Abstract
Microsporidia infections occur in virtually all invertebrate and vertebrate hosts, including humans. The aim of this study is detection of microsporidiosis in various samples of Iranian immunosuppressed patients during 2011–2012 by molecular methods. The samples included stool samples from the healthy participants and samples from biological fluids of the patients according to consult of their physician and the site of infection. The sample size was determined as 258 for each group. Clinical and demographical data related to each participant was collected. DNA extraction and nested polymerase chain reaction were carried out on all the samples. In the control group, the rates of Encephalitozoon and Enterocytozoon infections were 5.3 and 4 % respectively higher in males and in the age range of 30–45, and all positive cases had gastrointestinal symptoms. In the patient group, most infection cases occurred in male patients and in the age range of 60 and above. Patients with microsporidiosis mostly had the symptoms of chronic diarrhea, vomiting, weight loss, dyspepsia, and malabsorption. In BAL samples from patients 2 % Encephalitozoon and 0.7 % Enterocytozoon, in the sampling bone marrow transplantation from patients 5.7 % Encephalitozoon, 1.43 % Enterocytozoon and from patients who underwent kidney transplantation 5.26 % Enterocytozoon were detected. The most cases of human microsporidiosis are associated with human immunodeficiency virus infection or other states of immunosuppression, particularly in organ transplant recipients; the result of this study confirms this claim.
Keywords: Enterocytozoon bieneusi, Encephalitozoon spp., rRNA, BAL
Introduction
Microsporidia is a group of obligatory intracellular parasitic fungi that over the past two decades have risen from obscure organisms to well recognized human pathogens. Several species of microsporidia can cause disease in humans. Intestinal microsporidiosis due to Encephalitozoonintestinalis and Enterocytozoon bieneusi are most frequently reported among immunocompromised people including patients with acquired immune deficiency syndrome (AIDS). With heightened awareness and improved diagnostic methods, microsporidia infections have been recognized in a wide range of human populations including organ transplant recipients, travelers, children, contact lens wearers, and the elderly. In addition, microsporidia species that infect humans have been identified in animals and water sources, raising public health concerns about zoonotic and waterborne transmission of microsporidia Weiss (2001).
The spectrum of diseases includes gastrointestinal, pulmonary, nasal, ocular, muscular, cerebral, and systemic infections. Since 1985, several reports have confirmed that E. bieneusi is associated with persistent diarrhea in human immunodeficiency virus (HIV)-infected patients (Didier 1998; Schwartz et al. 1996). Recently, another microsporidian species has been reported to cause intestinal and extraintestinal infections in patients with AIDS. This organism is designated Encephalitozoon-like microsporidian (Cali et al. 1993; Canning 2001). In contrast to E. bieneusi, E. intestinalis infects not only enterocytes, but also fibroblasts, macrophages, and endothelial cells and has been reported to disseminate to respiratory and urinary tract epithelium. As microsporidia seems to play an important role in causing chronic diarrhea among HIV-infected immunocompromised patients, these patients should be monitored for detection of these organisms in stool and body fluids (Schwartz et al. 1996; Brusseau et al. 2005).
The current study was carried out over a period of 1 year. The aim of this study was molecular detection of microsporidiosis in various samples of Iranian immunocompromised patients. The comprehensive study was not done ever on the basis of epidemiological and incidence in humans in Iran with PCR method for species detection.
Materials and methods
Among the target population, sampling was performed with regard to the patients’ file, duration of the treatment, and instructions of the physician in charge. The sampling time should be at least 3 months after transplantation and initiation of the chemotherapy and administration of immunosuppressive drugs. The suppressive therapy was done with Acyclovir or Valacyclovir, hormone in cancer, Cholestasis after renal transplantation, Bone marrow suppression with Radiation therapy.
We chose patients with the same or similar information. The kind of study was Case/Control therefore healthy people were included as the control. The control group included all people who did not have the history of using any corticosteroid because of any specific diseases, according to the consult of an immunologist. For each participant, the data related to age, gender, and disease symptoms were recorded in a checklist. This study was done from 2010 to 2011.
The sampling was performed by non-probability and accessible sampling. The samples included 258 stool samples from the healthy participants and samples from biological fluids of the patients according to consult of their physician and the site of infection. With regard to the literature review and the approximately 15 % prevalence of the infection for patients with special medical conditions and 3 % prevalence for healthy population, the sample size was determined as 258 for each group.In this study, 150 broncho-alveolar lavage samples from respiratory patients, 70 blood and feces samples from bone marrow transplantation patients and 38 urine samples from kidney transplantation patients were obtained from Shariati and Masih Daneshvari hospital in Tehran. Following screening, all specimens underwent polymerase chain reaction (PCR) amplification for detection of microsporidia. Clinical and demographical data related to each participant was collected by interviewing the participant and the data recorded in the patients’ files. After registering the patients’ data and obtaining written informed consents from the participants, the samples were obtained and transferred to the laboratory under appropriate conditions. The patients identity were not disclosed. All samples with standard conditions and the correct principles of integrity with the easiest method and least painful for patients were prepared. All of patients had received treatment.
DNA extraction processes were carried out on all the samples. DNA of stool samples were extracted by alkaline digestion method as previously described. BAL, blood, and urine samples were extracted by QIAamp DNA Mini Kit Qiagene (Lobo et al. 2006; Sulaiman et al. 2003, 2004).
A nested PCR protocol was used to amplify the ITS region of the rRNA gene as well as the flanking regions of the coding sequences for rRNA of the small (SSU) and large (LSU) subunits for E. bieneusi and Encephalitozoon spp.
In the first PCR, three outer primers (MSP-1, TGAATG(G,T)GTCCCTGT; MSP-2A, TCACTCGCCGCTACT; and MSP-2B, GTTCATTCGCACTACT) were used. The second PCR was run by taking 2 μl of the first PCR product to the mixture containing three inner primers (MSP-3, GGA ATT CAC ACC GCC CGT C(A,G)(C,T) TAT; MSP-4A, CCA AGC TTA TGC TTA AGT (C,T)(A,C)AA(A,G)GGGT; and MSP-4B, CCA AGC TTA TGC TTA AGT CCA GGGAG). Amplification was carried out in 25 μl reactions containing 1× PCR buffer, 2.5 mM MgCl2, 200 μM deoxynucleotide triphosphate mix, 12.5 pmol of each primer, 1 U Taq DNA polymerase (Fermentas). The secondary PCR was performed using 0.5 U Taq. Each reaction set contained a negative control of ultrapure water and a positive control containing template DNA obtained from the cultures. For visualization of the products, amplified products were run on a 1.5 % agarose gel at 80 V for 70 min, then the gel was stained with 2 μg/ml ethidium bromide and viewed under ultraviolet light (Katzwinkel-Wladarsch et al. 1997; Rinder et al. 1997).
Results
The data related to the patients are provided in the following tables. As can be observed, most infection cases occurred in male patients, and considering the age range, the highest infection rate occurred in the age range of 60 and above. Patients with microsporidiosis mostly had the symptoms of chronic diarrhea, vomiting, weight loss, dyspepsia, malabsorption, stomach pain, heartburn, constipation, nausea. To evaluate respiratory microsporidiosis in people with respiratory complications, 150 BAL samples were obtained. The samples were evaluated using molecular techniques and three cases of Encephalitozoon (2 %) and one case of Enterocytozoon (0.7 %) infections were detected. As was mentioned, the sampling was performed from patients hospitalized in the Shariati Hospital to undergo bone marrow transplantation. From 70 patients, 70 whole blood and 70 stool samples were obtained. Using the molecular techniques, among the 70 blood samples, four samples were found to be infected by Encephalitozoon (5.7 %), while from the 70 stool samples, one was infected by Enterocytozoon (1.43 %). Moreover, from 38 patients who underwent kidney transplantation, only urine samples were obtained. Among the urine samples, two were detected to be infected by Enterocytozoon (5.26 %). The prevalence rate in the patient group was 15.09 %, which is provided with regard to gender and age (Fig. 1).
Fig. 1.
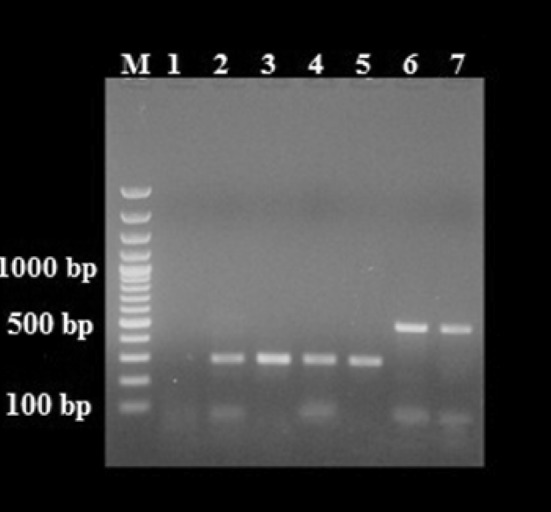
Agarose gel analysis of PCR-amplified products with genus-specific primers diagnostic for Encephalitozoon and Enterocytozoon. M 100 bp DNA ladder, 1 negative control, 2–5 ~300 bp PCR-amplified products isolated from patients with respiratory symptoms, bone marrow transplantation and kidney transplantation, 6–7 ~500 bp PCR-amplified products isolated from patients with respiratory symptoms and bone marrow transplantation
In the control group, the rates of Encephalitozoon and Enterocytozoon infections were 5.3 and 4 %, respectively, and all positive cases had Gastrointestinal (GI) disturbances. In the control group, the rate of infection was higher in males, and the highest rate of infection was observed in the age range of 30-45. The prevalence rate in the control group was 9.33 %, which is provided with regard to gender and age.
Discussion
Microsporidia infections affect all vertebrate and invertebrate hosts. In humans, these tiny single-celled organisms are known as emerging opportunistic pathogens that are present in HIV-positive patients, travellers, transplant recipients, and the elderly with diverse clinical symptoms. The commonest microsporidia in humans are E. bieneusi and then Encephalitozoon spp. Microsporidia have not been widely studied in Iran, and we do not have precise and update statistics on their spread among the control and experiment groups, particularly in our target population.
Diagnosis of the disease in different stages, especially in the acute phase is of great importance. This is of higher importance in the immunocompromised patients. Therefore, employment of an appropriate method that can demonstrate the presence or absence of the disease is necessary. Such method is required in treatment and prevention of the disease. Using different molecular methods such as PCR, the load and type of organism can be determined with higher precision.
We included the control group to be compared with the patients having special diseases to demonstrate the epidemiological status of the infection and prevalence of the infection in both groups. Teachey et al. (2004) reported a case of microsporidia pneumonia in a patient who underwent bone marrow transplantation. The patient was a 21-year-old woman with acute myelocytic leukemia, in whom the infection was confirmed 63 days after the transplantation using the lung biopsy.
Karaman et al. (2008) carried out a study on 320 cancer patients and reported the prevalence of microsporidia infection as 10.9 % using staining of the stool samples.
Jamet et al. (2009) reported the microsporidia infection in a leukemia patient using staining methods for urine and stool samples.
In a study, Angela and Suresh (2007) regarded various factors such as age, gender, ethnicity, disease symptoms, co-infections, and treatment stage (before or after treatment) and performed a study on the stool specimens of 311 cancer patients using modified trichrome staining and PCR. Among the specimens evaluated, microsporidia infection was detected in 68 patients (21.9 %). E. intestinalis and E. hellem were two of the microsporidia species detected.
Chabchoub et al. (2009) carried out a study on 51 patients with AIDS and 35 patients with hematological malignancies and demonstrated that 10.5 % of the patients (nine patients) were infected by microsporidia; six AIDS patients and three patients with hematological malignancy.
In a serological study, Bohumil et al. (2010) searched for the 32-kDa protein and among 115 serum samples studied, detected 20 % infection among the HIV-positive people, 33 % in people with occupational exposure to animals, and 10 % from healthy persons without any specified medical conditions.
Champion et al. (2010) studied 10 kidney transplant recipients, and reported the rate of infection with E. bieneusi in stool of the patients 68 months after the transplantation using molecular diagnostic tests.
Kahler and Thurston-Enriquez (2007) reported detection of human pathogenic microsporidia such as E.intestinalis in livestock fecal and wastewater samples using PCR.
Ghorbanzadeh et al. (2012) demonstrated the sensitivity of PCR evaluation (16 s and rRNA) in comparison with the staining methods in HIV-positive patients. Briefly, different authors have reported microsporidiosis with different rates in immunocompromised and healthy individuals using various methods; particularly precise molecular ones. In the current study, the rate of infection was higher in male patients. Moreover, the highest infection rate was observed in the age group of 60 and above. To evaluate respiratory microsporidiosis in people with respiratory complications, 150 BAL samples were obtained. The samples were evaluated using molecular techniques and three cases of Encephalitozoon (2 %) and one case of Enterocytozoon (0.7 %) infections were detected. As was mentioned, the sampling was performed from patients hospitalized in the Shariati Hospital to undergo bone marrow transplantation. From 70 patients, 70 whole blood and 70 stool samples were obtained. Using the molecular techniques, among the 70 blood samples, four samples were found to be infected by Encephalitozoon (5.7 %), while from the 70 stool samples, one was infected by Enterocytozoon (1.43 %). Moreover, from 38 patients who underwent kidney transplantation, only urine samples were obtained. Among the urine samples, two were detected to be infected by Enterocytozoon (5.26 %). The patients positive for microsporidia mainly had chronic diarrhea, vomiting, weight loss, dyspepsia, and malabsorption. In the control group, the infection rate was higher in males. Moreover, the highest infection rate in the control group was observed in the age group of 30–45. The prevalence rate in the control group was 9.33 %. In the control group, the rates of Encephalitozoon and Enterocytozoon infections were 5.3 and 4 %, respectively, and all positive cases had GI symptoms.
Variation in detection rate may be due to environmental, seasonal, and geographical factors and/or differences in sensitivity of diagnostic technique used in each study. Also, this may be due to lack of experienced examiners, especially as the spores are very minute (Sianongo et al. 2001).
In previous studies, regarding sex, the infection rate was slightly higher in females than males (53.3 vs. 46.7 %). However, the difference was not statistically significant. In some other studies a higher percentage of microsporidiosis patients were male, similar to what we found. In our study, the difference in infection rate between the two genders was statistically significant.
In previous studies, most microsporidia infections were detected among immunocompromised patients above the age of 18. Our results are not consistent with findings of previous studies in this respect. In the present study, most microsporidiosis cases were detected among immunocompromised patients in the age group of above 60 and in the control group, most microsporidiosis cases were in the age range of 30–45. For both the case and control groups, the differences in infection rates of various age groups were statistically significant.
The present study showed that patients with microsporidiosis suffered from clinical gastrointestinal symptoms; chronic diarrhea was the main complaint followed by abdominal pain and anorexia, watery non-bloody diarrhea, and weight loss. This finding is in accordance with those reported in other studies (Procop 2007; Endeshaw and Kebede 2006; Tumwine et al. 2005). The immunocompromised cellular or humoral responses undergo qualitative and/or quantitative alterations and cannot act efficiently against the infections and manifested in the downfall of their overall condition (Nkinin et al. 2007). In contrast, others found no significant relationship between diarrhea and microsporidiosis and it was suggested that most cases of microsporidia infections are asymptomatic (Botero et al. 2003). Unexpectedly, the prevalence of microsporidiosis among the immunocompetent people surveyed was unusually high. Hence, there was no apparent correlation between diarrhea and microsporidiosis. Absence of significant relationship between microsporidiosis and diarrhea might be due to small sample size of the study.
There is only a temporary correlation between detection of microsporidia in stool and gastrointestinal symptoms, and it is suggested that microsporidia infection may cause clinical symptoms during the early stages of infection. However, the role of microsporidia as an enteropathogen is uncertain and requires further investigation (Chacin-bonilla et al. 2006; Kumar et al. 2005).
Moreover, true prevalence of microsporidia has to be determined using highly sensitive techniques such as PCR.
Among the molecular techniques, PCR has shown a high sensitivity, in spite of being costly and time-consuming. PCR is a noninvasive, safe, sensitive, and specific technique and is able to detect a higher number of cases compared to microscopic evaluations, with greater ease of interpretation by non-specialists. Moreover, the technique is capable of determining the microorganism species, which is necessary for complete clinical management and therapy (Wichro et al. 2005; Müller et al. 2001).
It is concluded that microsporidia should be included in screening of opportunistic pathogens and PCR using species–specific primers could become the method of choice for identification of microsporidia in clinical specimens.
Acknowledgments
We would like to thank of Tehran University of Medical Sciences for financial support this work. The authors wish to thank the personnel of Shariati Hospital for their assistance.
Contributor Information
Fatemeh Tabatabaie, Email: dr.f.taba@hotmail.com, Email: fatemeh_tabatabaie@yahoo.com.
Majid Pirestani, Email: majid_pirestani@yahoo.com, Email: pirestani@modares.ac.ir.
References
- Angela R, Suresh K. Microsporidia in stools from cancer patients. Res J Med Sci. 2007;1(2):88–90. [Google Scholar]
- Bohumil S, Zuzana K, Martin K, Dana K, Michael R. Seropositivity for Enterocytozoon bieneusi, Czech Republic and Evan W. Secor Emerg Infect. 2010;16(2):35–337. doi: 10.3201/eid1602.090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botero J, Castano A, Montoya M, Ocampo N, Hurtado M, Lopera MA. Preliminary study of the prevalence of intestinal parasites in immunocompromised patients with and without gastrointestinal manifestations. Rev Inst Med Trop Sao Paulo. 2003;45:197–200. doi: 10.1590/S0036-46652003000400004. [DOI] [PubMed] [Google Scholar]
- Brusseau ML, Oleen JK, Santamaria J, Cheng L, Orosz-Coghlan P, Chetochine AS, et al. Transport of microsporidium Encephalitozoon intestinales spores in sandy porous media. Water Res. 2005;39:3636–3642. doi: 10.1016/j.watres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Cali A, Kotler DP, Orenstein JM. Septata intestinalis N. G., N. Sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J Eukaryot Microbiol. 1993;40(1):101–112. doi: 10.1111/j.1550-7408.1993.tb04889.x. [DOI] [PubMed] [Google Scholar]
- Canning EU. Microsporidia. In: Gillespie S, Pearson RD, editors. Principles and practice of clinical parasitology. Virginia: Wiley; 2001. pp. 171–204. [Google Scholar]
- Chabchoub N, Abdelmalek R, Mellouli F, Kanoun F, Thellier M, Bouratbine M, et al. Genetic Identification of intestinal microsporidia species in immunocompromised patients in Tunisia. Am J Trop Med Hyg. 2009;80(1):24–27. [PubMed] [Google Scholar]
- Chacin-bonilla L, Panunzio A, Castillo F, Cepeda I, Martinez R. Microsporidiosis in venezuela: prevalence of intestinal microsporidiosis and its contribution to diarrhea in a group of human immunodeficiency virus–infected patients from zulia state. Am J Trop Med Hyg. 2006;74(3):482–486. [PubMed] [Google Scholar]
- Champion L, Durrbach A, Lang P, Delahousse M, Chauvet C, Sarfati C and et al (2010) Fumagillin for treatment of intestinal microsporidiosis in renal transplant recipients. Am J Transpl 10(8):1925–1930 [DOI] [PubMed]
- Didier ES. Microsporidiosis. Clin Infect Dis. 1998;27:1–7. doi: 10.1086/514607. [DOI] [PubMed] [Google Scholar]
- Endeshaw T, Kebede A, Verweij J, Zewide A, Tsige K, Abraham Y, Wolday D, Woldeichael T, Messele T, Polderman A, Petros B. Intestinal microsporidiosis in diarrheal patients infected with human immunodeficiency virus-1 in Addis Ababa, Ethiopia. Jpn J Infect Dis. 2006;59:306–310. [PubMed] [Google Scholar]
- Ghorbanzadeh, et al. Diagnosis of cryptosporidium and intestinal microsporidia in HIV/AIDS patients with staining and PCR methods on 16srRNA gen. Arak Med Univ J (AMUJ) 2012;15(66):37–47. [Google Scholar]
- Jamet D, Quinio D, Moalic E, Ianotto JC, Dalbies F, Guillerm G et al (2009) Systemic microsporidiosis and toxoplasmosis in a patient with T prolymphocytic leukemia. Médecine et Maladies Infectieuses 39(6):406–408 [DOI] [PubMed]
- Kahler AM, Thurston-Enriquez JA. Human pathogenic microsporidia detection in agricultural samples: method development and assessment. Parasitol Res. 2007;100:529–538. doi: 10.1007/s00436-006-0300-2. [DOI] [PubMed] [Google Scholar]
- Karaman U, Atambay M, Daldal N, Çolak C. The prevalence of Microsporidium among patients given a diagnosis of cancer. Türkiye Parazitol Derg. 2008;32(2):109–112. [PubMed] [Google Scholar]
- Katzwinkel-Wladarsch S, Deplazes P, Weber R, Loscher T, Rinder H. Comparison of polymerase chain reaction with light microscopy for detection of microsporidia in clinical specimens. Eur J Clin Microbiol Infect Dis. 1997;16(1):7–10. doi: 10.1007/BF01575111. [DOI] [PubMed] [Google Scholar]
- Kumar S, Ananthan S, Joyee A. Detection of Enterocytozoon bieneusi (Microsporidia) by polymerase chain reaction (PCR) using species-specific primer in stool samples of HIV patients. Indian J Med Re. 2005;121:215–219. [PubMed] [Google Scholar]
- Lobo ML, Xiao L, Cama V, Stevens T, Antunes F, Matos O. Genotypes of Enterocytozoon bieneusi in mammals in Portugal. J Eukaryot Microbiol. 2006;53(1):61–64. doi: 10.1111/j.1550-7408.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Müller A, Bialek R, Kämper A, Tkenheuer G, Salzberger B, Franzen C. Detection of microsporidia in travelers with diarrhea. J Clin Microbiol. 2001;39(4):1630–1632. doi: 10.1128/JCM.39.4.1630-1632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkinin S, Asonganyi T, Didier E, Kaneshiro E. Microsporidian infection is prevalent in healthy people in Cameroon. J Clin Microbiol. 2007;45(9):2841–2846. doi: 10.1128/JCM.00328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procop G. Molecular diagnostics for the detection and characterization of microbial pathogens. Clin Infect Dis. 2007;45(2):99–111. doi: 10.1086/519259. [DOI] [PubMed] [Google Scholar]
- Rinder H, Katzwinkel-Wladarsch S, Loscher T. Evidence for the existence of genetically distinct strains of Enterocytozoon bieneusi. Parasitol Res. 1997;83(7):670–672. doi: 10.1007/s004360050317. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Sobottka I, Leitch GJ, Cali A, Visvesvara GS. Pathology of microsporidiosis: emerging parasitic infections in patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1996;120(2):173–188. [PubMed] [Google Scholar]
- Sianongo S, McDonald V, Kelly P. A method for diagnosis of microsporidiosis adapted for use in developing countries. Trans R Soc Trop Med Hyg. 2001;95(6):605–607. doi: 10.1016/S0035-9203(01)90093-8. [DOI] [PubMed] [Google Scholar]
- Sulaiman IM, Fayer R, Lal AA, Trout JM, Schaefer FW, 3rd, Xiao L. Molecular characterization of microsporidia indicates that wild mammals Harbor host-adapted Enterocytozoon spp. as well as human-pathogenic Enterocytozoon bieneusi. Appl Environ Microbiol. 2003;69(8):4495–4501. doi: 10.1128/AEM.69.8.4495-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman IM, Fayer R, Yang C, Santin M, Matos O, Xiao L. Molecular characterization of Enterocytozoon bieneusi in cattle indicates that only some isolates have zoonotic potential. Parasitol Res. 2004;92(4):328–334. doi: 10.1007/s00436-003-1049-5. [DOI] [PubMed] [Google Scholar]
- Teachey D, Russo P, Orenstein J, Didier E, Bowers C, Bunin N. Pulmonary infection with microsporidia after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33:299–302. doi: 10.1038/sj.bmt.1704327. [DOI] [PubMed] [Google Scholar]
- Tumwine J, Kekitiinwa A, Bakeera-Kitaka S, Ndeezi G, Downing R, Feng X, Akiyoshi D, Tzipori S. Cryptosporidiosis and microsporidiosis in Ugandan children with persistent diarrhea with and without concurren infection with the human immunodeficiency virus. Am J Trop Med Hyg. 2005;73:921–925. [PubMed] [Google Scholar]
- Weiss LM. Microsporidia: emerging pathogenic protists. Acta Trop. 2001;78:89–102. doi: 10.1016/S0001-706X(00)00178-9. [DOI] [PubMed] [Google Scholar]
- Wichro E, HoelzL D, Krause R, Bertha G, Reinthaler F, Wenisch c. Microsporidiosis in travel-associated chronic diarrhea in immune-competent patients. Am J Trop Med Hyg. 2005;73(2):285–287. [PubMed] [Google Scholar]


