | ||
| May | 39 | 9 | 23.08 | ||
| June | Summer | 26 | 6 | 23.08 | 30.84 ± 6.86 |
| July | 30 | 10 | 33.33 | ||
| August | 36 | 12 | 36.11 | ||
| September | Autumn | 40 | 19 | 47.50 | 45.12 ± 2.55 |
| October | 33 | 14 | 42.42 | ||
| November | 22 | 10 | 45.46 | ||
| December | Winter | 29 | 8 | 27.59 | 20.29 ± 6.40 |
| January | 34 | 6 | 17.65 | ||
| February | 32 | 5 | 15.63 | ||
| Total | 375 | 112 | 29.87 | P < 0.05 | |
*All numerical values are the number of organisms taken for the study
Fig. 3.
Prevalence of coccidian infection in poultry birds of kashmir valley
Table 2.
Prevalence of five Eimeria spp. among broiler farms in Kashmir valley
| Species of Eimeria | No. of positive | % positive | % Prevalence of each species in infected birds |
|---|---|---|---|
| E. tenella | 68 | 18.13 | 40 |
| E. acervulina | 38 | 10.13 | 22 |
| E. maxima | 31 | 8.26 | 18 |
| E. necatrix | 27 | 7.2 | 16 |
| E. mitis | 7 | 1.86 | 4 |
Fig. 4.
Showing the overall prevalence of each species
Discussion
Prevalence study provides the basic foundation for any parasite control measures. The study aimed to find out the correlation of occurrence of disease in a particular season with distinct environmental conditions. During the study period sampling was done throughout the year to find out the correlation of occurrence of disease in a particular season with distinct environmental conditions. In the present study, data showed that coccidiosis was more prevalent in autumn (September 47.5 %, October 42.42 % and November 45.46 %) followed by summer, spring and winter. This prevalence pattern of the disease may be correlated with the fact that ambient temperature and relatively higher humidity (>60 %) favour the disease by promoting the oocyst sporulation and survivability (Razmi and Kalideri 2000). The degree and rate of sporulation of excreted oocysts are important factors affecting the infection pressure in a flock of birds, thus influencing the epidemiology of the infections. Moist litter will favor the development of coccidiosis, because of the higher sporulation ability thus induced (Card and Nesheim 1972; Matter and Oester 1989).
When the temperature starts falling towards the end of summer, there is need for building temperature inside the broiler farm, which urges farmers to cut off the ventilation resulting in the buildup of relative moisture content inside resulting in wet litter. Wet litter is essential for sporulation of oocysts, despite winter recorded the lowest prevalence which might be attributed to unfavourable temperature, unsuitable for sporulation. There was, however, little difference in prevalence between winter and spring. Although environmental factors have positive impact on the occurrence of disease, results showed that higher prevalence of diseases was due to the combined effect of high level relative humidity and ambient temperature, and this was in agreement to the findings of Anderson et al. (1976).
The most prevalent species was E. tenella which is the most pathogenic species, causing haemorrhagic typhlitis in chickens (McDougald and Reid 1997), followed by E. acervulina, E. maxima,E. necatrix and E. mitis. These findings are partially in line with Ayaz et al. (2003), who also recorded highest prevalence of E. tenella in poultry birds. Eimeria species reproduce asexually (schizogony or merogony) and sexually (gametogony) in the intestinal cells to produce large numbers of progeny (oocysts), which are excreted in the faeces and subsequently undergo sporulation (sporogony) in the environment to become infective to susceptible chickens (McDougald and Reid 1997).
In the autumn, relatively higher humidity and ambient temperature might be responsible for increased sporulation and thus high prevalence of disease in this season. Heavy rainfall and afterwards evaporation due to high temperature in the end of summer are considered to be responsible for high humidity in autumn. In summer, prevalence of disease was lower from May to August that might be due to unfavourable climatic conditions (high temperature and low relative humidity) but afterwards prevalence of disease rose up from September to November when there was decrease in temperature and increase in relative humidity that favoured the developmental stages of coccidial life cycle (Rodríguez-Vivas et al. 1996). The findings of the present results are in accordance with the studies of Dar and Anwar (1981) and Khan et al. (2006) who also found higher prevalence of coccidiosis in the months of the year with higher level of relative humidity. Braunius (1988) and Graat et al. (1996) found coccidial infections to occur more often in autumn with high humidity in Netherlands. From the results of the study, it was concluded that coccidiosis is highly prevalent in the broiler chickens of Kashmir valley and parasite buildup shows dependence on ambient temperature and relative humidity.
Acknowledgments
The authors are highly thankful to Department of Zoology and Centre of Research for Development, University of Kashmir for providing the laboratory facilities. Besides the help provided by Prof. M. Z. Chishti during this work is highly acknowledged.
Contributor Information
Shazia Ahad, Phone: +91-9797143058, Email: shaziaahad19@gmail.com.
Syed Tanveer, Phone: + 91-9622661820, Email: syedtnvr@gmail.com.
Tauseef Ahmad Malik, Phone: +91-9622578349, Email: maliktsf@gmail.com.
References
- Anderson WI, Reid WM, Johnson JK. Effects of high environmental temperatures on cecal coccidiosis. Poult Sci. 1976;55:1429–1435. doi: 10.3382/ps.0551429. [DOI] [PubMed] [Google Scholar]
- Anonymous . Manual of veterinary parasitological laboratory techniques. Ministry of agriculture, fisheries and food (MAFF). Reference book 418. London: Her Majesty’s Stationary Office; 1984. [Google Scholar]
- Ayaz M, Akhtar M, Hayat CS, Hafeez MA, Haq A. Prevalence of coccidiosis in broiler chickens in Faisalabad, Pakistan. Pakistan Vet J. 2003;23(1):51–52. [Google Scholar]
- Bera AK, Bhattacharya D, Pan D, Dhara A, Kumar S, Das SK. Evaluation of economic losses due to coccidiosis in poultry industry in India. Agric Econ Res Review. 2010;23:91–96. [Google Scholar]
- Biggs PM. The world of poultry disease. Avian Pathol. 1982;11:281–300. doi: 10.1080/03079458208436101. [DOI] [PubMed] [Google Scholar]
- Braunius WW. Coccidiosis in broiler chicks: the prevalence of oocysts in feces in relation to necropsy findings in (sub) clinical coccidiosis and the effect of nicarbazin on these findings. Tijdschr Diergeneeskd. 1988;113(3):132–140. [PubMed] [Google Scholar]
- Card LE, Nesheim MC. Poultry production. 11. Philadelphia: Lea and Febiger; 1972. Chapter 10. Diseases and parasites; pp. 244–273. [Google Scholar]
- Collett SR. Proceedings of the 19th Annual Australian Poultry Science Symposium. Sydney: University Publishing Services; 2007. Strategies to manage wet litter; pp. 134–144. [Google Scholar]
- Collett SR. Nutrition and wet litter problems in poultry. Animal Feed Sci Technol. 2012;173:65–75. doi: 10.1016/j.anifeedsci.2011.12.013. [DOI] [Google Scholar]
- Danforth HD, Augustine PC. Coccidia vaccines. In: Wright IG, editor. Veterinary protozoan and hemoparasite vaccines. Boca Raton: CRC Press; 1989. pp. 165–175. [Google Scholar]
- Dar SA, Anwar AH. Incidence and pathogenesis of coccidiosis in chicken around Faisalabad. Pakistan Vet J. 1981;1:20–21. [Google Scholar]
- Graat EAM, Ploeger HW, Henken AM, Reilingh DG, Noordhuizen JPT, Beek PNGV. Effects of initial litter contamination level with Eimeria acervulina in population dynamics and production characteristics in broilers. Vet Parasitol. 1996;65:223–232. doi: 10.1016/S0304-4017(96)00952-1. [DOI] [PubMed] [Google Scholar]
- Khan MQ, Irshad H, Anjum R, Jahangir M, Nasir U. Eimeriosis in poultry of Rawalpindi/Islamabad area. Pakistan Vet J. 2006;26:85–87. [Google Scholar]
- Matter F, Oester H. Hygiene and welfare implications of alternative husbandry systems for laying hens. In: Faure JM, Mills D, editors. Proceedings from the 3rd European symposium on poultry welfare. France: Tours; 1989. pp. 201–212. [Google Scholar]
- Mattiello R (1990) Detect subclinical coccidiosis. Misset’s World Poultry. Misset Oct./Nov., p 82–83
- McDougald LR, Reid WM. Coccidiosis. In: Calnek BW, Barnes HJ, Beard CW, McDougald LR, Saif YM, editors. Diseases of poultry. Iowa: Iowa State University Press, Ames; 1997. pp. 865–883. [Google Scholar]
- Razmi GR, Kalideri AG. Prevalence of subclinical coccidiosis in broiler-chicken farms in the municipality of Mashhad, Khorasan, Iran. Prev Vet Med. 2000;44(3–4):247–253. doi: 10.1016/S0167-5877(00)00105-7. [DOI] [PubMed] [Google Scholar]
- Reid WM. Coccidiosis. In: Hofstad MS, Calnek BW, Helmboldt CF, Reid WM, Yoder JHW, editors. Diseases of Poultry. 7. Ames: Iowa State University Press; 1978. pp. 784–805. [Google Scholar]
- Rodríguez-Vivas RI, Dominguez-Alpizar JL, Torres-Acosta JF. Epidemiological factors associated to bovine coccidiosis in calves (Bos indicus) in a subhumid tropical climate. Revista Biomedica. 1996;7:211–218. [Google Scholar]
- Rose ME. Immunity to Eimeria infections. Vet Immunol Immunopathol. 1987;17:333–343. doi: 10.1016/0165-2427(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Shirley MW. New methods for the identification of species and strains of Eimeria. In: McDougald LR, Long PL, Joyner LP, editors. Research in avian coccidiosis. Athens: University of Georgia; 1986. pp. 13–35. [Google Scholar]
- Shirley MW, Lillehoj HS. The long view: a selective review of 40 years of coccidiosis research. Avian Pathol. 2012;41(2):111–121. doi: 10.1080/03079457.2012.666338. [DOI] [PubMed] [Google Scholar]
- Shirley MW, Smith AL, Tomley FM. The biology of avian Eimeria with an emphasis on their control by vaccination. Adv Parasitol. 2005;60:285–330. doi: 10.1016/S0065-308X(05)60005-X. [DOI] [PubMed] [Google Scholar]
- Soulsby EJL. Helminth, arthropods and protozoa of domestic animals. 7. Baillere Tindall: English Language Book Society; 1982. [Google Scholar]
- Talebi A, Mulcahy G. Correlation between immune responses and oocysts production in chickensmonospecifically infected with E. maxima. Avian Pathol. 1995;24:485–495. doi: 10.1080/03079459508419088. [DOI] [PubMed] [Google Scholar]
- Williams RB. Epidemiological aspects of the use of live anticoccidial vaccines for chicks. Int J Parasitol. 1998;28:1089–1098. doi: 10.1016/S0020-7519(98)00066-6. [DOI] [PubMed] [Google Scholar]
- Williams RB. A compartmentalized model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int J Parasitol. 1999;29:1209–1229. doi: 10.1016/S0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Yun CH, Lillehoj HS, Lillehoj EP. Intestinal immune responses to coccidiosis. Dev Comp Immunol. 2000;24:303–324. doi: 10.1016/S0145-305X(99)00080-4. [DOI] [PubMed] [Google Scholar]