Abstract
Beauveria bassiana HQ917687 virulence to housefly larvae and adult was assessed at different relative humidity, RH (50, 75, 90, and 100 %) and temperature (15, 20, 25, 30, 35, 40, 45 °C) conditions at the fungal dose of 108 conidia/ml. Depending on the temperature and RH regime tested, difference in mortality rates of housefly adult and larvae were detected. During assay on adult housefly, 100 % mortality was achieved at RH, 90 and 100 % while the temperature of 30 °C showed maximum mortality at all the tested humidity conditions. Lethal time, LT50 was 2.9 days at 100 % RH. Larval mortality at different humidity conditions varied between 30 and 74 %, with maximum mortality at 100 % RH and 30 °C. Optimum temperature for B. bassiana virulence to housefly larvae was also found to be 30 °C. The interaction between temperature and RH revealed significant effect of RH at moderate temperature range (20–35 °C), while such an interaction was not observed at extreme temperatures. The results obtained in this study have useful implications in understanding the pathogen behavior under actual field conditions. This in turn may help devising suitable entomopathogen release schedules for maximum fungal infection.
Keywords: Beauveria bassiana, Musca domestica, Temperature, Humidity, Virulence
Introduction
Biocontrol strategies based on entomopathogenic fungi are not only dependent upon interaction between host and pathogen, but also on the environment to which they are exposed. Variety of factors, including, temperature, relative humidity (RH), light, air, nutrient availability and host physiological status influences fungal pathogenicity (Padmini and Padmaja 2010). Of the various factors, temperature and humidity are the most important environmental factors affecting survival and effectivity of entomopathogenic fungi (Bugeme et al. 2008). Temperature significantly affects growth, germination, survival and virulence of the pathogens (Kiewnick 2006; Bugeme et al. 2008). Six and Mullens (1996) observed seasonal variation in E. muscae infection in housefly while Carswell et al. (1998) reported temperature susceptibility of adult house flies and fruit flies to infection by Metarhiziumanisopliae. Humidity affects rate of pathogenecity, by it being essential for fungal germination, infection and subsequent sporulation on insect cadavers (Luz and Fargues 1997; Devi et al. 2005). While Fargues and Luz (2000) reported requirement of high humidity for the optimal Beauveria infection, others studies (Barson et al. 1994; Devi et al. 2005) indicated RH to be subservient factor, with fungal infectivity being majorly governed by microclimate of insect cuticle.
Although the significance of temperature and RH to entomopathogenic fungi pathogenecity is recognized, their sensitivity to environmental factors varies with isolate/strain specificity (Bugeme et al. 2008). Hence, it is important to establish the effect of temperature and RH on the virulence of any new isolates, before it being considered for insect control and formulation design. Mechanism through which entomopathogenic fungi acts on host insect could be divided into three parts: adhesion of the fungal spore, penetration through the cuticle, and establishment within the host (Mishra 2013). Attachment of conidia to insect’s epicuticle is accompanied by electrostatic and hydrophobic interactions. Subsequent to the attachment, germination of fungal conidia occurs subjected to the availability of favorable temperature, humidity and nutrients on insect cuticle. Cuticle penetration of the germinating conidia is mediated by mechanical and enzymatic degradation, with later one being highly sensitive to temperature, RH and pH conditions (Goettel et al. 1989). After penetration, fungus grows in the host haemolymph, taking up nutrients, producing toxins, destroying host cells and eventually killing the insect (Anderson et al. 2011). The defense mechanism of the insect is based on innate components consisting of cellular and humoral factors. The ability of entomopathogenic fungi to cause disease in host insects, without the requirement of it being ingested through food, adds to attraction in their usage for control application.
House fly, Musca domestica L. (Diptera: Muscidae) is a major domestic, medical, and veterinary pest that causes irritation, spoils food, and acts as a vector of many medical and veterinary pathogenic organisms (Forester et al. 2009; Mishra et al. 2011). Control of houseflies through entomopathogenic fungi is well reported in the literature (Barson et al. 1994; Watson et al. 1995; Carswell et al. 1998; Mishra et al. 2011); however, very few of these studies explored the effect of temperature (Mullens 1990; Watson et al. 1993) and humidity on virulence potential of entomopathogenic fungi for housefly control. A B. bassiana strain previously found to be highly effective against housefly adults and larvae (Mishra and Malik 2012) was used in the present study. The objective of the present study was to evaluate the impact of temperature and RH on the virulence of this native isolate so as to maximize its pathogenecity potential by possible control of experimental environment and suitable modification in design for formulations.
Materials and methods
Musca domestica
All the bioassays were performed with lab reared flies obtained by the earlier described method (Mishra et al. 2011). Field collected flies were placed done in a cylindrical boxes (90 × 140 mm2), pasted with diet mixture (groundnut oil cake:wheat bran :: 1:3), and incubated at 28 ± 5 °C, 65 % RH. Hatched larvae were transferred individually to cylindrical vials (28 × 12 mm2) containing semi-synthetic diet (constituents: 2 g groundnut oil cake, 5 g wheat bran, 2 g milk powder, 1 g honey mixed with 10 ml of water), which was changed daily until pupal stage. Pupae were transferred to a netted chamber (20 × 20 × 20 cm3) and adult flies emerged were fed on diet mixture of groundnut oil cake and wheat bran (1:3). About 2–3 days old flies were used for the bioassays. For the larvicidal assay, second instar larvae were used.
Fungal culture
Beauveria bassiana HQ917687 used in the present study was isolated in our laboratory from soil samples collected from different region of North India using Galleria bait method (Zimmermann 1986). Fungal isolate was characterized for its morpho-biological traits and identified using 18s-rRNA (Mishra 2013). The fungal isolate was maintained on Potato Dextrose Agar slants at 4 °C. Spore suspensions were prepared by harvesting spore in 10 ml of distilled water containing 0.1 % sterile Tween 80, followed by vortexing for 5 min and filtration through sterilized membrane filter (8 μm) disk (Mishra et al. 2011). The filtered spore suspension was enumerated and checked for viability using an Automatic Cell Counter (Cellometer® Vision HSL, Nexcelom Bioscience). For determination of conidial viability, conidia were stained with Trypan blue. For each bioassay, freshly prepared spore suspensions were used.
Effects of temperature and humidity on Beauveria bassiana virulence
The effects of temperature and RH on B. bassiana virulence to adult and larvae of housefly were assessed through a series of bioassays done at four different RH (50, 75, 90, and 100 %) and seven different temperature (15, 20, 25, 30, 35, 40, and 45 °C). The experimental set-ups included all combinations of RH and temperature.
For the bioassay against adult houseflies, ten flies were placed on a filter paper (Whatmann No.1), in petri plate (dia.−150 mm, area = 17,662.5 mm2) along with diet. Diet was uniformly sprayed with 1 ml of B. bassiana spore suspensions (~2.3 × 108 conidia/ml) and incubated at designated temperature and RH. Deposition rate of spores from application of conidial suspension amounted to (108 spores/17,662.5 mm2) ≈ 5,662 spores/mm2. For each treatment, four replications were incorporated. Control was sprayed with 1 ml of sterile distilled water containing 0.1 % Tween 80. Dead flies were counted daily during 5 days of monitoring. Whole experimental setup was repeated twice.
Larvicidal bioassay was performed with ten housefly larvae. Larvae were taken on petri plates covered with filter paper along with larval diet which were uniformly sprayed with 1 ml of B. bassiana spore suspensions (~2.3 × 108 conidia/ml). Controlled treatment sprayed with 1 ml of sterile distilled water containing 0.1 % Tween 80, was also incorporated. All the petri plates were incubated at designated temperature and RH. Larvae were monitored daily during 6 days of monitoring and mortality was adjudged by larval wasting and immobility.
Statistical analysis
Mortality data were corrected for control mortality using Abbott’s (1925) formula and normalized using arcsine transformation before being subjected to a two-way factorial analysis of variance using StatPlus (2007). The means were separated using LSD and differences between them were considered significant at P < 0.05. LT50 values were determined for each replicate using the probit analysis method for correlation data using Statistical Package for Social Sciences Inc. 17.5 (2008).
Results
Temperature and humidity effect on B. bassiana virulence to housefly adults
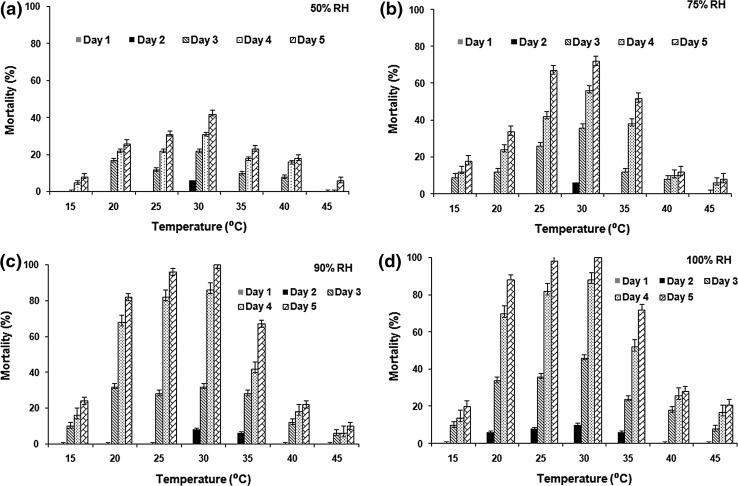
Beauveria bassiana infectivity to housefly adults at varying temperature and relative humidity condition in different experimental repeats was found to be insignificantly different (P > 0.05). Effect of different temperature and relative humidity on B. bassiana virulence to adult housefly is represented in Fig. 1. Mortality of adult flies varied significantly between different temperatures at the relative humidity of 50 % (F = 6.47; df = 6, 24; P < 0.001), 75 % (F = 5.05; df = 6, 24; P < 0.01), 90 % (F = 4.32; df = 6, 24; P < 0.01) and 100 % (F = 5.48; df = 6, 24; P < 0.001). The mortality of adult housefly was highest at 30 °C at all the humidity variations, suggesting it to be optimum for B. bassiana virulence.
Fig. 1.
Mortality (mean ± SD) of adult houseflies by B. bassiana (2.3 × 108 conidia/ml) at different temperatures a at 50 % RH, b at 75 % RH, c at 90 % RH, and d at 100 % RH
The relative humidity of 50 % showed less than 50 % mortality of adult flies at all the tested temperatures (Fig. 1a). At 75 % RH, maximum housefly adult mortality was 72 % (Fig. 1b), which increased to 100 % mortality at 90 % (Fig. 1c) and 100 % RH (Fig. 1d), achieved at the temperature of 30 °C. Also, LT50 (lethal time to kill 50 % of adult flies) was lowest at 30 °C, for all the RH (Table 1), implying rapid kill activity at this temperature. Considering LT50 as indicator of B. bassiana virulence, lowest LT50 of 2.9 days was observed at 100 % RH, however, at lower temperature (15 °C), 90 % RH was found to be more effective (LT50-6.2 days) for B. bassiana virulence.
Table 1.
Chi square and LT50 values for B. bassiana (108 conidia/ml) in adult housefly at different humidity for three temperature variations (minimum, optimum, maximum)
| Temperature | Humidity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 75 | 90 | 100 | |||||||||
| LT50 (in days) | 95 % CI | χ 2 | LT50 (in days) | 95 % CI | χ 2 | LT50 (in days) | 95 % CI | χ 2 | LT50 (in days) | 95 % CI | χ 2 | |
| 15 | 7.3 | 6.2–11.5 | 2.62 | 6.9 | 5.3–42.0 | 6.93 | 6.2 | 5.0–14.1 | 6.69 | 6.6 | 5.1–35.3 | 7.70 |
| 30 | 5.5 | 4.9–6.7 | 7.22 | 3.7 | 3.5–3.9 | 10.66 | 3.1 | 2.5–3.6 | 4.62 | 2.9 | 2.8–3.1 | 1.44 |
| 45 | 6.2 | – | 0.33 | 7.3 | 6.2–11.4 | 3.80 | 6.7 | 5.9–8.2 | 4.75 | 6.4 | 5.1–15.8 | 6.46 |
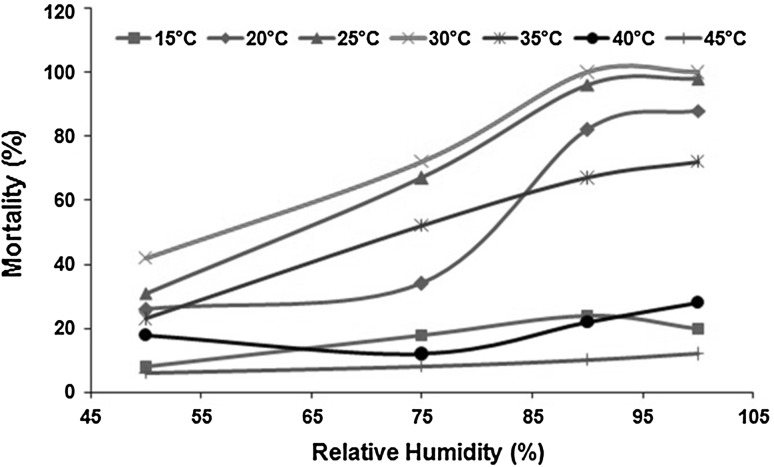
Interaction between temperature and humidity on adult housefly mortality is represented in Fig. 2. At 15 °C, linear increase in adult mortality was observed with increase in RH till 90 %, with linear decrease in mortality with further increase in value of RH. At 20 °C, adult mortality showed a little increase (≈X1/3) with increase in value of RH (till 75 %). Afterwards, RH played a critical role, and a cubic relation for increase in mortality (≈X3) was observed with increase in RH value. The temperature of 25 and 30 °C, showed an almost similar relationship for adult fly mortality with increase in RH, revealing linear increase in mortality data till 90 % RH, while becoming stable for any change in RH afterward. At 35 °C, a continuous linear increase in adult mortality was observed with increase in RH. Mortality data at 40 °C showed deviation in comparison to other temperatures, where an initial decrease (linear) in mortality was observed with increase in RH till 75 %, where after a linear increase in fly mortality was observed with increase in RH value. At 45 °C, no effect on adult housefly mortality was observed with change in humidity. The interaction trend reveals significant effect of RH at moderate temperature range (20–35 °C), whereas at extremes, the effect of RH gets nullified with prevailing effect of temperature.
Fig. 2.
Interaction between temperature and percentage relative humidity on adult housefly mortality
Temperature and humidity effect on B. bassiana virulence to housefly larvae
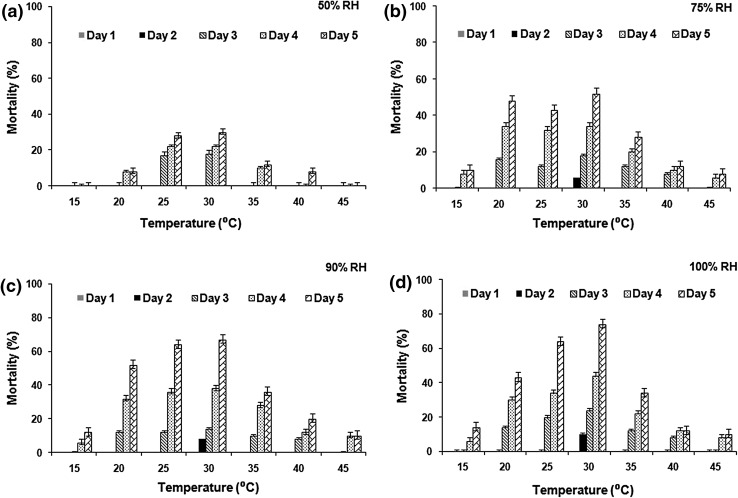
Figure 3 represents larval mortality due to B. bassiana application at different temperature and humidity. Highly significant value was observed for the mortality of housefly larvae between different temperature at relative humidity of 50 % (F = 5.16; df = 6, 24; P < 0.01), 75 % (F = 4.72; df = 6, 24; P < 0.01), 90 % (F = 3.99; df = 6, 24; P < 0.01) and 100 % (F = 5.35; df = 6, 24; P < 0.01).
Fig. 3.
Housefly larval mortality (mean ± SD) by B. bassiana (2.3 × 108 conidia/ml) at different temperatures a at 50 % RH, b at 75 % RH, c at 90 % RH, and d at 100 % RH
Temperature of 30 °C was observed to be optimum for B. bassiana virulence, at all the humidity. Lethal time, LT50 varied between 4.4 and 6.8 days for 90 % RH while for 100 % RH, it was 4.0–6.7 days (Table 2). Time of kill indicated 90 and 100 % humidity variation as the most effective for B. bassiana pathogenecity to housefly larvae. At 50 % RH, maximum mortality observed was 30 % (Fig. 3a), which increased to 52 % at 75 % RH (Fig. 3b). Relative humidity of 100 % showed maximum larval mortality at 30 °C (74 %), followed by that at 35 °C (72 %), at the same humidity (Fig. 3d). Effect of B. bassiana towards housefly larvae mortality was insignificant at different temperature and relative humidity condition for different experimental repeats (P > 0.05).
Table 2.
Chi square and LT50 values for B. bassiana (108 conidia/ml) in housefly larvae at different humidity for three temperature variations (minimum, optimum, maximum)
| Temperature | Humidity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 75 | 90 | 100 | |||||||||
| LT50 (in days) | 95 % CI | χ 2 | LT50 (in days) | 95 % CI | χ 2 | LT50 (in days) | 95 % CI | χ 2 | LT50 (in days) | 95 % CI | χ 2 | |
| 15 | – | – | – | 7.0 | 5.34 | 6.6 | 5.9–8.9 | 2.36 | 6.4 | 5.7–8.2 | 1.91 | |
| 30 | 6.5 | – | 15.32 | 4.9 | 4.5–5.6 | 2.55 | 4.4 | 3.7–6.2 | 3.71 | 4.0 | 3.7–4.3 | 3.26 |
| 45 | – | – | – | 7.3 | 6.2–11.4 | 3.80 | 6.8 | – | 6.88 | 6.7 | 5.9–8.9 | 4.38 |
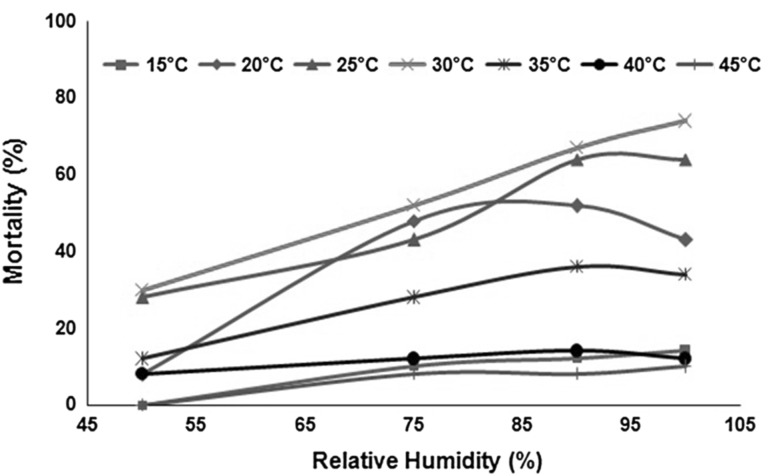
Interaction between temperature and humidity on housefly larvae mortality is shown in Fig. 4. At the temperature of 15 °C, larvae mortality varied linearly (with a small slope) till 75 % RH, and became stable for further increase in value of RH. Variation in larvae mortality followed quadratic relationship with increase in RH at 20 °C. The mortality increased quadratically till 80 % RH, whereas decreased quadratically with further increase in RH. At 25 °C, mortality of larvae increases linearly till 75 % RH, while with further increase in RH, mortality varies polynomially. At 30 °C, a continuous linear increase in larval mortality with increase in RH was observed. The temperature of 35 °C showed a linear increase in larval mortality till 90 % RH, showing a stable mortality data for further increase in RH at the temperature. At 40 °C, no effect of RH was observed on percentage larval mortality while negligible effect of RH was observed on mortality at 45 °C. Interaction data between temperature and RH for housefly larvae mortality again showed RH as having prominent effect at moderate temperature range (20–35 °C). At temperature extremes, temperature showed prevailing effect.
Fig. 4.
Interaction between temperature and percentage relative humidity on housefly larvae mortality
Discussion
Environmental factors, such as temperature and humidity not only affect survival and virulence of entomopathogenic fungi, but also influence their host–pathogen interaction (Kiewnick 2006). Moreover, strain variation in fungi influences optimal temperature and humidity requirement for survival as well as virulence (Fargues et al. 1997). Carswell et al. (1998) studied the effect of temperature on susceptibility of adult houseflies and fruit flies to an isolate of M.anisopliae and reported higher rate of mortality at 25 and 30 °C (100 % mortality in 7–9 days) as compared to that at 20 °C which showed only 50 % of mortality. Also, they reported disease development to be slow at lower temperatures. The present study showed 30 °C as the optimum temperature for B. bassiana infection to adults and larvae of housefly, which corroborated the earlier findings. Comparatively good mortality was also observed at 25 and 35 °C which could be explained by wide adaptability of B. bassiana of different origins owning to its cosmopolitan and facultative nature (Fargues et al. 1997).
Higher thermal tolerance of the native isolate obtained in this study, is also supported by results of Vidal et al. (1997), where entomopathogenic fungus isolates from Indian Subcontinent was found to be better temperature tolerant. Although, the effect of external temperature on pathogenecity of native fungal isolates is confirmed in the present study, the factor of host thermoregulation, which may have influence on its infectivity, need not be overlooked. Host thermoregulation in response to pathogen invasion have been shown to reduce entomopathogenic fungi infectivity in grasshoppers (Inglis et al. 1996), locusts (Ouedraogo et al. 2003), and crickets (Adamo 1998). Although no such response was observed in mosquito, Anopheles stephensi (Blanford et al. 2009), a behavioral fever in housefly was reported in response to Entomophthoramuscae infection (Mullens 1990; Watson et al. 1993). Watson et al. (1993) reported decrease/elimination of E.muscae infection in housefly, when exposed to the higher temperature during initial incubation period. The above result was similar to that obtained in the present study, where drastic reduction in adult and larval mortality was observed at 40 and 45 °C. Moreover, thermoregulation reduced insect mortality but did not completely eliminate the fungus from infected hosts and the fungus grew and killed the insects when thermoregulation was interrupted (Ouedraogo et al. 2004).
Humidity requirement by fungus for infection is complicated and literature is replete with contradictory findings. High ambient humidity was reported to be essential for entomopathogenic fungi infection and subsequent sporulation on insect cadavers (Luz and Fargues 1997; Sivasankaran et al. 1998) while contrary reports indicate that infection from an initial conidial application is unaffected by low ambient relative humidity when the microclimate on the insect cuticle is suitable for infection (Barson et al. 1994). Fargues and Luz (2000) reported dependence of infection potential of Beauveria upon humidity as well as time of exposure to favourable humidity, i.e. longer the time of exposure for favorable humidity, higher was the infection. They also reported humidity to be deciding factor for infection over temperature as high infection was observed for even an unfavourable temperature condition.
In the present study, comparatively lesser mortality was obtained for housefly larvae compared to adults, at all the temperature and humidity variation. The phenomenon could be explained by the physiological state of the host including insects’ cuticle properties (McCoy et al. 1988). Although the mechanism by which physiological state influences entomopathogenic fungi infectivity is uncertain, low prevalence of entomopathogenic fungi has been reported in larvae, nymphs and males of ticks, compared to female ticks (McCoy et al. 1988). The preference of entomopathogenic fungi may be correlated with inherent cuticle hydrocarbons associated with different life stages of insects.
From the results of present study, the optimum temperature and RH for field evaluation of the entomopathogen in Indian climatic conditions are 30–35 °C and 75–100 %, respectively. As revealed by this study, availability of suitable RH (≈90 %) conditions can tremendously influence the pathogenecity in summer when temperature is between 35 and 40 °C. Hence, efforts can be directed towards such formulations (e.g. spray formulation) that may help create suitable microclimate by lowering the temperature and enhancing the RH. Moreover, it is desirable that the strain chosen for commercialization should have the ability to control the fly menace when the populations are high. Life cycle of housefly varies between 12 and 42 days, depending upon temperature and humidity conditions. Optimum temperature for housefly reproduction and propagation is between 30 and 37 °C (USDA 1976) which corresponds with the optimum temperature for B. bassiana pathogenecity obtained in the above study. Although, optimum RH for B. bassiana pathogenecity is at ≈90 %, good activity of fungus was also observed at 75 % RH. In this connection the optimum RH for housefly development is 65–75 %. In view of the above, it is anticipated that the strain investigated in the present study would display reasonably good performance when the housefly densities in the environment are at the peak. Moreover, B. bassiana investigated in this study, occur naturally in soil and insects, apparently with no side effect to human health and vegetation. The fungus rarely infects humans or other animals, and is thus considered safe as an insecticide. However, one study by Tucker et al. (2004) reported disseminated B. bassiana infection in a patient with acute lymphoblastic leukemia.
Conclusions
The present study revealed optimal conditions for virulence of new isolate of B. bassiana against housefly. The temperature was found to be significant factor affecting housefly mortality, whereas relative humidity played prominent role in regulating the mortality at moderate temperature range. The result suggests effective housefly control over wide temperature range in favourable humidity conditions. However, further work is required to ascertain the critical thresholds of RH beyond which the fungus is ineffective. Also, field evaluation of B. bassiana in Indian climatic conditions is warranted to establish its effectivity in temperature and RH condition favourable for housefly growth and dissemination.
Acknowledgments
This work was partially supported by Indian Council of Medical Research, India. CSIR fellowship to one of the authors (SM) is gratefully acknowledged. The authors acknowledge Mr. Sudheer and Mr. Satendar Singh (both IIT Delhi, India) for their help in statistical analysis and support in experimental work, respectively.
References
- Abbott WS. A method for computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- Adamo SA. The specificity of behavioral fever in the cricket Acheta domesticus. J Parasitol. 1998;84:529–533. doi: 10.2307/3284717. [DOI] [PubMed] [Google Scholar]
- Anderson RD, Bell AS, Blanfor S, Paaijmans KP, Thomas MB. Comparative growth kinetics and virulence of four different isolates of entomopathogenic fungi in the housefly (Musca domestica L.) J Invertebr Pathol. 2011;107:179–184. doi: 10.1016/j.jip.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Barson G, Renn N, Bywater AF. Laboratory evaluation of six species of entomopathogenic fungi for the control of house fly (Musca domestica L.), a pest of intensive animal units. J Invertebr Pathol. 1994;64:107–113. doi: 10.1006/jipa.1994.1078. [DOI] [PubMed] [Google Scholar]
- Blanford S, Read AF, Thomas MB. Thermal behaviour of Anopheles stephensi in response to infection with malaria and fungal entomopathogens. Malar J. 2009;8:1–9. doi: 10.1186/1475-2875-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugeme DM, Maniania NK, Knapp M, Boga HI. Effect of temperature on virulence of Beauveria bassiana and Metarhizium anisopliae isolates to Tetranychus evansi. Exp Appl Acarol. 2008;46:275–285. doi: 10.1007/s10493-008-9179-1. [DOI] [PubMed] [Google Scholar]
- Carswell I, Spooner-Hart R, Milner RJ. Laboratory susceptibility of Musca domestica L. (Diptera: Muscidae) and Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) to an isolate of Metarhizium anisopliae (Metsch.) Sorokin. Aust J Entomol. 1998;37:281–284. doi: 10.1111/j.1440-6055.1998.tb01584.x. [DOI] [Google Scholar]
- Devi KU, Sridevi V, Mohan CM, Padmavathi J. Effect of high temperature and water stress on in vitro germination and growth in isolates of the entomopathogenic fungus Beauveria bassiana (Bals.) Vuillemin. J Invertebr Pathol. 2005;88:181–189. doi: 10.1016/j.jip.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Fargues J, Luz C. Effects of fluctuating moisture and temperature regimes on the infection potential of Beauveria bassiana for Rhodnius prolixus. J Invertebr Pathol. 2000;75:202–211. doi: 10.1006/jipa.1999.4923. [DOI] [PubMed] [Google Scholar]
- Fargues J, Goettel MS, Smits N, Ouedraogo A, Rougier M. Effect of temperature on vegetative growth of Beauveria bassiana isolates from different origins. Mycologia. 1997;89:383–392. doi: 10.2307/3761032. [DOI] [Google Scholar]
- Forester M, Klimpel S, Sievert K. The house fly (Musca domestica) as a potential vector of metazoan parasites caught in a pig-pen in Germany. Vet Parasitol. 2009;160:163–167. doi: 10.1016/j.vetpar.2008.10.087. [DOI] [PubMed] [Google Scholar]
- Goettel MS, St Leger RJ, Rizzo NW, Staples RC, Roberts DW. Ultrastructural localization of a cuticle-degrading protease produced by the entomopathogenic fungus Metarhizium anisopliae during penetration of host (Manduca sexta) cuticle. J Gen Microbiol. 1989;135:2233–2239. [Google Scholar]
- Inglis DG, Johnson DL, Goettel MS. Effects of temperature and thermoregulation on mycosis by Beauveria bassiana in grasshoppers. Biol Control. 1996;7:131–139. doi: 10.1006/bcon.1996.0076. [DOI] [Google Scholar]
- Kiewnick S. Effect of temperature on growth, germination, germ-tube extension and survival of Paecilomyces lilacinus strain 251. Biocontrol Sci Technol. 2006;16:535–546. doi: 10.1080/09583150500532766. [DOI] [Google Scholar]
- Luz C, Fargues J. Temperature and moisture requirements for conidial germination of an isolate of Beauveria bassiana, pathogenic to Rhodnius prolixus. Mycopathologia. 1997;138:117–125. doi: 10.1023/A:1006803812504. [DOI] [PubMed] [Google Scholar]
- McCoy CW, Samson RA, Boucias DG. Entomogenous fungi. In: Ignoffo CM, editor. CRC handbook of natural pesticides. Microbial insecticides, part A: entomogenous protozoa and fungi. Boca Raton: CRC Press; 1988. pp. 151–236. [Google Scholar]
- Mishra S (2013) Development of fungal formulations for house fly control. Ph D thesis, Indian Institute of Technology Delhi
- Mishra S, Malik A. Nutritional optimization of a native Beauveria bassiana isolate (HQ917687) pathogenic to housefly, Musca domestica L. J Parasitic Dis. 2012 doi: 10.1007/s12639-012-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Kumar P, Malik A, Satya S. Adulticidal and larvicidal activity of Beauveriabassiana and Metarhizium anisopliae against housefly, Musca domestica (Diptera: Muscidae), in laboratory and simulated field bioassays. Parasitol Res. 2011;108:1483–1492. doi: 10.1007/s00436-010-2203-5. [DOI] [PubMed] [Google Scholar]
- Mullens BA. Entomophthora muscae (Entomophthorales: Entomophthoraceae) as pathogen of filth flies. In: Rutz DA, Patterson RS, editors. Biocontrol of livestock pests. Boulder: Westview; 1990. [Google Scholar]
- Ouedraogo MR, Cusson M, Goettel MS, Brodeur J. Inhibition of fungal growth in thermoregulating locusts, Locusta migratoria, infected by the fungus Metarhizium anisopliae var acridum. J Invertebr Pathol. 2003;82:103–109. doi: 10.1016/S0022-2011(02)00185-4. [DOI] [PubMed] [Google Scholar]
- Ouedraogo RM, Goettel MS, Brodeur J. Behavioral thermoregulation in the migratory locust: a therapy to overcome fungal infection. Oecologia. 2004;138:312–319. doi: 10.1007/s00442-003-1431-0. [DOI] [PubMed] [Google Scholar]
- Padmini PCP, Padmaja V. Impact of different relative humidities on in vitro growth and sporulation of entomopathogenic fungal isolates of Beauveria species. Int J Pharm Biol Sci Arch. 2010;1:355–359. [Google Scholar]
- Sivasankaran P, Easwaramoorthy S, David H. Influence of temperature and relative humidity on growth, sporulation and pathogenecity of Beauveria bassiana. J Biol Control. 1998;12:71–76. [Google Scholar]
- Six DL, Mullens BA. Seasonal prevalence of Entomophthora muscae and introduction of Entomophthora schizophorae (Zygomycotina: Entomophthorales) in Musca domestica (Diptera: Muscidae) populations on California dairies. Biol Control. 1996;6:315–323. doi: 10.1006/bcon.1996.0040. [DOI] [Google Scholar]
- SPSS (2008) Statistical product and service solution, system user’s guide, version 17.0.0.0. Polar Engineering and Consulting, United State. http://www.winwrap.com/. Accessed Aug 2011
- StatPlus (2007) Professional build 4.9.0.0. AnalystSoft
- Tucker DL, Beresford CH, Sigler L, Rogers K. Disseminated Beauveria bassiana infection in a patient with acute lymphoblastic leukemia. J Clin Microbiol. 2004;42(11):5412–5414. doi: 10.1128/JCM.42.11.5412-5414.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA Report (1976) Control of insects affecting livestock. USDA Agricultural Research Service National Research Programs 2048
- Vidal C, Fargues J, Lacey LA. Intraspecific variability of Paecilomyces fumosoroseus: effect of temperature on vegetative growth. J Invertebr Pathol. 1997;70:18–28. doi: 10.1006/jipa.1997.4658. [DOI] [Google Scholar]
- Watson DW, Mullens BA, Petersen JJ. Behavioral fever response of Musca domestica (Diptera: Muscidae) to infection by Entomophthora muscae (Zygomycetes: Entomophthorales) J Invertebr Pathol. 1993;61:10–16. doi: 10.1006/jipa.1993.1003. [DOI] [Google Scholar]
- Watson DW, Geden CJ, Long SJ, Rutz DA. Efficacy of Beauveria bassiana for the controlling of the house fly and stable fly (Diptera: Muscadiae) Biol Control. 1995;5:405–411. doi: 10.1006/bcon.1995.1048. [DOI] [Google Scholar]
- Zimmermann G. The Galleria bait method for detection of entomopathogenic fungi in soil. J Appl Entomol. 1986;102:213–215. doi: 10.1111/j.1439-0418.1986.tb00912.x. [DOI] [Google Scholar]