Abstract
The present work reports the larvicidal potential of microbially synthesised silver nanoparticles (AgNPs) by using an actinobacterium, Streptomyces sp. M25 isolated from Western Ghats, Tamil Nadu, India. The biomass of Streptomyces sp. was exposed to 1 mM silver nitrate (AgNO3) solution. The synthesis of AgNPs was confirmed by visual inspection followed by instrumental analysis such as UV–vis spectroscopy, fourier transform infrared spectroscopy, X-ray diffraction (XRD), energy dispersive X-ray spectroscopy, scanning electron microscopy, atomic force microscopy and transmission electron microscopy (TEM) with selected area electron diffraction. Based on the TEM and XRD analysis, the average size of the AgNPs was determined to be 10–35 nm. The biosynthesised AgNPs exhibited significant larvicidal activity against malarial vector, Anopheles subpictus (LC50 51.34 mg/L and χ2 value of 8.228), filarial vector, Culex quinquefasciatus (LC50 48.98 mg/L and χ2 value of 14.307) and dengue vector, Aedes aegypti (LC50 60.23 mg/L and χ2 value of 4.042), respectively. Similarly, AgNO3 had larvicidal activity against malarial vector, A. subpictus (LC50 42.544 mg/L and χ2 value of 2.561), filarial vector, C. quinquefasciatus (LC50 44.922 mg/L and χ2 value of 1.693) and dengue vector, A. aegypti (LC50 39.664 mg/L and χ2 value of 5.724), respectively. The current study is a rapid, cost effective, eco-friendly and single step approach. The Streptomyces sp. M25 is a newly added source for the synthesis of AgNPs with improved larvicidal activity.
Keywords: Biosynthesis, Silver nanoparticles, Mosquito vector, Larvicidal, Streptomyces sp.
Introduction
Mosquitoes are the most prevalent vector species, which can able to transmit diseases like, malaria, dengue fever, yellow fever, filariasis, chickungunya, etc., in worldwide (Matasyoh et al. 2011). It also causes some skin and systemic allergic reactions such as angioedema on human beings (Peng et al. 1999). In worldwide, the diseases like malaria, dengue and filariasis are the most significant cause of morbidity and mortality every year (WHO 1996, 2009). In India, these diseases are still the most crucial cause of morbidity and mortality with arising of totally two to three million new cases every year (Sharma et al. 2009). Among Aedes species present in India, the dengue vector A. aegypti was found as the most prevalent species (86.89 %) occurring throughout the year, while A. albopictus and A. vitattus were found mostly during monsoon periods in the tropical and subtropical zones. Of the 53 Anopheles species present in India, only nine species are most important vectors of malarial disease. The lymphatic filarial vector Culex quinquefasciatus is one of the most important vectors and annoying man—biting mosquitoes (Mourya et al. 1989; Das et al. 2002). Lymphatic filariasis is second to malaria as the most significant mosquito borne diseases in India.
Vector control is one of the most serious concerns in developing countries like India, due to the lack of general awareness and socio-economic reasons (Kovendan et al. 2012). The control of these vectors is being strengthened in many areas, but there are significant drawbacks and challenges including the development of resistance to routinely used synthetic insecticides and phytochemicals day by day and a lack of alternative, cost-effective and safer insecticides led to resurgences in mosquito populations. Increasing insecticide resistances requires the development of new strategies for elongating the use of highly effective mosquito control compounds. Thus, the novel attempts to develop for the synthesis of nanomaterials for control of mosquito larvae in worldwide with the progress of nanotechnology (Patil et al. 2012).
The synthesis of nanoparticles of specific composition and size is a rapidly growing area of materials science research. New methods to the preparation of these materials extend the choice of different properties that can be obtained. Various physical and chemical (Mandal et al. 2005) synthesis methods are aimed for controlling the properties of the particles and for the production of metal nanoparticles. Most of the techniques are still in the developmental stage and various problems are often experienced with the stability of the nanoparticles preparations (Vahabi et al. 2011). One of the major developments in nanotechnology is the synthesis of nanoparticles using living organisms such as plants, microbes, marine organisms and their components (Sadhasivam et al. 2012).
At present, different types of metal nanoparticles are being produced including copper, zinc, titanium, magnesium, gold, alginate and silver (Bhaskara Roa et al. 2010) using microbial sources (Sastry et al. 2003). Among these, AgNPs have received considerable attention due to their attractive physico-chemical properties, surface plasmon resonance, surface-enhanced raman scattering (SERS) or biological applications. Biologically synthesised AgNPs could have many applications, including for both in vivo and in vitro biomedical and industrial research. Addition to this, the AgNPs act as the very good insecticide for the control of mosquito vectors nowadays (Patil et al. 2012).
The present study reports, the mosquito larvicidal activity of silver nanoparticles against A. subpictus, C. quinquefasciatus and A. aegypti has been synthesised using actinobacterium, Streptomyces sp. M25 from Western Ghats ecosystem, Tamil Nadu, India.
Materials and methods
Description of producing strain and biomass preparation
Streptomyces sp. M25 was isolated from soil samples collected from Western Ghats (Lat: 8°25′N; Long: 77°10′E), Tamil Nadu. Viability of culture was maintained on yeast extract malt extract agar (YEME) (Shirling and Gottileb 1966) slants at 4 °C. For the preparation of biomass, mycelial growth was inoculated into 100 mL of YEME broth and incubated at 28 °C for 5 days in rotary shaker with 120 rpm speed. After incubation, the mycelium was separated by centrifugation (5,000 rpm for 10 min at 4 °C). The biomass was collected in sterile screw cap vials after washing twice with sterile distilled water and stored at 4 °C until further use (Balagurunathan et al. 2011).
Biosynthesis of silver nanoparticles
About 10 g of Streptomyces biomass was exposed to a 100 mL aqueous solution of 1 mM silver nitrate (AgNO3) in a 500 mL Erlenmeyer flask. The flask was incubated in rotary shaker with 120 rpm at 28 °C. During biosynthesis, the reaction flask was observed for every 24 h for visual colour change from white to brown, which indicated the biosynthesis of AgNPs (Senapati et al. 2005). After colour change, the whole mixture was centrifuged at 5,000 rpm for 15 min and the cells and supernatant was observed to determine the intra (or) extra cellular synthesis of AgNPs. After centrifugation, the quantity of AgNPs was weighed per 100 ml.
Characterisation of silver nanoparticles
UV–Visible and FT-IR analysis
The bioreduction of Ag+ ions in solution was monitored using UV–vis spectroscopy (Cyberlab double beam model) at a resolution of 1 nm between 190 and 1,000 nm range (Balagurunathan et al. 2011). For the FT-IR analysis, a small amount of the sample was mixed with 100 mg KBr FT-IR grade and pressed into a pellet. The pellet was placed into the sample holder and the FT-IR spectra were recorded in the range of 4,000–400 cm−1 in FT-IR spectroscopy at a resolution of 4 cm−1 (Bhaskara Rao et al. 2010).
XRD and EDX analysis
Structural characterisation was carried out by X-ray diffraction spectroscopy (Shimadzu XRD 6000), in the operating voltage of 40 kV and a current of 30 mA with Cu Kα radiation (λ = 1.540 A°). The AgNPs were mixed into 10 mL of deionized water. Then the biosynthesised AgNPs were freeze–dried, powdered and used for XRD analysis (Sadhasivam et al. 2012). EDX measurements of the AgNPs were performed by SEM–EDX (Model Jeol JFSM 6390). The sample was prepared by casting the nanoparticle solution on a glass substrate (Du et al. 2010).
SEM and TEM observation
Scanning electron microscopy (Model Jeol JFSM 6390) and TEM (Model Hitachi H-500) analysis as well as SAED pattern was performed for identify the size and shape of the nanoparticles. After synthesis of AgNPs, thin film of the sample that was prepared on a carbon coated copper grid by just dropping a very small amount of the sample on the grid. Extra solution was removed using a blotting paper and then the film on the grid was allowed to dry by putting it under a mercury lamp for 5 min. Then, the film was magnified at different magnifications for the observation of AgNPs (Mukherjee et al. 2001).
Atomic force microscope observations
To perform AFM analysis, the nanoparticles were mixed in methanol and a drop of the mixture was deposited on a silicon slide and the solvent was allowed to evaporate. Then, the thin film (1 × 1 cm2) containing the sample was analysed using AFM (Model Nanosurf A) (Sadhasivam et al. 2010).
Larvicidal activity
Test larvae
For preliminary screening, the malarial vector larvae of A. subpictus, filariasis vector larvae of C. quinquefasciatus and dengue vector A. aegypti were obtained from the National Center for Disease Control Research Institute, Coonoor, Tamil Nadu, India. The larvae were kept in plastic enamel trays containing dechlorinated tap water and maintained (Patil et al. 2010).
Mosquito larvicidal bioassay
The toxicity test was performed by placing 25 mosquito larvae into 200 mL of sterilized double–distilled water with microbially synthesised AgNPs and AgNO3 to the designed concentrations (25, 20, 15, 10 and 5 mg/L) into a 250 mL beaker. Distilled water used as control for each individual concentrations. Mortality was estimated after 24 h to calculate the acute toxicities on larvae of A. subpictus, C. quinquefasciatus and A. aegypti (Jayaseelan et al. 2011). The 16:8 h light/dark cycle was tested using the AgNPs concentrations of 100, 80, 60, 40 and 20 mg/L and the AgNO3 concentrations of 50, 40, 30, 20 and 10 mg/L with a final volume of 200 mL in a 250 mL glass beaker. A negative control (Mosquito larvae without nanoparticles and silver nitrate) was used in all experiments, and all conditions were tested in five replicates.
Dose–response bioassay
Based on the preliminary screening results, microbially synthesised AgNPs and AgNO3 were subjected to dose–response bioassay for larvicidal activity against the larvae of A. subpictus, C. quinquefasciatus and A. aegpyti. Different concentrations ranging from 20 to 100 mg/L (for microbially synthesised AgNPs) and 10–50 mg/L (for silver nitrate) were prepared for larvicidal activity. The numbers of dead larvae were counted after 24 h of exposure, and the mortality percentage was reported from the average of five replicates. However, at the end of 24 h, the selected test samples turned out to be equal in their toxic potential (Rahuman et al. 2008).
Statistical analysis
All results were revealed as the mean ± standard deviation (SD) of the mean (SEM) values. Statistical significant difference was calculated using Chi square followed by regression co-efficient to compare with control group. A significance level of P < 0.05 was considered to be statistically significant.
Results and discussion
Biosynthesis of silver nanoparticles
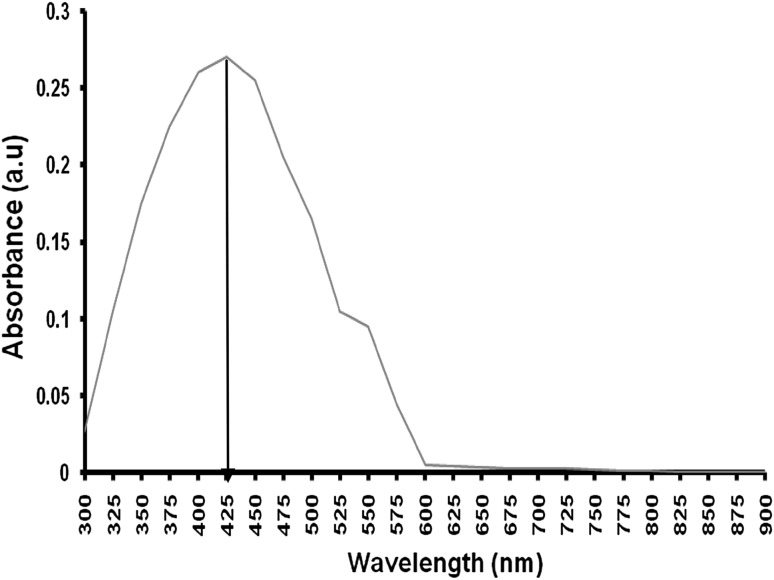
Observed that upon the addition of biomass into the flask containing the AgNO3 solution the colour of the solution was changed into brown colour within 72 h. This indicates the extracellular synthesis of AgNPs. The visual observation of colour change in AgNO3 solution was reported by various research groups (Vahabi et al. 2011). In UV–spectral analysis, the maximum absorbance peak observed at 425 nm in the visible region (Fig. 1). These colour changes arise because of the excitation of surface plasmon vibrations with the AgNPs (Ahmad et al. 2003). Dry weight of the biosynthesised AgNPs is 65 mg/100 ml.
Fig. 1.
UV–vis spectrum shows a characteristics peak at 425 nm
Characterisation of silver nanoparticles
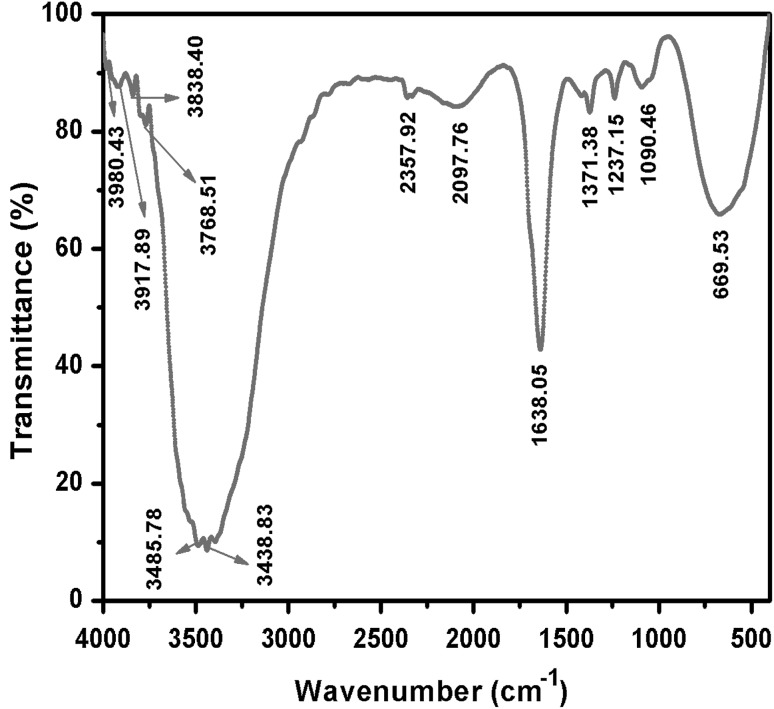
The FT-IR spectrum shows the prominent absorption bands at 3485, 3438, 1638, 1371, 1237, 1090 and 669 cm−1 (Fig. 2). The bands observed at 3,485 cm−1 is due to OH stretching of water molecules. Another band observed at 3,438 cm−1 corresponds to the stretching vibration of primary amines respectively and their corresponding bending vibrations were observed at 1,638 cm−1. The two bands observed at 1,371 and 1,237 cm−1 can be assigned to the C–N stretching vibration of aromatic and aliphatic amines respectively. NO3− ions were free in the actinobacterial surface; it was proved by two bands observed at 1,090 and 669 cm−1. Based on the overall observations, protein can bind to nanoparticles either through free amine groups or cysteine residues. Additionally, it binds with electrostatic attraction of negatively charged carboxylate groups of enzymes present in the cell wall of actinobacterial mycelium (Vahabi et al. 2011).
Fig. 2.
FT-IR spectrum shows different functional peaks
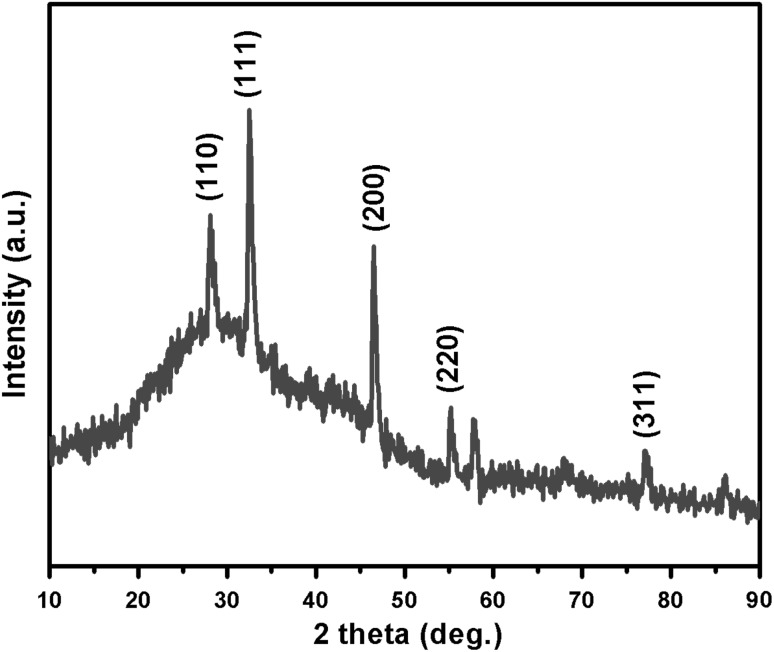
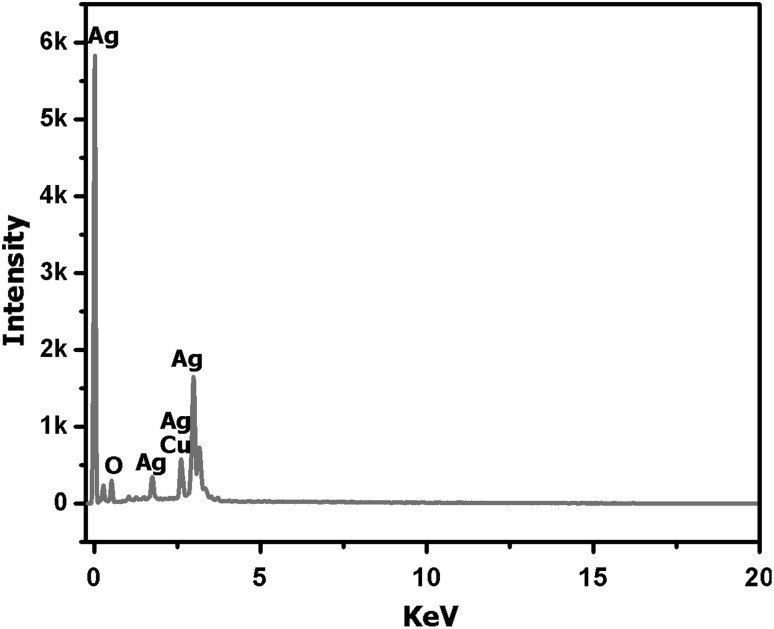
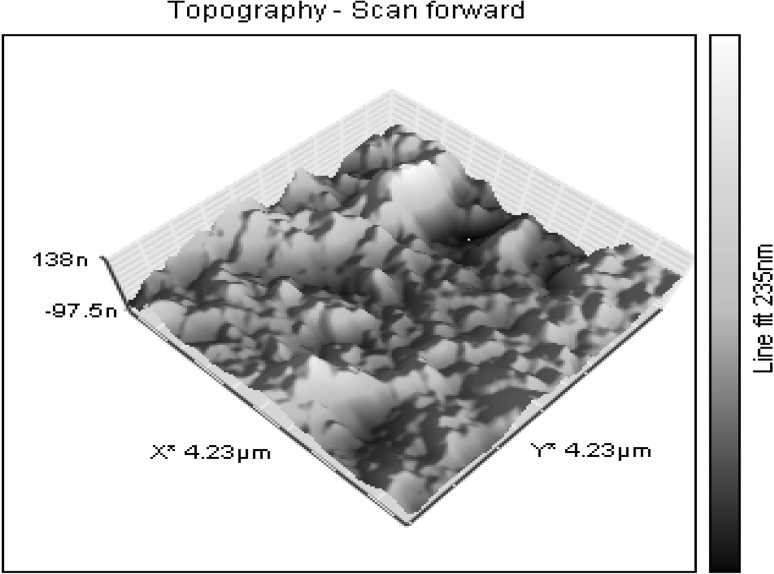
XRD pattern shows sharp peaks at the diffraction angles at 32.57°, 46.55°, 55.21° and 77.11° (Fig. 3) Corresponding to (111), (200), (220) and (311) Bragg’s reflection, in the whole spectrum of 2θ values ranges from 10° to 90° respectively. The sharp intense peaks of the spectrum clearly indicated that the particles are in the nanoregime and also crystalline in nature. The size of the crystallite AgNPs was calculated using Debye–Scherrer formula (Sadhasivam et al. 2012). The calculated crystallite size of Ag particle is ~11.5 nm. The EDX analysis study shown in Fig. 4. The strong Ag signal confirms the presence of metallic AgNPs and their crystalline nature (Ahmad et al. 2003). The SEM observation showed that the presence of AgNPs clearly (Fig. 5). The topography of biosynthesised AgNPs were obtained from 3D image (Fig. 6) of AFM. The surface morphology was predicted, through image scanning in an area of 4.23 × 4.23 μm. Representative TEM images in Fig. 7a shows the well dispersed AgNPs and the morphology and size of the AgNPs are displayed more clearly in the figure at higher magnification. The AgNPs were mostly spherical in shape and showed a large distribution of sizes in the range of 10–35 nm. The SAED pattern of the AgNPs suggests clear single crystallinity showed in Fig. 7b. The nanoparticle shapes are resolved by the relative growth rate in different crystallographic directions. Based on the experimental results the interaction among biomolecules and surface of AgNPs were very strong. The crystal growth prejudiced by the inhibition of silver atom accumulation on the bacterial surface (Sadhasivam et al. 2012; Ahmad et al. 2003).
Fig. 3.
XRD pattern of actinobacterially synthesised AgNPs
Fig. 4.
EDX pattern shows strong Ag signals
Fig. 5.
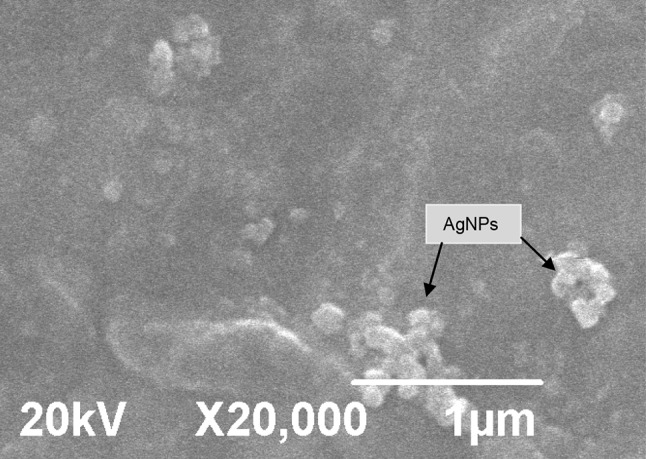
Typical SEM image showing the spherical morphology and agglomeration of AgNPs at 45,000×
Fig. 6.
Typical AFM image showing the 3D image topography of AgNPs at 235 nm
Fig. 7.
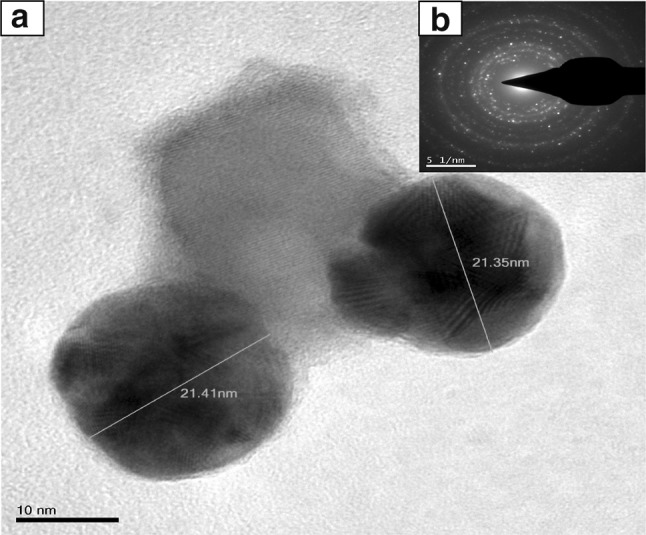
a TEM image shows the clear shape and size of the AgNPs at 10 nm scale. b SAED pattern of microbially synthesised AgNPs
Larvicidal activity
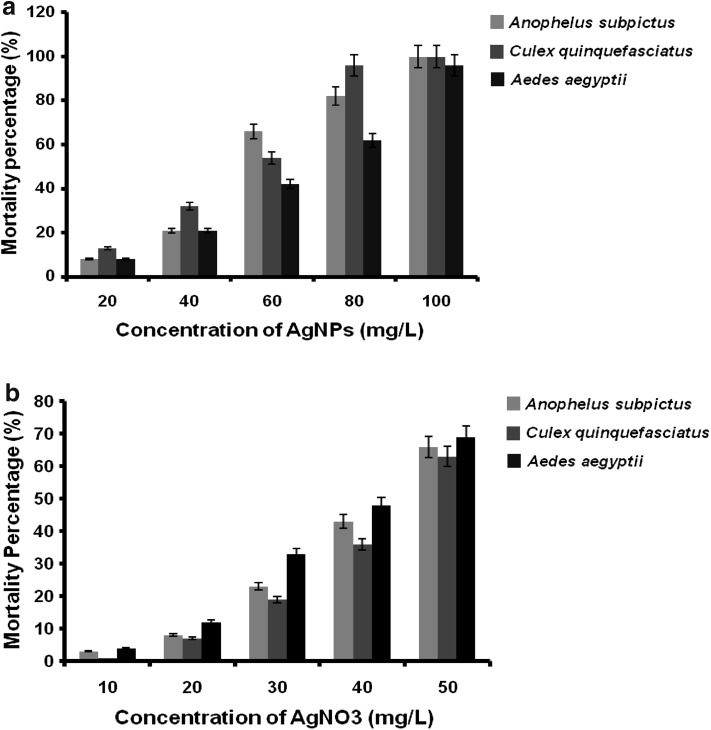
In the present study, the larvicidal effect of AgNPs synthesised using actinobacterial strain M25 and AgNO3 is noted. The highest mortality was found in synthesised AgNPs against the larvae of A. subpictus (LC50 51.34 mg/L, r2 = 0.962 and χ2 value of 8.228), against the larvae of C. quinquefasciatus (LC50 48.98 mg/L, r2 = 0.956 and χ2 value of 14.307) and against the larvae of A. aegpyti (LC50 60.23 mg/L, r2 = 0.972 and χ2 value of 4.042), respectively (Table 1; Fig. 8a). Similarly, the precursor chemical AgNO3 was found mortality against the larvae of A. subpictus (LC50 42.544 mg/L, r2 = 0.954 and χ2 value of 2.561), against the larvae of C. quinquefasciatus (LC50 44.922 mg/L, r2 = 0.936 and χ2 value of 1.693) and against the larvae of A. aegpyti (LC50 39.664 mg/L, r2 = 0.983 and χ2 value of 5.724), respectively (Table 2; Fig. 8b).The Chi square value was significant at P < 0.05. The result indicates, the biosynthesised AgNPs were showed higher toxic effect than their precursor AgNO3 against all the three mosquito species.
Table 1.
Larvicidal activity of the microbially synthesised AgNPs
| Species | Concentrations (mg/L) | Percent mortality ± SD | LC50 (mg/L) | 95 % confidence interval for LC50 | r 2 | χ 2 (df = 4) | Regression equation | |
|---|---|---|---|---|---|---|---|---|
| LCL (mg/L) | UCL (mg/L) | |||||||
| Anopheles subpictus | 100 | 100 ± 0.00 | 51.34 | 47.72 | 55.22 | 0.962 | 8.228 | y = −24.5x + 128.9 |
| 80 | 82 ± 2.36 | |||||||
| 60 | 66 ± 1.89 | |||||||
| 40 | 21 ± 3.33 | |||||||
| 20 | 8 ± 1.00 | |||||||
| Culex quinquefasciatus | 100 | 100 ± 0.00 | 48.98 | 44.81 | 53.53 | 0.956 | 14.307 | y = −23.8x + 130.4 |
| 80 | 96 ± 2.25 | |||||||
| 60 | 54 ± 1.96 | |||||||
| 40 | 32 ± 2.50 | |||||||
| 20 | 13 ± 3.78 | |||||||
| Aedes aegypti | 100 | 96 ± 2.24 | 60.232 | 55.854 | 65.032 | 0.972 | 4.042 | y = −21.7x + 110.9 |
| 80 | 62 ± 2.74 | |||||||
| 60 | 42 ± 2.73 | |||||||
| 40 | 21 ± 3.33 | |||||||
| 20 | 8 ± 1.00 | |||||||
Control−nil mortality, a mean value of five replicates, significant at P < 0.05 level, LC50 lethal concentration that kills 50 % of the exposed larvae
UCL upper confidence limit, LCL lower confidence limit, r2 regression co-efficient, χ2 Chi square, df degrees of freedom
Fig. 8.
a Mean mortality rate of microbially synthesised AgNPs on three mosquito species at different concentrations. b Mean mortality rate of AgNO3 on three mosquito species at different concentrations
Table 2.
Larvicidal activity of the silver nitrate (AgNO3)
| Species | Concentrations (mg/L) | Percent mortality ± SD | LC50 (mg/L) | 95 % confidence interval for LC50 | r 2 | χ 2 (df = 2) | Regression equation | |
|---|---|---|---|---|---|---|---|---|
| LCL (mg/L) | UCL (mg/L) | |||||||
| Anopheles subpictus | 50 | 66 ± 2.36 | 42.544 | 38.41 | 47.126 | 0.954 | 2.561 | y = −16.1x + 76.9 |
| 40 | 43 ± 2.74 | |||||||
| 30 | 23 ± 2.73 | |||||||
| 20 | 8 ± 1.00 | |||||||
| 10 | 3 ± 2.74 | |||||||
| Culex quinquefasciatus | 50 | 63 ± 2.73 | 44.922 | 42.388 | 47.612 | 0.936 | 1.693 | y = −15.3x + 71.1 |
| 40 | 36 ± 2.23 | |||||||
| 30 | 19 ± 2.23 | |||||||
| 20 | 7 ± 2.74 | |||||||
| 10 | 1 ± 1.00 | |||||||
| Aedes aegypti | 50 | 69 ± 4.18 | 39.664 | 36.296 | 43.356 | 0.983 | 5.724 | y = −16.6x + 83 |
| 40 | 48 ± 2.74 | |||||||
| 30 | 33 ± 2.73 | |||||||
| 20 | 12 ± 3.33 | |||||||
| 10 | 4 ± 2.23 | |||||||
Control–nil mortality, a mean value of five replicates, significant at P < 0.05 level, LC50 lethal concentration that kills 50 % of the exposed larvae
UCL upper confidence limit, LCL lower confidence limit, r2 regression co-efficient, χ2 Chi square, df degrees of freedom
The larvicidal efficacy of Cochlioboluslunatus synthesised silver nanoparticles (AgNPs) was checked against larva of A. aegypti and A. stephensi at different concentrations (10, 5, 2.5, 1.25, 0.625 and 0.3125 ppm). The test was determined against second, third, and fourth instar larvae of A. aegypti (LC50-1.29, 1.48 and 1.58; LC90-3.08, 3.33 and 3.41 ppm) and A. stephensi (LC50-1.17, 1.30 and 1.41; LC90-2.99, 3.13 and 3.29 ppm) were observed (Salunkhe et al. 2011). The larvicidal activity of silver nanoparticles against malarial vector, A. subpictus and filariasis vector, C. quinquefasciatus was reported by Jayaseelan et al. (2011). The results suggested that the optimal times for measuring mortality effects of synthesised AgNPs were 33 % at 5 min, 67 % at 15 min, and 100 % after 1 h. The maximum activity was observed in the synthesised silver nanoparticles against lice, A. subpictus and C. quinquefasciatus(LC50 = 12.46, 6.43 and 6.96 mg/L; γ2 = 0.978, 0.773 and 0.828), respectively. The maximum efficacy of AgNPs was observed against the larvae of A. subpictus, C. quinquefasciatus, and Rhipicephalus microplus (LC50 = 13.90, 11.73 and 8.98 mg/L, r2 = 0.411, 0.286 and 0.479), respectively (Marimuthu et al. 2011). Priyadarshini et al. (2012) revealed that the biolarvicidal and pupicidal activity of silver nanoparticles against A. stephensi. Based on their report, the highest larval mortality was found in the synthesised silver nanoparticles against the first to fourth instar larvae and pupae of values LC50 (10.14, 16.82, 21.51 and 27.89 ppm, respectively), LC90 (31.98, 50.38, 60.09 and 69.94 ppm, respectively), and the LC50 and LC90 values of pupae of 34.52 and 79.76 ppm, respectively. The larvicidal activity of silver nanoparticles from plant source against A. aegypti and A. stephensi was also a very successful one (Patil et al. 2012). Based on these reports, the AgNPs synthesised in this present study showed improved toxicity against all the three mosquito vectors. Additionally, based on the available literatures this study is a novel report on the synthesis of AgNPs with larvicidal activity by using bacterial strains, especially in the genus of Streptomyces.
Conclusion
The present study demonstrated that the excellent larvicidal activity of actinobacterially synthesised AgNPs against mosquito vectors. Thus, the AgNPs have potential to be developed a novel larvicidal agents. However, the larvicidal action mode of AgNPs needs further investigations. This study is a newly added source for the synthesis of AgNPs by using actinobacteria in particularly Streptomyces sp. Moreover, these results could be very useful in the research for selecting novel and more selective larvicidal compounds. This report will also lead to the development of a coherent biosynthetic procedure for other metal nanomaterials such as gold and platinum with the actinobacterial strains from various ecosystems against almost all the vectors, which are responsible for transmit many diseases among human civilization.
Acknowledgments
The authors thank the Vice-Chancellor and Registrar of Periyar University, Salem for providing the research facilities. One of the authors, T. Shanmugasundaram (INSPIRE Fellow), wishes to thank the DST, India for financial support.
References
- Ahmad A, Senapathi S, Khan MI, Kumar R, Sastry M. Extracellular biosynthesis of gold nanoparticles by a novel extremophilic actinomycetes, Thermomonospora sp. Langmuir. 2003;19:3550–3553. doi: 10.1021/la026772l. [DOI] [Google Scholar]
- Balagurunathan R, Radhakrishnan M, Babu Rajendran R, Velmurugan D. Biosynthesis of gold nanoparticles by actinomycete Streptomycesviridogens strain HM10. Indian J Biochem Biophys. 2011;48:331–335. [PubMed] [Google Scholar]
- Bhaskara Rao KV, Hemath Naveen KS, Kumar G, Karthik L. Extracellular biosynthesis of silver nanoparticles using the filamentous fungus Penicillium sp. Arch Appl Sci Res. 2010;2:161–167. [Google Scholar]
- Das PK, Pani SP, Krishnamoorthy K. Prospects of elimination of lymphatic filariasis in India. ICMR Bull. 2002;32:41–54. [Google Scholar]
- Du L, Xian L, Feng J-X. Rapid extra/intracellular biosynthesis of gold nanoparticles by the fungus Penicillium sp. J Nanopart Res. 2010;13:921–930. doi: 10.1007/s11051-010-0165-2. [DOI] [Google Scholar]
- Jayaseelan C, Rahuman AA. Acaricidal efficacy of synthesised silver nanoparticles using aqueous leaf extract of Ocimumcanum against Hyalommaanatolicumanatolicum and Hyalommamarginatumisaaci (Acari: Ixodidae) Parasitol Res. 2011;111(3):1369–1378. doi: 10.1007/s00436-011-2559-1. [DOI] [PubMed] [Google Scholar]
- Kovendan K, Murugan K, Vincent S. Evaluation of larvicidal activity of Acalyphaalnifolia Klein ex Wild. (Euphorbiaceae) leaf extract against the malarial vector, Anophelesstephensi, dengue vector, Aedesaegypti and Bancroftian filariasis vector, Culexquinquefasciatus (Diptera: Culicidae) Parasitol Res. 2012;110:571–581. doi: 10.1007/s00436-011-2525-y. [DOI] [PubMed] [Google Scholar]
- Mandal S, Sujatha K, Pasricha R, Arumugam, Sastry M. Silver nanoparticles of variable morphology synthesised in aqueous foams as novel templates. Bull Mater Sci. 2005;28:503–510. doi: 10.1007/BF02711244. [DOI] [Google Scholar]
- Marimuthu S, Rahuman AA, Rajakumar G, Santhoshkumar T, Kirthi AV, Jayaseelan C, Bagavan A, Zahir AA, Elango G, Kamaraj C. Evaluation of green synthesised silver nanoparticles against parasites. Parasitol Res. 2011;108(6):1541–1549. doi: 10.1007/s00436-010-2212-4. [DOI] [PubMed] [Google Scholar]
- Matasyoh JC, Dittrich B, Schueffler A, Laatsch H. Larvicidal activity of metabolites from the endophytic Podospora sp. against the malaria vector Anophelesgambiae. Parasitol Res. 2011;108:561–566. doi: 10.1007/s00436-010-2098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourya DT, Iikal MA, Mishra AC, Jacob PG, Pant U, Ramanujam S, Mavale MS, Bhat HR, Dhanda V. Isolation of Japanese encephalitis virus from mosquitoes collected in Karnataka state, India from 1985 to 1987. Trans R Soc Trop Med Hyg. 1989;83:550–552. doi: 10.1016/0035-9203(89)90288-5. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Ramani R, Parischa R, Kumar PAV, Alam M, Sastry M, Kumar R. Bioreduction of AuCl4-ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew Chem Int Ed. 2001;40:3585–3588. doi: 10.1002/1521-3773(20011001)40:19<3585::AID-ANIE3585>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Patil SV, Patil CD, Salunke BK, Salunkhe RB. Larvicidal efficacy of six plants against two mosquito species Aedesaegypti and Anophelesstephensi. Trop Biomed. 2010;27(3):360–365. [PubMed] [Google Scholar]
- Patil CD, Patil SV, Borase HP, Salunke BK, Salunkhe RB. Larvicidal activity of silver nanoparticles synthesised using Plumeriarubra plant latex against Aedesaegypti and Anophelesstephensi. Parasitol Res. 2012;110:1815–1822. doi: 10.1007/s00436-011-2704-x. [DOI] [PubMed] [Google Scholar]
- Peng Z, Yang J, Wang H, Simons FER. Production and characterisation of monoclonal antibodies to two new mosquito Aedes aegypti salivary proteins. Insect Biochem Mol Biol. 1999;29:909–914. doi: 10.1016/S0965-1748(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Priyadarshini KA, Murugan K, Panneerselvam C, Ponarulselvam S, Hwang J, Nicoletti M. Biolarvicidal and pupicidal potential of silver nanoparticles synthesised using Euphorbiahirta against Anophelesstephensi Liston (Diptera: Culicidae) Parasitol Res. 2012;111(3):997–1006. doi: 10.1007/s00436-012-2924-8. [DOI] [PubMed] [Google Scholar]
- Rahuman RR, Venketesan P. Larvicidal efficacy of five cucurbitaceous plant leaf extracts against mosquito species. Pararsitol Res. 2008;103:133–139. doi: 10.1007/s00436-008-0940-5. [DOI] [PubMed] [Google Scholar]
- Sadhasivam S, Shanmugam P, Yun K. Biosynthesis of silver nanoparticles by Streptomyceshygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf B: Biointerfaces. 2010;81:358–362. doi: 10.1016/j.colsurfb.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Sadhasivam S, Shanmugam P, Veerapandian M, Subbiah R, Yun K. Biogenic synthesis of multidimensional gold nanoparticles assisted by Streptomyceshygroscopicus and its electrochemical and antibacterial properties. Biometals. 2012;25:351–360. doi: 10.1007/s10534-011-9506-6. [DOI] [PubMed] [Google Scholar]
- Salunkhe RB, Patil SV, Patil CD, Salunke BK. Larvicidal potential of silver nanoparticles synthesised using fungus Cochlioboluslunatus against Aedesaegypti (Linnaeus, 1762) and Anophelesstephensi Liston (Diptera; Culicidae) Parasitol Res. 2011;109(3):823–831. doi: 10.1007/s00436-011-2328-1. [DOI] [PubMed] [Google Scholar]
- Sastry M, Ahmad A, Khan MI, Kumar R. Biosynthesis of metal nanoparticles using fungi and actinomycetes. Curr Sci. 2003;85:162–170. [Google Scholar]
- Senapati S, Ahmad A, Khan MI, Sastry M, Kumar R. Extracellular biosynthesis of bimetallic Au–Ag alloy nanoparticles. Small. 2005;1(5):517–520. doi: 10.1002/smll.200400053. [DOI] [PubMed] [Google Scholar]
- Sharma P, Mohan L, Srivastava CN. Amaranthusoleracea and Euphorbiahirta: natural potential larvicidal agents against the urban Indian malaria vector, Anopheles stephensi Liston (Diptera: Culicidae) Parasitol Res. 2009;106:171–176. doi: 10.1007/s00436-009-1644-1. [DOI] [PubMed] [Google Scholar]
- Shirling EB, Gottileb D. Methods for characterisation of Streptomyces sp. Int J Syst Bacteriol. 1966;16:313–340. doi: 10.1099/00207713-16-3-313. [DOI] [Google Scholar]
- Vahabi K, Mansoori GA, Karimi S. Biosynthesis of silver nanoparticles by fungus Trichoderma reesei. Insciences J. 2011;1(1):65–79. doi: 10.5640/insc.010165. [DOI] [Google Scholar]
- WHO (1996) Report of the WHO informal consultation on the evaluation on the testing of insecticides CTD/WHO PES/IC/96.1:69
- WHO-World Health Organisation (2009) Available from: http://www.who.int/mediacentre/factsheets/fs117/en/index.html. Accessed June 2011