Abstract
The objective of this work was to study gastrointestinal nematode community infecting Acomysdimidiatus in different wadis of St. Katherine, South Sinai, Egypt. Fieldwork was conducted in three Wadis over a 4 weeks period during April–May, 2003 in St. Katherine, South Sinai, Egypt. Faecal samples from 47 spiny mice were analysed for gastrointestinal nematode community. The nematodes community consisted of four genera Dentostomella spp., Syphacia spp., Aspicularis spp. and Spirurids species. The overall prevalence of infection was 55.3 %. A significant difference in prevalence was found per wadis. Wadi Toffaha showed the highest diversity when compared to other Wadis. Mean species richness was higher in Wadi Tlah (0.87) when compared to other Wadis. Syphacia spp. was frequently found coexisting with other nematodes. A significant interaction was found between both site and co-infection for Aspicularis spp. The spatial stability of nematode community was discussed compared to other related studies. In terms of similarity, the nematode community from Wadi Toffaha was closest to Wadi Tlah. In conclusion, this study showed that there is spatial variation in the distribution of nematode community. Possible factors affecting the stability of parasite community were discussed and further studies are needed.
Keywords: Gastrointestinal nematodes, Acomys dimidiatus, Spatial variation
Introduction
Parasite species are not randomly distributed among host species or geographical areas (Brouat et al. 2007). Many factors are known to affect the parasites community structure (Kehr et al. 2000). The extrinsic factors such as seasonal variation and the intrinsic ones such as host age and sex are now well documented for some host–parasite systems such as those involving rodents and their parasites (Behnke et al. 2004; Pawelczyk et al. 2004; Hawlena et al. 2005; Krasnov et al. 2005; Brouat et al. 2007; Behnke et al. 2009). However, the considerable variability of parasite community structure between different populations of the same host species remains largely unexplained (Behnke et al. 2001). Parasite communities and infracommunities are usually described only numerically, especially in rodents (Simões et al. 2012). Nevertheless, the size of the parasites is relevant information in order to understand how much parasite biomass can be sustained and how it can determine the helminth community structure (Simões et al. 2012).
Fragmentation of the environment has important consequences for the local flora and fauna, and human activities in particular have exacerbated naturally occurring barriers to animal movement and hence gene flow through the construction of towns, major roads and through agricultural activities (Bajer et al. 2005). Animals from such isolated or semi-isolated patches may be subject to different stresses in each site, including different infections, and hence experience different selection pressures created by the specific conditions in their home range (Thompson 1994; Bajer et al. 2005).
It has been shown that spiny mice (Acomys dimidiatus) from semi-isolated wadis in the montane wadis in the south of the Sinai Peninsula in Egypt experienced differences in parasite exposure and that mice from wadis where parasite challenge was high were less inquisitive and aggressive compared with those from wadis where they experienced less helminth infection (Behnke et al. 2000, 2003, 2004). In addition, site of capture was more important than the intrinsic factors in explaining variation in helminth communities in such region (Behnke et al. 2004). They expected that each wadi is distinct in terms of its rodent parasites, spatially different coevolutionary pressures on their hosts, with resultant variation in life-histories. The objective of this work was to study gastrointestinal nematodes community infecting A. dimidiatus in different wadis of St. Katherine, South Sinai, Egypt.
Materials and methods
Study area
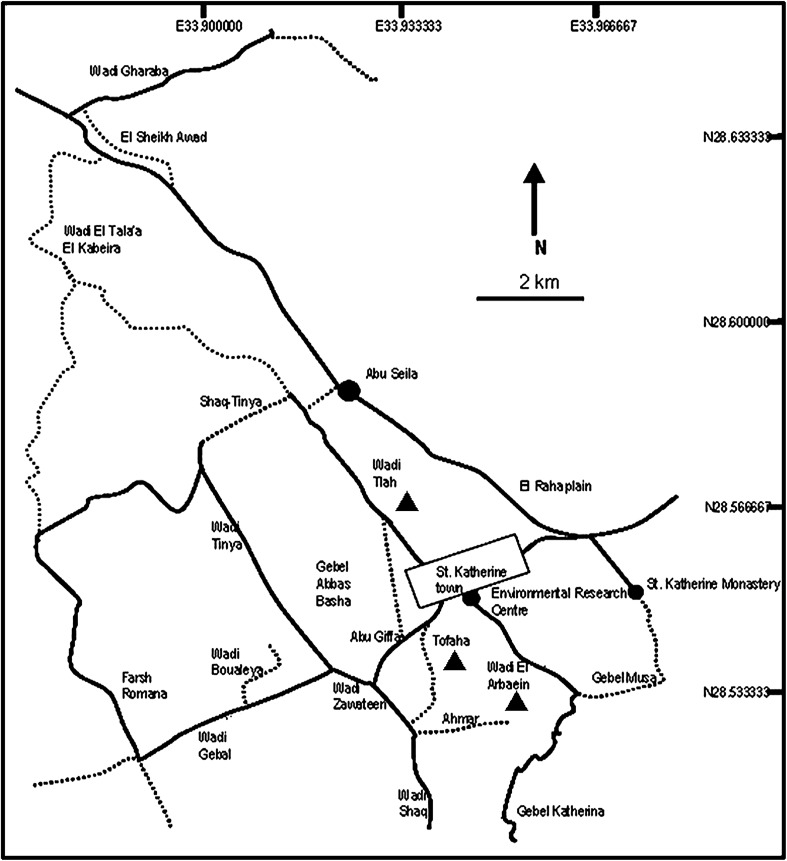
Fieldwork was conducted over a 4 weeks period during April–May, 2003 in St. Katherine, South Sinai, Egypt. Trapping was carried out in three sites (Fig. 1) namely Wadi Arbaein (N28.533, E33.966), Wadi Tlah (N28.566, E33.933) and Wadi Tofaha (N28.533, E33.933).
Fig. 1.
Map of the study area (modified from Behnke et al. 2004). Sites of the study area were indicated by arrow heads
Collection of rodents
At each site, rodents were captured alive using Sherman traps placed selectively among the rocks and boulders, mostly in the wadi bases, around walled gardens, and occasionally along the lower slopes (Behnke et al. 2004). These were set out at dusk, and inspected in the early morning before exposure to direct sunlight. All traps were brought into the local camp where the animals were removed, identified and processed. The rodents were identified according to Atallah (1967). Total examined mice were 47 divided among Wadis as follows: Wadi Arbaein, 9 (19.2 % of total), Wadi Tofaha 22 (46.8 %) and Wadi Tlah, 16 (34.0 %). The captured rodents were released after examination.
Faecal egg counts
Faecal samples were recovered from traps after the animals had been removed and were preserved in SAF (0.9 % w/v sodium acetate, 2 % acetic acid, 4 % formaldehyde, pH 4.15) fluid in 1.5 ml sample tubes (Behnke et al. 2004). All samples were centrifuged at 1,000 rpm for 10 min, and the volume of the deposit was recorded. The deposit was then re-suspended in a known volume of water and ten 10 μl samples examined on a microscope slide, and all the eggs of each type counted. Total egg counts were converted into eggs/ml (EPM) of faecal deposit (mean no. of eggs/10 μl sample × 100 × total volume of suspension (ml), divided by total volume (ml) of the original faecal deposit). Eggs were identified based on the morphology and morphometric parameters according to Ashour (1980) and Ashour and Lewis (1982). Eggs from the 4 species of spirurids (Protospirura muricola, Mastophorus muris, Streptopharagus kuntzi and Streptopharagus numidicus) could not be distinguished from one another (Behnke et al. 2004) therefore, they indicated in the text as spirurids egg. The species P. muricola, M. muris, S. kuntzi and S. numidicus were already previously described in A. dimidiatus from wadis of the study area (Behnke et al. 2004) and so, the eggs of spirurids found probably belong to some of these species.
Statistical analysis
Summary of statistics are presented as mean number ± standard error of EPM infection. Testing of effects of both individual and interacted factors (site and co-infection) on infection rate and eggs count was statistically analyzed using the General Linear Interactive Model (GLIM) after normalization of the data by log 10(x + 1) transformation (Crawley 1993; Wilson and Grenfell 1997; Behnke et al. 1999). At the component community level, two indices of diversity were quantified: numerical species richness (total number of species) and the Simpson’s index which weight for abundance of the most common species (Magurran 2004). Simpson’s Diversity Index is a measure of diversity which takes into account the number of species present, as well as the relative abundance of each species. All indices were calculated according to Magurran (2004) and Krebs (1998). All the statistical tests were performed by using the software packages SPSS 15.0.0 (USA).
Results
Fecal samples from the captured mice were analyzed for gastrointestinal nematodes. There was a significant difference in the numbers of animals sampled by Wadis (χ2 = 8.4, df = 2, P = 0.04). The gastrointestinal nematodes community consisted of four genera (Table 1). They were Dentostomella spp., Syphacia spp., Aspicularis spp. and Spirurids species.
Table 1.
Prevalence and number of EPM of nematodes community in the study area
| Overall infection | Helminths | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study site | Total examined mice | Number of infected mice | Total infection prevalence (%) | Mean species richness ± SE | Dentostomella spp. | Syphacia spp. | Aspicularis spp. | Spirurids egg | ||||
| Prevalence (%) | EPM (rang) | Prevalence (%) | EPM (rang) | Prevalence (%) | EPM (rang) | Prevalence (%) | EPM (rang) | |||||
| Wadi Arbaein | 9 | 5 | 55.6 | 0.44 ± 0.72 | 22.2 | 1–198 (n = 2) | 11.1 | 8 (n = 1) | 0 | 0 | 0 | 0 |
| Wadi Toffaha | 22 | 9 | 40.9 | 0.50 ± 0.74 | 4.5 | 1 (n = 1) | 18.2 | 8–83 (n = 3) | 22.7 | 4–113 (n = 5) | 9.1 | 5–20 (n = 2) |
| Wadi Tlah | 16 | 12 | 75 | 0.87 ± 0.80 | 6.3 | 8 (n = 1) | 18.8 | 7–63 (n = 3) | 62.5 | 4–163 (n = 10) | 0 | 0 |
| Total | 47 | 26 | 55.3 | 0.62 ± 0.76 | 8.5 % | 14.9 % | 31.9 % | 4.3 % | ||||
SE standard error, EPM egg/ml, rang minimum and maximum value of egg/ml, n number of infected animals
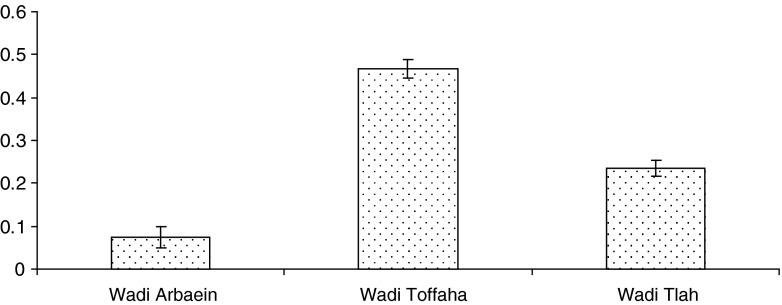
Table 1 shows that all nematodes that found during the study represented by 50 % in Wadi Arbaein, 100 % in Wadi Toffaha and 75 % in Wadi Tlah. In terms of similarity, the helminth component community from Wadi Toffaha was closest to Wadi Tlah (the same three genera found in both wadis). The percentage of similarity among communities was 58.2 %. The diversity of helminths component community was differed according to site. The diversity in Wadi Arbaein, Wadi Toffaha and Wadi Tlah were 0.07 ± 0.03, 0.46 ± 0.02 and 0.23 ± 0.02, respectively (Fig. 2). Wadi Toffaha has the highest diversity compared with other Wadis.
Fig. 2.
Diversity of helminth community per site
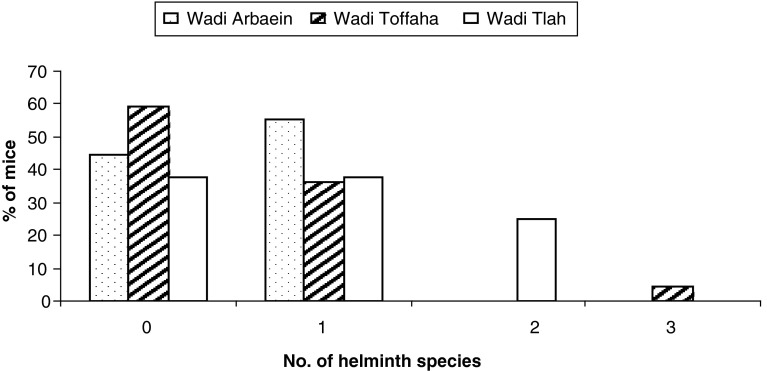
The frequency of the distribution of nematode community species richness is shown in Fig. 3. Overall mean number of helminth harboured per host (all mice combined, n = 47) was 0.62 ± 0.76 (variance to mean ratio = 0.59). Mean species richness was higher in Wadi Tlah (0.87 ± 0.8) and lower in Wadi Arbaein (Table 1, 0.44 ± 0.72). There was no significance difference among the Wadis (P = 0. 25) (Table 1).
Fig. 3.
Frequency distribution of infracommunity species richness per site
The overall prevalence of infection was 55.3 %. A significance difference in prevalence was found in Wadis (P = 0.04). The combined prevalences were 55.6, 40.9, 75 % in Wadi Arbaein, Toffaha, Telah respectively (Table 1). Aspicularis spp. (31.9 %) and Syphacia spp. (14.9 %) were the most prevalent nematode among all the mice combined. Spirurids species were found only in Wadi Toffaha. There was a significant difference in prevalence among different sites in Aspicularis spp. (Table 1, P = 0.003). The numbers of nematodes recorded in the mice from Wadis Toffaha and Tlah were higher than that from mice in Wadis Arbaein (Table 1). The number of EPM of Aspicularis spp. differed significantly among sites (Table 1, P = 0.004).
Dentostomella spp. was found as a single infection in all wadis except in Wadi Tlah, while Syphacia spp. was frequently coexisted with other genera (Table 2). Analysis of these data with GLIM (two ways ANOVA in GLIM with normal errors and site and co-infection as factors) revealed that there was interaction between site and co-infection in Aspicularis spp. (Table 3).
Table 2.
Percentage of occurrence of either single or co- infection in the study sites
| Study site | Dentostomella spp. | Syphacia spp. | Aspicularis spp. | Spirurids egg | ||||
|---|---|---|---|---|---|---|---|---|
| Single infection | Co-infection | Single infection | Co-infection | Single infection | Co-infection | Single infection | Co-infection | |
| Wadi Arbaein | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wadi Toffaha | 100 | 0 | 66.7 | 33.4 | 80 | 20 | 0 | 0 |
| Wadi Tlah | 0 | 100 | 100 | 0 | 66.7 | 33.3 | 50 | 50 |
Table 3.
Test of interaction between site and co-infection status of Aspicularis spp.
| Source | Type III sum of squares | df | Mean square | F | Sig. |
|---|---|---|---|---|---|
| Corrected model | 28544.58(a) | 3 | 9514.86 | 6.10 | 0.011 |
| Intercept | 60024.27 | 1 | 60024.27 | 38.53 | 0.000 |
| Co-infection | 3873.47 | 1 | 3873.47 | 2.48 | 0.143 |
| Site | 1862.45 | 1 | 1862.45 | 1.19 | 0.298 |
| Co-infection × site | 15961.25 | 1 | 15961.25 | 10.24 | 0.008 |
| Error | 17134.75 | 11 | 1557.70 | ||
| Total | 112346.00 | 15 | |||
| Corrected total | 45679.33 | 14 |
df degree of freedom, F value of F test, Sig significant difference
Discussion
The aim of this work was to study the nematode community of spiny mice (A. dimidiatus) in different wadis. As in numerous other studies (e.g. Behnke et al. 2000, 2001, 2004, 2008; Brouat et al. 2007) on gastrointestinal helminths of rodents, we found a considerable variability of parasite community structure between the different sites as indicated by species richness and diversity index. Helminths infracommunity structure was clearly dominated by the site effect when examined at a variety of levels (e.g. prevalence, aggregation, abundance etc.) for individual parasite species that were common and which could be treated quantitatively (Behnke et al. 2008). Such variability in community structure should be attributed to the fact that gastro-intestinal helminths of terrestrial mammals spend at least one part of their life-cycle in the external environment outside their host, and habitat characteristics might be crucial for the survival of eggs or larvae (Brouat et al. 2007).
Nematodes community structure of the spiny mice collected from Wadi Arbaein or Wadi Tlah apparently differed from those recorded by Behnke et al. (2004) when compared with our study. Aspicularis spp. and Spirurids spp. were found in Wadi Arbaein by Behnke et al. (2004) and not found in the present study. Spirurids species were found by Behnke et al. (2004) in Wadi Tlah but not found in our study. A variation was also recorded regarding nematode community of Wadi Toffaha compared with that reported by Behnke et al. (2000). Syphacia spp. and Aspicularis spp. were recorded in the present study and were not reported by Behnke et al. (2000). Although there are noticeable spatial variations through time in nematode community, species richness in Wadi Tlah has remained the highest as previously recorded by Behnke et al. (2004). Recording of two new species namely, Syphacia spp. and Aspicularis spp. in Wadi toffaha; which has not been reported by Behnke et al. (2000), pointed also to a possible spatial instability through time. EPM recorded in this study greatly varied compared to those recorded by Behnke et al. (2004). The prevalence of Dentostomella spp. and Syphacia spp. was different as compared to that recorded by Behnke et al. (2004). The possible instability of nematodes community as represented in prevalence, intensity and species richness may be attributed to factors such as sample size, possible host migration and some ecological factors. Hence, further investigation is needed. In contrast, some authors (Mollhagan 1978; Thul et al. 1985; Calvete et al. 2004; Booth 2006; Behnke et al. 2008) demonstrated that there is considerable medium-term stability in the parasite communities as in other helminth and protozoan infections. In addition, Bajer et al. (2005) reported that the structure of helminth communities in wild rodents is subject to seasonal variation, and is dependent on host age within year.
Single and co-infection as mentioned in the present results regarding Dentostomella spp. or Syphacia spp. for example, and the significant effect of both site and co-infection on Aspicularis spp. may indirectly result in instability of parasite community through times. In this regards, Géraldine et al. (2007) reported that among the potential biotic factors affecting population dynamics, relations between different parasite communities has received relatively little attention. Similarly, Brooker and Clements (2009) pointed to the importance of mono- and co-infection with helminth parasites in spatial epidemiology especially in developing world.
In conclusion, this study showed that there is instability in gastrointestinal nematode community in the semi isolated and xeric habitat. Further studies and analyses are needed to test such factors in controlling the stability or instability of parasite community.
Acknowledgments
The authors thank Dr. Ehab Sayed, lecturer, Department of Zoology, Faculty of Science, Suez Canal University, Ismailia, Egypt, for his help in this work.
References
- Ashour AA (1980) Ultrastructural and other studies on intestinal nematodes of small mammals from Egypt. PhD thesis, Ain Shams University, Cairo, Egypt
- Ashour AA, Lewis JW. The morphology of Dentostomella kuntzi(Nematoda: oxyuroidea) from Egyptian rodents. J Helminthol. 1982;56:159–168. doi: 10.1017/S0022149X00034398. [DOI] [PubMed] [Google Scholar]
- Atallah A new species of spiny mouse (Acomys) from Jordan. J Mammal. 1967;48(2):258–261. doi: 10.2307/1378029. [DOI] [Google Scholar]
- Bajer A, Behnke JM, Pawełczyk A, Kuliś K, Sereda MJ, Siński E. Medium-term temporal stability of the helminth component community structure in bank voles (Clethrionomys glareolus) from the Mazury Lake District region of Poland. Parasitology. 2005;130:213–228. doi: 10.1017/S0031182004006389. [DOI] [PubMed] [Google Scholar]
- Barnard CJ, Sayed E, Barnard LE, Behnke JM, Abdel Nabi I, Sherif N, Shutt A, Zalat S. Local variation in helminth burdens of Egyptian spiny mice (Acomys cahirinus dimidiatus) from ecologically similar sites: relationships with hormone concentrations and social behaviour. J Helminthol. 2003;77:197–207. doi: 10.1079/JOH2003189. [DOI] [PubMed] [Google Scholar]
- Behnke JM, Lewis JW, Mohd Zain SN, Gilbert FS. Helminth infections in Apodemus sylvaticus in southern England: interactive effects of host age, sex and year on the prevalence and abundance of infections. J Helminthol. 1999;73:31–44. [PubMed] [Google Scholar]
- Behnke JM, Barnard CJ, Mason N, Harris PD, Sherif NE, Zalat S, Gilbert FS. Intestinal helminths of spiny mice (Acomys cahirinus dimidiatus) from St Katherine’s Protectorate in the Sinai Egypt. J Helminthol. 2000;74:31–44. [PubMed] [Google Scholar]
- Behnke JM, Barnard CJ, Bajer A, Bray D, Dinmore J, Frake K, Osmond J, Race T, Sinski E. Variation in the helminth community structure in bank voles (Clethrionomys glareolus) from three comparable localities in the Mazury Lake District region of Poland. Parasitology. 2001;123:401–414. doi: 10.1017/S0031182001008605. [DOI] [PubMed] [Google Scholar]
- Behnke JM, Harris PD, Bajer A, Barnard CJ, Sherif N, Cliffe L, Hurst J, Lamb M, Rhodes A, James M, Clifford S, Gilbert FS, Zalat S. Variation in the helminth community structure in spiny mice (Acomys dimidiatus) from four montane wadis in the St Katherine region of the Sinai Peninsula in Egypt. Parasitology. 2004;129:379–398. doi: 10.1017/S003118200400558X. [DOI] [PubMed] [Google Scholar]
- Behnke JM, Bajer A, Harris PD, Newington L, Pidgeon E, Rowlands G, Sheriff C, Kulis K, Malkowska K, Siński E, Gilbert FS, Barnard CJ. Temporal and between-site variation in helminth communities of bank voles (Myodes glareolus) from N.E. Poland. 2. The infracommunity level. Parasitology. 2008;135:999–1018. doi: 10.1017/S0031182008004484. [DOI] [PubMed] [Google Scholar]
- Behnke JM, Eira C, Rogan M, Gilbert FS, Torres J, Miquel J, Lewis JW. Helminth species richness in wild wood mice, Apodemus sylvaticus, is enhanced by the presence of the intestinal nematode, Heligmosomoides polygyrus. Parasitology. 2009;136:793–804. doi: 10.1017/S0031182009006039. [DOI] [PubMed] [Google Scholar]
- Booth M. The role of residential location in apparent helminth and malaria associations. Trends Parasitol. 2006;22:359–362. doi: 10.1016/j.pt.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Brooker S, Clements AC. Spatial heterogeneity of parasite co-infection: determinants and geostatistical prediction at regional scales. J Parasitol. 2009;39:591–597. doi: 10.1016/j.ijpara.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouat C, Kane M, Diouf M, Bâ K, Sall-Dramé R, Duplantier JM. Host ecology and variation in helminth community structure in Mastomys rodents from Senegal. Parasitology. 2007;134:437–450. doi: 10.1017/S003118200600151X. [DOI] [PubMed] [Google Scholar]
- Calvete C, Blanco-Aguiar JA, Virgo E, Cabezas-Dias S, Villafuerte R. Spatial variation in helminth community structure in the red-legged partridge (Alectoris rufa L.): effects of definitive host density. Parasitology. 2004;129:101–113. doi: 10.1017/S0031182004005165. [DOI] [PubMed] [Google Scholar]
- Crawley MT. GLIM for ecologists. Oxford: Blackwell Scientific Press; 1993. [Google Scholar]
- Géraldine LG, de Montaudouin X, Soudant P, Christine PC. Parasite co-infection of two sympatric bivalves, the Manila clam (Ruditapes philippinarum) and the cockle (Cerastoderma edule) along a latitudinal gradient. Aquat Living Resour. 2007;20:33–42. doi: 10.1051/alr:2007013. [DOI] [Google Scholar]
- Hawlena H, Abramsky Z, Krasnov BR. Age-biased parasitism and density-dependent distribution of fleas (Siphonaptera) on a desert rodent. Oecologia. 2005;146:200–208. doi: 10.1007/s00442-005-0187-0. [DOI] [PubMed] [Google Scholar]
- Kehr AI, Manly BFJ, Hamann MI. Coexistence of helminth species in Lysapsus limellus (Anura: Pseudidae) from an Argentinean subtropical area: influence of biotic and abiotic factors. Oecologia. 2000;125:549–558. doi: 10.1007/s004420000480. [DOI] [PubMed] [Google Scholar]
- Krasnov BR, Morand S, Hawlena H, Khokhlova IS, Shenbrot GI. Sex-biased parasitism, seasonality and sexual size dimorphism in desert rodents. Oecologia. 2005;146:209–217. doi: 10.1007/s00442-005-0189-y. [DOI] [PubMed] [Google Scholar]
- Krebs CJ. Ecological methodology. 2. Menlo Park: Addison Wesley Longman; 1998. [Google Scholar]
- Magurran AE. Measuring biological diversity. Maldan: Blackwell Publishing; 2004. [Google Scholar]
- Mollhagan T. Habitat influence on helminth parasitism of the cotton rat in western Texas, with remarks on some of the parasites. Southwest Nat. 1978;23:401–407. doi: 10.2307/3670248. [DOI] [Google Scholar]
- Pawelczyk A, Ogrzewalska M, Zadrozna I, Siński E. The zoonotic reservoir of Borrelia burgdorferisensu lato in the Mazury Lakes district of North-Eastern Poland. Int J Med Microbiol. 2004;293:167–171. doi: 10.1016/s1433-1128(04)80033-0. [DOI] [PubMed] [Google Scholar]
- Simões RO, Maldonado-Júnior A, Luque JL. Helminth communities in three sympatric rodents from the Brazilian Atlantic Forest: contrasting biomass and numerical abundance. Braz J Biol. 2012;72:909–914. doi: 10.1590/S1519-69842012000500018. [DOI] [PubMed] [Google Scholar]
- Thompson JN. The co-evolutionary process. Chicago: University of Chicago; 1994. [Google Scholar]
- Thul JE, Forrester DJ, Abercrombie CL (1985) Ecology of parasitic helminths of wood ducks, Aix sponsa, in the Atlantic flyway. In: Proceeding of Helminthological Society of Washington 52: 297–310
- Wilson K, Grenfell BT. Generalized linear modelling for parasitologists. Parasitol Today. 1997;13:33–38. doi: 10.1016/S0169-4758(96)40009-6. [DOI] [PubMed] [Google Scholar]