Abstract
We investigated the occurrence of urinary schistosomiasis and estimated predictors for risk of infection among children in the Bunuso community of Ashanti Region of Ghana, West Africa. The cross-sectional survey was conducted between June and December 2009. Information was obtained on socio-demographic characteristics, schistosomiasis symptoms and other risk factors through interviews and questionnaires. Urine samples were analysed for Schistosoma haematobium ova using centrifugation and sedimentation, filtration and microscopy. Bivariate estimations and multiple logistic regression modelling with odds ratios (OR) were used to assess risk factors for S. haematobium infections. Inspections at River Nanakwaw revealed snail vectors, Bulinus truncatus. Overall, 95 out of 100 (95 % confidence interval [CI] 88.8–97.6) children tested positive for S. haematobium infection. The mean ova density (eggs/10 ml of urine) of infections was 58.12 (95 % CI 31.3–71.6) and varied significantly between age-group distributions (P value = 0.001; Post Hoc, P > 0.05 for ≤8 vs 15–17 years, and 9–11 vs 12–14 years), sources of house-hold water (P value = 0.019; Post Hoc, P < 0.05 for Borehole vs River Nanakwaw), children activities in River Nanakwaw (P value = 0.001), and haematuria (P value = 0.007). Independent variables significantly associated with S. haematobium infections were the use of River Nanakwaw as source of household water (OR 12.54; 95 % CI 3.932–42.12, P value = 0.003), engaging activities in River Nanakwaw (OR 8.76; 95 % CI 1.759–31.871; P value = 0.008) and haematuria (OR 36.71; 95 % CI 10.18–48.47; P value = 0.001). The passage of blood urine was prognostic of urinary schistosomiasis with a positive predictive value of 97.5 %. Our results demonstrate the endemicity of urinary schistosomiasis in Bunuso. Schistosomiasis remains a disease of great public health importance in Ghana, and there is the urgent need to intensify surveillance and control programs in remote riparian communities.
Keywords: Schistosomiasis, Children, Microscopy, Risk, Bunuso, Ghana
Introduction
When a river serves as a source of drinking water, it is natural to speculate that it will have an impact on the health status of the people. This may manifest itself as an increase in the incidence of water-related diseases, such as urinary schistosomiasis (Engels et al. 2002). Urinary schistosomiasis with its potential for polluting water bodies is generating tremendous health concerns. This is illustrated by their severe clinical and economic implications. Initially schistosomiasis was seen to cause haematuria in communities (Nash et al. 1982). Later on, they were shown to be involved with several clinical complications such as renal failure and cancer of the bladder (Davis 1996; Woolhouse 1998). Lately, several reports of impairment of cognitive function and stunted growth have been associated with the disease—significantly resulting in poor academic performances and social stigmatizations (Tetteh et al. 2004; King et al. 2005). Consequently, the duration of hospitalization and mortality rates have increased (Aryeetey et al. 2000; Lengeler et al. 2002; King et al. 2005). Urinary schistosomiasis is endemic in Ghana and affects mostly children between 5 and 14 years old (Gordon and Amatekpor 1999; Wagatsuma et al. 2003). Prior to 2007 most schistosomiasis control programs in Ghana were focused in the Volta River basin. In recent times, nationwide initiatives with health education, personal hygiene instructions, and mass chemotherapy treatment in populations of school-age children and high risk adults have been emphasized (Magalhaes et al. 2011, Clements et al. 2008). There is the Schistosomiasis Control Initiative Ghana (SCIG) which aims to implement an integrated national plan for sustainable control of schistosomiasis in Ghana. However, despite the improved control efforts, there remain many inhabitants of remote communities with little or no access to health facilities that suffer from urinary schistosomiasis. Our study was conducted in response to cases of Schistosoma haematobium infections at Ashante-Mampong’s Municipal hospital. A laboratory confirmed case of the infection, with visible haematuria in a child from Bunuso, prompted this investigation. We report the presence of high levels of S. haematobium infection among children in Bunuso, a remote rural community along River Nanakwaw in the Ashanti region of Ghana, West Africa.
Materials and methods
Study site
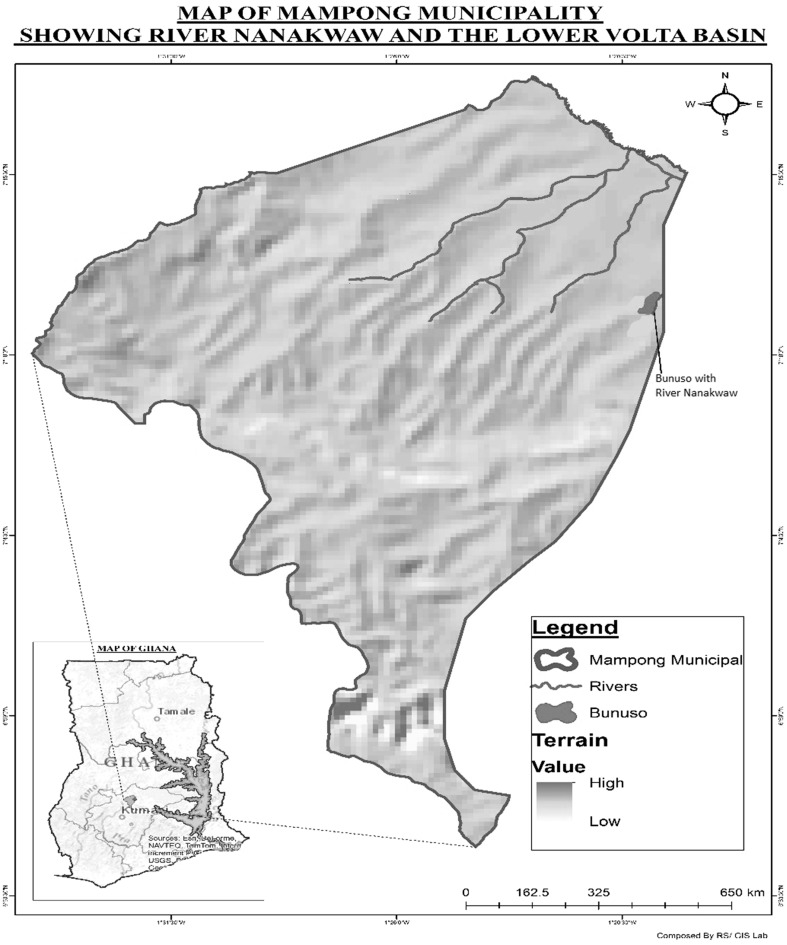
The present study was carried out in Bunuso (latitude 0007°4.123N and longitude 001°23.231W), a rural community in the Ashanti-Mampong Municipality of Ghana (Fig. 1). According to the Ghana Statistical Service, Bunuso has a land area of 700 m2, and lies about 45 km, west of the River Volta basin. Preliminary surveillance showed Bunuso to be a community with over 90 households and a human population of about 500, with children forming the majority. The illiteracy rate in Bunuso exceeds 50 %. The community has a bore hole that serves as the main source of drinking water but most inhabitants wash, bathe and drink from a nearby river called Nanakwaw which lies 200 m south of the community and runs through farms. The nearest health facility to Bunuso is the Ashanti Mampong Municipal Hospital, which lies about 2.4 km north of the village. The predominant occupations in Bunuso are fishing, farming and trading.
Fig. 1.
Map of Ghana (bottom left) showing Mampong municipal and Bunuso with River Nanakwaw (South west of the River Volta basin)
Study design
A cross-sectional study was conducted in Bunuso between June and December 2009 to determine the occurrence of urinary schistosomiasis. All children were registered in the community after a house-to-house census. A questionnaire was used to register children which documented their age and sex. For schistosomiasis control purposes, WHO recommends that 200–250 children are sampled per community (Montresor et al. 1998). Altogether, 171 children, aged 4–17 years, were enumerated and considered eligible to participate in our study. Due to the small number of children in Bunuso, all children who provided urine specimens for examination were enrolled in the study. Prior to this, the purpose of the study was explained to the community inhabitants. Parents and guardians who declined their wards to participate fully were excluded from the study. Children were interviewed by certified parasitology technicians using questionnaires. The questionnaires were structured in a more detailed and specific manner and the content explained in a language best understood by respondents as a way of reducing bias.
Data collection
Parents and guardians were requested for assistance in the provision of information. Interviews were conducted in the local dialect spoken by study participants. The local dialects spoken in Bunuso is Twi. Data were abstracted through interviews using standardized questionnaires with the help of trained parasitology technicians. Data were collected regarding the following: demographics (gender and age), educational level (Nursery; Primary; Junior Secondary School status, JSS; Senior Secondary School status, SSS; No education, none), sources of household water (Borehole water, River Nanakwaw, River Nanakwaw and Borehole water), engaging activities in River Nanakwaw (yes, no) and contact frequencies with River Nanakwaw per week (none, once, 2–4 days, 5–7 days). Children were also interviewed on the presence or absence of visible bloody urine, perception on bloody urine (maturity, sickness, nothing), knowledge of River Nanakwaw as source of bloody urine (yes, no) and any history of schistosomiasis treatment (yes, no). Parents were asked of their knowledge on schistosomiasis (yes, no). Also, the distance of each child’s home to River Nanakwaw was estimated and recorded (<100, 100–300, 300–500, 500–700 m).
Sample collection
Each child’s parent/guardian was requested to assist in the provision of urine samples. Labelled 20 ml screw-top sterile plastic containers were given to parents/guardians, who were also advised to collect about 20 ml terminally voided urine as these usually yield the most ova (Aboagye and Edoh 2009). Urine samples were split into two 10-ml aliquots for use in the centrifugation and sedimentation assay, and the filtration process for egg counting respectively. Samples were collected between 8.00 and 12.00 h, kept in a cool box with ice packs, and immediately transported to our team of trained laboratory technologist for analysis.
Parasitological analyses
Parasitological examinations were conducted on the field and validated by cross-checking microscopy at the regional Public Reference Laboratory in Kumasi, Ghana. Urine samples were tested for the presence of blood (microhaematuria) upon receipt using urine reagent strips (Uripath, Plasmatec Laboratory, UK) according to manufacturer’s instructions. Results were interpreted as negative, positive+, positive++, or positive+++. Our primary outcome was Shistosoma hematobium infection diagnosed by urine microscopy examination. 10 ml of each thoroughly mixed urine sample was transferred aseptically into centrifuge tubes and centrifuged at 25,000 revolutions per minute for 2 minutes to concentrate S. haematobium ova. The supernatants were discarded and the pellets re-suspended. Wet preparations of the urine sediments were examined for the presence of red blood cells to confirm microhaematuria. Also, smears were prepared from all the urine sediments on slides and in duplicates, and examined under the light microscope for S. haematobium ova. Thorough observation of all prepared slides was done under 40× objective lens magnification. Lugol’s iodine was used to enhance the clarification of S. haematobium ova for microscopic identification. Microscopy examinations were performed by separate operators blinded to the results of each other. Children with ova of S. haematobium observed in urine microscopy were described as infected. Parents and guardians were accordingly advised based on results of field laboratory diagnosis. In collaboration with the municipal hospital, children who were found to be positive through field microscopic examinations were referred for treatment.
Egg counting
Urine samples were also analyzed for S. haematobium eggs by nucleopore filtration method, as described by Mott et al. (1982). Briefly, 10 ml of urine was strained using nucleopore filter paper (Gelman Sciences, Michigan USA). The filter papers were stained with 5 % ninhydrin and examined for the presence of S. haematobium eggs when dry. The intensity of S. haematobium infections was calculated as the number of ova per 10 ml of urine. Light infections and heavy infections were defined as less than 50 ova per 10 ml urine and more than 50 ova per 10 ml urine respectively (WHO 2004).
Investigations for Bulinus species
Inspections were conducted for the snail vector Bulinus species and for ecological breeding conditions. We aimed to provide some evidence of Bulinus species in River Nanakwaw rather than study the epidemiology and spatial distribution of the snail Bulinus species in its natural habitat. In a convenient sampling, snails in shallow water along the shore of River Nanakwaw within Bunuso were collected by using dip-nets and long forceps to pick up vegetation (Chu and Vanderburg 1976). Ecological information were recorded with structured data sheets. We identified three habitats for sampling: first, locations bordered with floating masses of grass; second, pockets with aquatic weeds; third, shallow open beaches.
Ethical considerations
The study protocol was reviewed and approved by the ethical review board of the School of Allied Health Sciences, University of Ghana. Parents, guardians and community leaders were briefed on the objectives and procedures to be used prior to conducting the study. Written, verbal or fingerprinted consent was obtained from local leaders, parents or guardians. Verbal assent was subsequently obtained from the children.
Statistics
Data from interviews and parasitological investigations were captured into Microsoft Excel to generate a database, which was exported into Statistical Package for Social Sciences (SPSS, Version 16). Data editing and statistical analyses were conducted in SPSS. Descriptive statistics (frequencies and cross-tabulations) were used to determine the prevalence of S. haematobium infection among the study population. All analyses were two-tailed. Unadjusted odds ratios (OR) using the Chi square test was used to estimate the bivariate associations between urinary schistosomiasis and independent categorical variables among children in Bunuso. Variables that had a P value <0.05 were included in a multivariate logistic regression model to identify risk factors for S. hematobium infection. Covariates that showed independent association with S.haematobium infection at a significance level of P value <0.05 were noted. Pearson correlation coefficient was used to test for correlations involving continuous variables. Intensity of Schistosoma haematobium infections was compared using mean ova counts for variables significantly associated with schistosomiasis. Standard weighted-means analyses with One-away Analyses of Variance (One-way ANOVA) was used to estimate differences in mean values (of S. haematobium egg counts) between independent variables (Kruskal–Wallis or Mann–Whitney test for the mean comparison were used where appropriate). Post Hoc analyses were conducted with Turkey honest significant difference tests (Turkey HSD) for post-ANOVA pairwise comparisons.
Results
Socio-demographic characteristic
From a total of 171 children, 141 consented and were enrolled into the study. A hundred and thirty-nine children were interviewed but 39 declined to submit urine specimens. Overall, 100 children provided urine samples to be examined for S. hematobium ova. Most of the children (95 %) were of school going age (>5 years) with 32 % receiving formal education. The mean age of the study population was 11 years. Most of the children (84 %) were aged 8–17 years with a significant difference by gender (male 68 %; female 32 %; P value = 0.002). General characteristics of the study population are shown in Table 1.
Table 1.
Prevalence and intensity of Schistosoma haematobium infections across general characteristics and predictors of factors associated with schistosomiasis amongst children in Bunuso, Ghana
| Variables (number examined) | Number infected (%) | Intensity of infection (%) | Geometric mean egg count | ||
|---|---|---|---|---|---|
| Light | Heavy | Per 10 ml ± SEM | P value (Post Hoc) | ||
| Gender | =0.279 | ||||
| Female (32) | 30 (93.8) | 9 | 21 | 55 ± 3.11 | |
| Male (68) | 65 (95.5) | 21 | 44 | 61 ± 3.05 | |
| Age group (years) | =0.001 | ||||
| <8 (27) | 26 (96.3) | 6 | 20 | 40 ± 2.61 | (P > 0.05 for <8 vs 15–17; 9–11 vs 12–14) |
| 9–11 (34) | 33 (97.0) | 9 | 24 | 51 ± 1.56 | |
| 12–14 (21) | 20 (95.2) | 9 | 11 | 57 ± 1.38 | |
| 15–17 (18) | 15 (83.3) | 6 | 9 | 39 ± 1.68 | |
| Educational level | =0.299 | ||||
| None (68) | 67 (98.5) | 15 | 52 | 54 ± 3.13 | (P < 0.05 for none vs JSS) |
| Primary (22) | 21 (95.5) | 10 | 11 | 50 ± 4.54 | |
| JSS (8) | 6 (75.0) | 4 | 2 | 47 ± 3.21 | |
| SSS (2) | 1 (50.0) | 1 | 0 | 37 | |
| Sources of water | =0.019 | ||||
| Borehole (9) | 6 (66.7) | 6 | 0 | 14 ± 2.35 | (P < 0.05 for Borehole vs Nanakwaw) |
| River Nanakwaw (77) | 76 (98.7) | 17 | 59 | 61 ± 1.65 | |
| Bunuso/Borehole (14) | 13 (92.8) | 7 | 6 | 46 ± 3.82 | |
| Activities in River Nanakwaw | =0.001 | ||||
| No (8) | 6 (75.0) | 6 | 0 | 14 ± 1.45 | |
| Yes (92) | 89 (96.7) | 24 | 65 | 57 ± 3.24 | |
| Type of activity in River Nanakwawa | =0.092 | ||||
| No activity (8) | 6 | 6 | 0 | 14.1 ± 1.45 | |
| Work (52) | 48 | 20 | 28 | 51.2 ± 2.13 | |
| Bathe/swim (71) | 67 | 25 | 38 | 58.1 ± 1.78 | |
| Fetch water (81) | 78 | 30 | 48 | 50.1 ± 3.3 | |
| Wash (83) | 80 | 29 | 51 | 57.12 ± 2.1 | |
| Contact frequency with River Nanakwaw/week | =0.066 | ||||
| None (8) | 7 (87.5) | 5 | 2 | 45 ± 2.91 | |
| Once (12) | 11 (91.6) | 6 | 5 | 46 ± 2.23 | |
| 2–4 days (12) | 11 (91.6) | 3 | 8 | 50 ± 1.45 | |
| 5–7 days (68) | 66 (97.1) | 16 | 50 | 52 ± 1.61 | |
| Distance of home to River Nanakwaw | =0.056 | ||||
| <100 m (15) | 14 (93.3) | 3 | 11 | 46.8 ± 2.3 | |
| 100–300 m (31) | 30 (96.7) | 9 | 21 | 47.6 ± 1.1 | |
| 300–500 m (52) | 50 (96.2) | 10 | 40 | 46.7 ± 1.2 | |
| 500–700 m (12) | 11 (91.6) | 8 | 5 | 53.1 ± 1.9 | |
| Haematuria | =0.007 | ||||
| No (19) | 16 (84.2) | 5 | 11 | 39 ± 1.50 | |
| Yes (81) | 79 (97.5) | 25 | 54 | 63 ± 3.50 | |
| Knowledge of River Bunuso as source of bloody urine | =0.173 | ||||
| No (92) | 91 (98.9) | 28 | 63 | 57 + 3.11 | |
| Yes (8) | 4 (50 %) | 2 | 2 | 49 ± 4.45 | |
| Parents knowledge of Schistosomiasis as disease | =0.381 | ||||
| No (79) | 77 (97.5) | 20 | 57 | 49 ± 3.89 | |
| Yes (21) | 18 (85.7) | 10 | 8 | 55 ± 4.11 | |
SEM Standard error of mean
aNumber of children engaging each activity overlap due to multiple responses
Prevalence and distribution of infection
Urine samples identified as positive or negative for S. haematobium eggs using centrifugation and sedimentation were all accordingly confirmed as such by the filtration method. A total of 95 out of 100 (95 % confidence interval [CI] 88.8–97.6) children tested positive for S. haematobium infection. Table 1 illustrates the significant differences in infection across demographic and other independent variables of the children participants. Nearly two-thirds (65.0 %; [95 % CI 55.2–73.6]) of the study participants had heavy infections while the overall prevalence of light infections was 35.0 % (95 % CI 26.4–44.7). Across all age ranges, boys had similar infection prevalence compared to girls. Among parents knowledgeable on the subject of Schistomiasis (n = 21 vs naïve parents, n = 79) borehole was more likely to be the source of household water (23.8 and 5.6 % respectively, P value = 0.007) and their children were less likely to report activities in River Nanakwaw (19.1 and 5.1 % respectively, P value = 0.04). Compared to children who did not engage activities in River Nanakwaw (8), children reporting activities in the River (92) had higher prevalence of haematuria (83.7 and 50.0 % respectively, P value = 0.02) and were more likely to be infected with S. haematobium (97.3 and 62.5 % respectively, P value <0.02).
Bloody urine
Of the 100 children participants, 42 (95 % CI 32.3–52.2) passed visible blood in urine and 80 (95 % CI 71.7–86.9) were diagnosed with microhaematuria based on the urine reagent strips. Using urine microscopy examination as the gold standard, 81 (95 % CI 71.6–87.9) children had haematuria. The use of visible bloody urine for prognosis of urinary schistosomiasis recorded a sensitivity of 83.2 %, specificity of 60.0 %, negative predictive value of 15.7 %, positive predictive value of 97.5 %, negative likelihood ratio of 0.281, and a positive likelihood ratio of 2.071.
Intensity of infections
The mean ova density (eggs/10 ml of urine) of light and heavy infections were 37.2 (95 % CI 31.9–41.9) and 63.9 (95 % CI 59.2–67.1) respectively. The mean ova density (eggs/10 ml urine) of total infections was 58.12 (95 % CI 31.3–71.6) and varied significantly between age-group distributions (P value = 0.001; Post Hoc, P > 0.05 for ≤8 vs 15–17 years, and 9–11 vs 12–14 years), sources of household water (P value = 0.019; Post Hoc, P < 0.05 for Borehole vs River Nanakwaw), children activities in River Nanakwaw (P value = 0.001) and haematuria (P value = 0.007) (Table 1). The mean ova densities within age-group distributions, sources of household water, children activities in River Nanakwaw and haematuria were compared to the prevalence of S. haematobium infection. There were no significant correlations between prevalence of S. haematobium infection and mean ova density for age-group distributions (Pearson Correlation coefficient, r = 0.545, P value = 0.554) and for sources of household water (r = 0.99, P value = 0.09). No correlation between prevalence of S. haematobium infection and mean ova densities for children activities in River Nanakwaw and for haematuria in children were recorded.
Factors associated with infections with Schistosoma haematobium
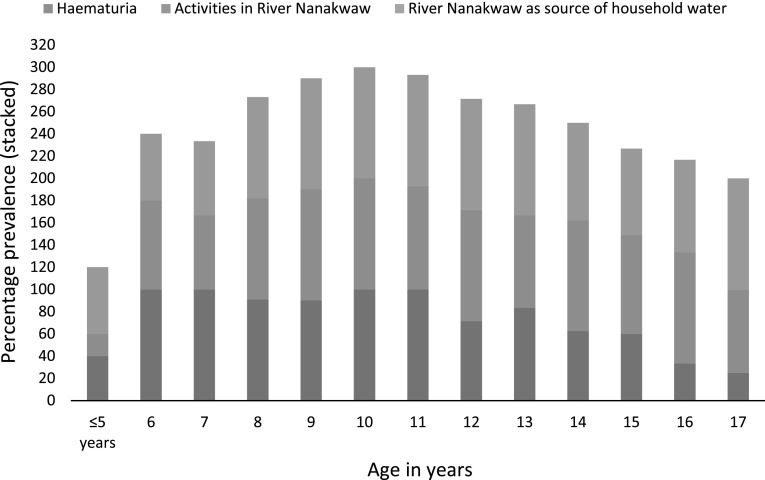
Table 2 summarizes the risk of infection with urinary schistosomiasis expressed as odds ratio (OR), and the levels of association with Wald’sP value. No significant associations between urinary schistosomiasis and age or gender were observed. In bivariate analyses, the following factors were significantly associated with S. haematobium infection: educational levels at JSS and SSS, sources of water, activities in River Nanakwaw, haematuria, knowledge of River Nanakwaw as source of bloody urine and parent’s knowledge of schistosomiasis as disease. Bivariate factors associated with presence of Schistosomiasis were entered into multivariate logistic regression model. Independent variables that remained significantly associated with S. hematobium infections were the use of River of Nanakwaw as source of household water (OR, 12.54; 95 % CI 3.932–42.12, P value = 0.003), engaging activities in River Nanakwaw (OR, 8.76; 95 % CI 1.759–31.871; P value = 0.008) and the presence of haematuria (OR 36.71; 95 % CI 10.18–48.47; P value = 0.001) (Table 3). When the prevalence of schistosomiasis among children with haematuria or engaging activities in River Nanakwaw or using River Nanakwaw as source of household water was compared across age, a trend towards low prevalence was observed at extreme age-boundaries (≤8 and 15–17 years) (Fig. 2). A fairly weak but significant correlation was noted between age and haematuria (r = −0.559; P value = 0.044).
Table 2.
Bivariate analyses for predictors of factors associated with Schistosoma haematobium infections among children in Bunuso
| Variables (number examined) | Infected children | Unadjusted odds ratio | Walds’ |
|---|---|---|---|
| (95 % CI) | P valuea | ||
| Gender | |||
| Female (N = 32) | 30 (31.6 %) | 1.0 | – |
| Male (N = 68) | 65 (68.4 %) | 1.44 (0.229–9.102) | 0.695 |
| Age group (years) | |||
| <8 (27) | 26 (27.0 %) | 1.0 | – |
| 9–11 (34) | 33 (34.7 %) | 1.290 (0.08–21.30) | 0.868 |
| 12–14 (21) | 20 (21.1 %) | 0.769 (0.05–13.10) | 0.856 |
| 15–17 (18) | 16 (16.8 %) | 0.308 (0.03–3.67) | 0.352 |
| Educational level | |||
| None (68) | 67 (70.5 %) | 1.0 | – |
| Primary (22) | 21 (22.1 %) | 0.313 (0.019–5.231) | 0.395 |
| JSS (8) | 6 (6.3 %) | 0.045 (0.004–0.569) | 0.001 |
| SSS (2) | 1 (1.15 %) | 0.015 (0.00–0.449) | 0.003 |
| Sources of water | |||
| Borehole (9) | 6 (6.3 %) | 1.0 | – |
| River Nanakwaw (77) | 76 (80.0 %) | 38.00 (3.410–423.497) | 0.000 |
| River Nanakwaw/Borehole (14) | 13 (13.7 %) | 15.5 (1.370–175.383) | 0.003 |
| Activities in River Nanakwaw | |||
| No (8) | 6 (6.3 %) | 1.0 | – |
| Yes (92) | 89 (96.7 %) | 10.00 (1.393–71.766) | 0.006 |
| Type of activity in River Nanakwawb | |||
| No activity (8) | 6 | 1.0 | – |
| Work (52) | 50 | 8.33 (0.985–70.5) | 0.085 |
| Bathe/swim (71) | 68 | 7.55 (1.05–53.15) | 0.077 |
| Fetch water (81) | 76 | 6.33 (0.952–41.93) | 0.09 |
| Wash (83 | 80 | 8.88 (1.237–63.88) | 0.06 |
| Contact frequency with River Nanakwaw/week | |||
| None (8) | 7 (7.3 %) | 1.0 | – |
| Once (12) | 11 (11.6 %) | 1.571 (0.084–29.409) | 0.761 |
| 2–4 days (12) | 11 (11.6 %) | 1.571 (0.084–29.409) | 0.761 |
| 5–7 days (68) | 66 (69.5 %) | 0.333 (0.028–3.996) | 0.365 |
| Haematuria | |||
| No (19) | 16 (31.3 %) | 1.0 | – |
| Yes (81) | 79 (68.6 %) | 40.7 (11.78–40.87) | <0.001 |
| Perception on bloody urine | |||
| Nothing (23) | 22 | 1.0 | – |
| Maturity (66) | 64 | 1.455 (0.126–16.836) | 0.763 |
| Sickness (11) | 9 | 0.205 (0.016–2.549) | 0.183 |
| Knowledge of River Nanakwaw as source of bloody urine | |||
| No (92) | 91 | 1.0 | – |
| Yes (8) | 4 | 0.011 (0.001–0.122) | 0.000 |
| History of Schistosomiasis treatment | |||
| No (91) | 87 | 1.0 | – |
| Yes (9) | 8 | 6.44 (0.095–43.79) | 0.090 |
| Distance of home to River Nanakwaw | |||
| <100 m (15) | 14 | 1.0 | – |
| 100–300 m (31) | 30 | 2.143 (0.125–36.804) | 0.592 |
| 300–500 m (52) | 50 | 1.786 (0.151–21.165) | 0.642 |
| 500–700 m (12) | 11 | 0.786 (0.044–14.026) | 0.869 |
| Parents knowledge of Schistosomiasis as disease | |||
| No (79) | 77 | 1.0 | – |
| Yes (21) | 18 | 0.156 (0.024–1.002) | 0.028 |
CI Confidence interval
a P values less than 0.05 were considered significant
bNumber of children engaged in each activity overlap due to multiple responses
Table 3.
Risk factors for infections with Schistosoma haematobium among children in Bunuso using Multivariate adjusted associations
| Variable | Adjusted odds ratio | Confidence interval | P valuea |
|---|---|---|---|
| Educational level | |||
| None (68) | 1.0 | – | |
| Primary (22) | 0.571 | 0.231–9.451 | 0.395 |
| JSS (8) | 0.255 | 0.118–6.261 | 0.081 |
| SSS (2) | 0.019 | 0.012–0.612 | 0.062 |
| Sources of water | |||
| Borehole (9) | 1.0 | – | |
| Nanakwaw (77) | 12.541 | 3.932–42.12 | 0.003 |
| Nanakwaw/Borehole (14) | 6.112 | 1.410–13.721 | 0.002 |
| Activities in River Nanakwaw | |||
| No (8) | 1.0 | – | 0.008 |
| Yes (93) | 8.761 | 1.759–31.871 | |
| Haematuria | |||
| No (19) | 1.0 | 10.18–48.47 | 0.001 |
| Yes (81) | 36.7 | ||
| Knowledge of River Nanakwaw as source of bloody urine | |||
| No (92) | 1.0 | 0.015–1.182 | 0.074 |
| Yes (8) | 0.012 | ||
| Parents knowledge of Schistosomiasis as disease | |||
| No (79) | 1.0 | 0.452–4.112 | – |
| Yes (21) | 0.756 | 0.287 | |
a P values less than 0.05 were considered significant
Fig. 2.
Prevalence of Schistosoma haematobium infection among children in Bunuso according to age and in relation to haematuria, activities in River Nanakwaw and the use of River Nanakwaw as source of water
Inspections for Bulinus species
Only Bulinus truncatus specimens were found in our sampling (Table 4). Bulinus truncatus was focal and related to Ceratophyllum demersum vegetation. A few B. truncatus were attached to the under surface of decayed palm fronds.
Table 4.
Qualitative assessments of lacustrine conditions of River Nanakwaw
| Inspections at River Nanakwaw | Ecological habitats | ||
|---|---|---|---|
| Floating grasses | Open beaches | Aquatic weeds | |
| Bulinus species | None observed | Bulinus truncatus | Bulinus truncatus |
| Presence of aquatic weeds | Non observed | Ceratophyllum demersum | Ceratophyllum demersum |
| Other submerged vegetation | Yes | Yes | None observed |
| Other organic materials with B. truncatus | Decayed palm fronds | Decayed palm fronds | Decayed palm fronds |
| Flow rate of River | Slow | Slow | Slow |
| Occupational activities within 50 m | None observed | Farming, fishing | Farming, fishing |
| Human activities | Non observed | Washing, bathing, fetching water | Washing, fetching water |
| Presence of livestock | Yes | Yes | Yes |
| Urination/defecation | None observed | Non observed | Non observed |
Based on 3-days observations
Discussion
To our knowledge this is the first survey conducted in Bunuso, in the Ashante-Mampong district of Ghana, to determine the prevalence and risk factors associated with S. hematobium infection. Our study confirms a high prevalence (95 %) of urinary schistosomiasis among children in Bunuso studied between June and December 2009. The use of River Nanakwaw as source of house-hold water, children activity in River Nanakwaw and the presence of haematuria were independently and significantly associated with infection in our study.
The distribution of S. haematobium infections varies greatly within geographical regions and from country to country. The global status survey for schistosomiasis in Ghana notes an at-risk population of 17.1 million (Chitsulo et al. 2000), with reported national prevalences ranging from 20.0 to 60.0 % (Nsowah-Nuamah et al. 2001; Tay et al. 2011). Our findings are similar to reported surveillance estimates in 1970–1990 among school children along the Volta River basin: 88–95 % (Paperna 1969), 82–92 % (Klump 1982) and 70–95 % (Gordon and Amatekpor 1999). The results are higher than recent prevalence figures along some riparian communities: Mahem (58 %) and Galilea (49 %) (Aboagye and Edoh 2009), and the Lower River Volta Basin (2–21 %) (Nkegbe 2010). The observed difference in infection prevalences illustrates the focal distribution characteristic of urinary schistosomiasis. The difference is attributed to the fact that the schistosomiasis problem has long existed in the community, and the lack of awareness, coupled with the absence of control programs confounded the burden. Besides, most of the participants and parents would not consider urinary schistosomiasis as a health condition, in view of the belief that haematuria, a cardinal symptom of the infection formed part of their cultural orientation for initiation into adulthood. Schistosoma haematobium is endemic in Bunuso. Snail vectors, Bulinus truncatus were identified along the shore of River Nanakwaw. The lacustrine conditions observed at the River were favourable to growth of aquatic weeds and breeding of B. truncatus (Table 4).
We estimated associations between urinary schistosomiasis and intensity of Schistosoma haematobium ova across various children characteristics. No significantly strong correlation was identified between infection prevalence and mean density of Shistosoma haematobium ova for all independent variables (correlation coefficient, r < 1; P value > 0.05 in all cases). However, children were significantly more likely (OR > 8.8, P value ≤ 0.05) to suffer from S. haematobium infection, and with heavy ova densities ≥57 eggs/10 ml of urine, if they suffered from haematuria or engaged activities in River Nanakwaw or used the River as the source of household water. River Nanakwaw was noted as the focal contaminating source. Indeed, communities with high infection prevalence are often clustered around contaminated open water bodies (Tay et al. 2011). This could partly explain why children have been most affected wherever the disease is encountered (Aryeetey et al. 2000; World Healh Organisation (WHO) report (2002); Nkegbe 2010). Moreover, we observed a trend towards low infection prevalence among children engaging activities in River Nanakwaw at extreme age-boundaries (≤8 and 15–17 years). Older children were more likely to be conscious of their physical developments and less attracted to recreational water-contact behaviours. In a study conducted by Zhakari (1997) to investigate factors influencing the prevalence of urinary schistosomiasis in the Volta Region of Ghana, approximately 70 % of the infected were below the age of 15 years. Similar reports by Wagatsuma et al. (2003), and Aboagye and Edoh (2009) suggests a reduction in incidence of urinary schistosomiasis with age of the definitive host.
In this study, 42 (46.1 %) (95 % CI 32.3–52.2) of the 91 infected children passed visible blood in urine with a sensitivity and specificity of 83.2 % and of 60.0 % respectively for the prognosis of urinary schistosomiasis. The presence of bloody urine was highly predictive of urinary schistosomiasis with a positive predictive value of 97.5 %. The diagnosis of schistosomiasis remains easy microscopically, but the challenge is formidable in low income countries where clinical microbiological services are unaccessible to remote communities mostly affected. Our findings support the opinion that, in highly endemic areas, bloody urine could be used as an empiric indicator of urinary schistosomiasis (Erlanger et al. 2005). Anosike et al. (2001), studied the occurrence of haematuria as a common symptom of urinary schistosomiasis, and have validated the importance of screening for haematuria in the community diagnosis of urinary schistosomiasis infections. Based on endemicity of study sites, reported sensitivities and specificities of blood urine for predicting urinary schistosomiasis have ranged between 50–100 and 58–98 % respectively (Mafe et al. 2000; Lengeler et al. 2002).
Urinary schistosomiasis remains a disease of great public health importance in Ghana. The silver lining to this study is the fact that the results highlight an urgent need to intensify surveillance and control programs on urinary schistosomiasis in remote rural and riparian communities. Our results demonstrate the endemicity of Schistosoma haematobium infections in Bunuso. It also demonstrates the importance of active research participation at sub-national or sub-district levels in the prevention and control of urinary schistosomiasis. Educational and curative programs for urinary schistosomiasis should be integrated with communal participation and long term sanitary measures such as the control of aquatic weeds. A noteworthy limitation of this study is the rather few participants (100 children; range 4–17 years). A more large-scale survey is much more likely to be with little bias for high-at-risk children populations.
Acknowledgments
We acknowledge the hard work of staff of the microbiology unit of Asante-Mampong Municipal Hospital, whose contributions to this study and beyond have led to the improved outcome of infectious disease management in several communities. We extend our profound appreciation to the chief, elders and people of Bunuso community for their support towards the success of this study.
Conflict of interest
The Authors declare no conflict of interest.
Contributor Information
P. F. Ayeh-Kumi, Phone: +233-244042718, Email: payehkumi@yahoo.com
N. Obeng-Nkrumah, Phone: +45-71153705, Email: nbn@ssi.dk
D. Baidoo, Email: kwametops@yahoo.com
R. H. Asmah, Phone: +233-244266329, Email: rhasmah@chs.edu.gh
References
- Aboagye IF, Edoh D. Investigation of the risk of infection of urinary schistosomiasis at Mahem and Galilea communities in the Greater Accra Region of Ghana. West Afr J Appl Ecol. 2009;15:1–6. [Google Scholar]
- Anosike JC, Nwoke BEB, Njoku AJ. The validity of haematuria in the community diagnosis of urinary schistosomiasis infections. J Helminthol. 2001;75:223–225. [PubMed] [Google Scholar]
- Aryeetey ME, Wagatsuma Y, Yeboah G, Asante M, Mensah G, Nkrumah FK, Kojima S. Urinary schistosomiasis in southern Ghana: prevalence and morbidity assessment in three (defined) rural areas drained by the Densu River. Parasit Int. 2000;49(2):155–163. doi: 10.1016/S1383-5769(00)00044-1. [DOI] [PubMed] [Google Scholar]
- Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/S0001-706X(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu KY, Vanderburg JA. Techniques for estimating densities of Bulinus truncatus rohlfsi and its horizontal distribution in Volta Lake. Ghana Bull World Health Organ. 1976;54:411–416. [PMC free article] [PubMed] [Google Scholar]
- Clements AC, Garba A, Sacko M, Toure S, Dembele R. Mapping the probability of schistosomiasis and associated uncertainty. West Afr Emerg Infect Dis. 2008;14:1629–1632. doi: 10.3201/eid1410.080366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. Schistosomiasis. In: Cook CG, editor. Manson’s tropical diseases. 2. London: WB Saunders Company Ltd; 1996. pp. 1413–1456. [Google Scholar]
- Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002;82:139–146. doi: 10.1016/S0001-706X(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger TE, Keiser J, Castro MC. Effect of water resource development and management on lymphatic filariasis, and estimates of population at risk. Am J Trop Med Hyg. 2005;73:523–533. [PubMed] [Google Scholar]
- Gordon C, Amatekpor JK (1999) The sustainable integrated development of the Volta Basin in Ghana. Volta Basin Research Project, Accra, Ghana. Gold Time Press, pp 159–191
- King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helminth infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- Klump RK (1982) A study of the transmition of S. haematobium in Volta Lake Ghana. PhD thesis, University of London, Faculty of Medicine, London School Hygiene and Tropical Medicine, pp 111–280
- Lengeler C, Utzinger J, Tanner M. Questionnaires for rapid screening of schistosomiasis in sub-Saharan Africa. Bull World Health Organ. 2002;80:235–242. [PMC free article] [PubMed] [Google Scholar]
- Mafe MA, von Stamm T, Utzinger J, N’Goran EK. Control of urinary schistosomiasis: an investigation into the effective use of questionnaires to identify high-risk communities and individuals in Niger state, Nigeria. Trop Med Int Health. 2000;5(1):53–63. doi: 10.1046/j.1365-3156.2000.00508.x. [DOI] [PubMed] [Google Scholar]
- Magalhaes RJ, Biritwum NK, Gyapong JO, Brooker S, Zhang Y. Mapping helminth co-infection and co-intensity: geostatistical predictionin Ghana. PLoS Negl Trop Dis. 2011;5:e1200. doi: 10.1371/journal.pntd.0001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. Geneva: World Health Organization; 1998. [Google Scholar]
- Mott KE, Balters R, Bambagha J, Baidassini B. Field studies of a reusable polyamide filter for detection of Schistosoma haematobium eggs by urine filtration. Tropenmed Parasitol. 1982;33:227–228. [PubMed] [Google Scholar]
- Nash TE, Cheever AW, Ottesen EA, Cook JA. Schistosome infections in humans: perspectives and recent findings. Ann Int Med. 1982;97:740–754. doi: 10.7326/0003-4819-97-5-740. [DOI] [PubMed] [Google Scholar]
- Nkegbe E. Prevalence of Schistosomiasis among school children in the lower River Volta Basin in Ghana. Gomal J Med Sci. 2010;8(1):54–56. [Google Scholar]
- Nsowah-Nuamah NN, Mensah G, Aryeetey ME, Wagatsuma Y, Bentil G. Urinary schistosomiasis in southern Ghana: a logistic regression approach to data from a community-based integrated control program. Am J Trop Med Hyg. 2001;65(5):484–490. doi: 10.4269/ajtmh.2001.65.484. [DOI] [PubMed] [Google Scholar]
- Paperna I. Study of an outbreak of Schistosomiasis in the newly formed Volta Lake in Ghana. J Trop Med Parasitol. 1969;21:411–424. [PubMed] [Google Scholar]
- Tay SCK, Amankwa R, Yao-Gbedema S. Prevalence of Schistosoma Haematobium infection in Ghana: a retrospective case study in Kumasi. Int J Parasitol Res. 2011;3(2):48–52. doi: 10.9735/0975-3702.3.2.48-52. [DOI] [Google Scholar]
- Tetteh IK, Frempong E, Awuah E. An analysis of the environmental health impact of the Barekese dam in Kumasi, Ghana. J Environ Manage. 2004;72:189–194. doi: 10.1016/j.jenvman.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Wagatsuma Y, Aryeetey ME, Nkrumah FK, Sack DA, Kojima S. Highly symptom-aware children were heavily infected with urinary schistosomiasis in southern Ghana. Cen Afr J Med. 2003;49(1–2):16–19. [PubMed] [Google Scholar]
- Woolhouse MEJ. Patterns in parasite epidemiology: the peak shift. Parasitol Today. 1998;14:428–434. doi: 10.1016/S0169-4758(98)01318-0. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO) Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. WHO Tech Rep Ser. 2002;912:1–57. [PubMed] [Google Scholar]
- World Health Organization (WHO) (2004) Division of Control of Tropical Disease: Schistosomiasis. WHO Update October 19
- Zhakari K. Factors affecting the prevalence of Schistosomiasis in Volta Region of Ghana. MJM. 1997;3(2):93–101. [Google Scholar]