Abstract
Medication adherence monitoring has relied largely on indirect measures of pill ingestion including patient self-report, pharmacy refills, electronically triggered pill bottles, and pill counts. Our objective is to describe an ingestible biosensor system comprising a radio-frequency identification (RFID)-tagged gelatin capsule. Once the capsule dissolves in the stomach, the RFID tag activates to transmit a unique signal to a relay device which transmits a time-stamped message to a cloud-based server that functions as a direct measure of medication adherence. We describe a constellation of mobile technologies that provide real-time direct measures of medication adherence. Optimizing connectivity, relay design, and interactivity with users are important in obtaining maximal acceptability. Potential concerns including gut retention of metallic components of the ingestible biosensor and drug dissolution within a gelatin capsule should be considered. An ingestible biosensor incorporated into a medication management system has the potential to improve medication compliance with real-time monitoring of ingestion and prompt early behavioral intervention. Integration of ingestible biosensors for multiple disease states may provide toxicologists with salient data early in the care of poisoned patients in the future. Further research on device design and interventions to improve adherence is needed and will shape the evolving world of medication adherence.
Keywords: Medication adherence, Antiretroviral therapy, Biosensors, HAART
Introduction
Chronic disease prevention and management strategies have long been hindered by the challenge of maintaining appropriate adherence to oral medication regimens. Accurate adherence monitoring can facilitate delivery of support interventions to optimize prevention and treatment outcomes. In addition, valid measurement of oral medication adherence is critical to the conduct of clinical trials, as well as disease prevention and treatment. Clinical investigations at the proof-of-concept stage to assess new medication regimens cannot be interpreted without valid adherence data because null findings may arise not from lack of drug efficacy but from poor adherence [1].
Unfortunately, no gold standard exists for monitoring medication adherence. Commonly used methods include self-report, announced and unannounced pill counts, pharmacy refill measures, electronic measurement of pill bottle opening, and measurement of plasma drug concentrations (Table 1) [2–5]. These methods of assessing medication adherence are usually subjective and rely on indirect evidence of medication ingestion. For example, opening a pill bottle merely implies that a medication was ingested, pharmacy refill times do no more than suggest that a drug was ingested as prescribed, and self-report, relies on memory to recall episodes of nonadherence. The validity and precision of these tools vary, and each confers a different set of advantages and disadvantages depending upon the context of its use.
Table 1.
Methods to assess medication adherence
| Technique | Description | Example | Adherence category |
|---|---|---|---|
| Patient diary or report | Log of ingestion events by patient or family member | N/A | Indirect |
| Pill counts | Pharmacy pill counts to assess ingestion | N/A | Indirect |
| Pharmacy assessment | Pharmacy pill refills for adherence | N/A | Indirect |
| Smart pill bottle | Wireless pill bottle cap that acts as surrogate to ingestion event when bottle is opened | Medication Event Monitoring System (MEMS), AdhereTech, Abiogenix uBox, Wisepill | Indirect |
| Smart pill bottle prompts | Visual and audio prompts integrate to pill bottle to remind patient to take medication | Viality GlowCap, Medminder, MedSignal, e-Pill MedSmart PLUS | Indirect |
| Pharmacological measures | Drug levels in biomatrices to measure recent and cumulative drug exposure | Plasma, urine, saliva, cells, hair and dried blood spots | Direct |
| Ingestible biosensors | Integrated into medication or taken in addition to medication as a direct marker of ingestion | Proteus Raisin System, eTect ID Cap | Direct |
| Directly observed therapy | Direct observation by nursing staff of medication ingestion | N/A | Direct |
The recent development of ingestible biosensor systems that integrate into existing medication adherence programs provides a low cost solution for direct, accurate, real-time medication adherence monitoring [6, 7]. Because ingestible biosensors are adhered onto the desired drug, detection of ingestion events is direct and accurate [8]. Strategies that incorporate ingestible biosensors into a body-sensing network or existing mobile health-based (mHealth) adherence interventions may improve medication adherence both in clinical trials and in the management of chronic disease [9–12]. Here, we describe the current state of ingestible biosensors and provide formative guidance for their integration into systems for real-time medication adherence.
Components of Ingestible Biosensor Systems
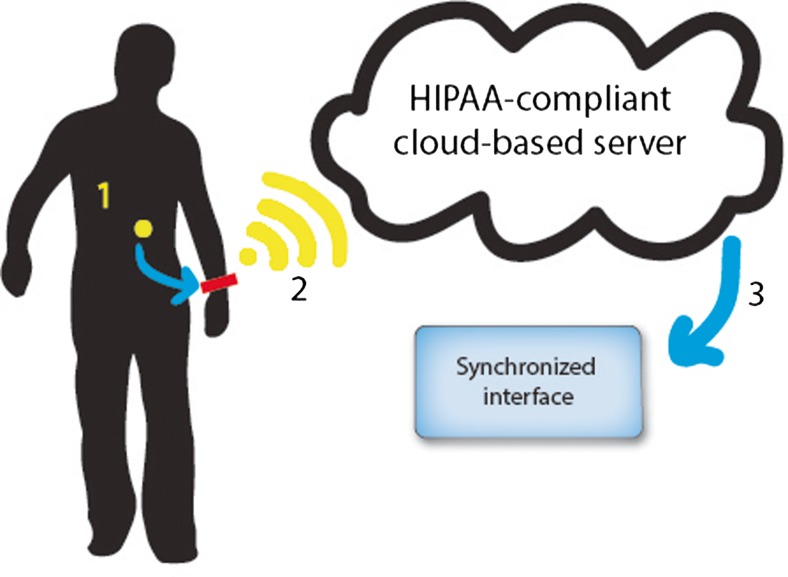
Ingestible biosensors comprise a constellation of advanced technologies, including radio-frequency identification (RFID) tags and wireless connectivity, to monitor and support oral medication adherence. An RFID tag is adhered to a gelatin capsule that is large enough to encapsulate the oral medication(s) under study (Fig. 1). The RFID-tagged digital pill dissolves in stomach acid, releasing the study medication and activating the RFID tag to emit a signal that is detected by a RFID reader that is worn on the body (Fig. 2). The reader wirelessly (2) transmits a time-stamped message with the pill’s unique RFID code to a cloud-based, Health Insurance Portability and Accountability Act (HIPAA)-compliant online server. The third component is an interface (3) that promotes collaborative awareness between clinician-patient dyads. The interface presents performance feedback describing medication adherence, responds to nonadherence with support interventions that are stored on the interface, and enhances communication between patient and healthcare provider.
Fig. 1.
A digital comprised of a standard gelatin pill capsule with an RFID tag (a) that can be integrated with any medication (b, c). For size reference, the digital pill (a) is the size of an 800 mg ibuprofen tablet
Fig. 2.
Ingestible biosensor system. (1) A radio-frequency identification (RFID)-tagged gelatin capsule. Once the capsule dissolves in the stomach, the RFID tag activates to transmit a unique signal to (2) a relay device which transmits a time-stamped message to (3) a cloud-based server that functions as a direct measure of medication adherence
After dissolution of the gelatin capsule and absorption of the study medication, the RFID tag transits the bowel and is eliminated in the stool.
Considerations for Implementing an Ingestible Biosensor
Technical Considerations
To avoid interference with other devices that rely on wireless data transmission, ingestible biosensors utilize different radio frequencies from other wireless implantable devices and commercial wearable devices to transmit ingestion data. Because of the low power generated by the electrochemical reaction between electrolytes in gastric acid and the digital pill’s battery, the RFID signal transmits over a very short range. Ingestible biosensors, therefore, require that the receiver be placed very close to the transmission source to collect and relay RFID transmissions [6, 13]. Although current receivers, typically worn on the hip, advances in data transmission will create a stronger signal that can be acquired by receivers integrated into common mobile devices like smartphones or smartwatches. Other low-energy short-range data transmission protocols including Low Energy Bluetooth (BLE) and ZigBee, may serve as alternatives to current RFID transmission and allow for improved range and more acceptable placement of RFID receivers [14, 15]. Battery-powered ingestible devices similar to pill endoscopy capsules can be developed with improved range and signal duration, but are limited by the physical size of components [16, 17]. Integration of receiver sensors in homes, hospitals, and chronic care facilities where patients tend to ingest their medications may allow for elimination of worn receivers and improved adherence to ingestible systems [18].
Clinical implementation of real-time medication adherence monitoring systems including ingestible biosensors relies heavily upon patient acceptability. Prior studies demonstrate the feasibility of ingestible biosensors in directly observed therapy, but field testing of an ingestible biosensor system in naturalistic patient populations has yet to be performed [8, 19]. Qualitative patient feedback from focus groups will pinpoint acceptable designs that support integration of the device use into a user’s daily routine and minimize missed adherence. Other strategies to improve acceptability may include newer iterations of RFID tags with greater signal strength, RFID readers with greater sensitivity, smaller RFID tags, and positive patient feedback [7].
Security and Privacy
Ingestible biosensors must meet security requirements for protecting patient information as set forth by the Health Information Technology for Economic and Clinical Health (HITECH) and HIPAA standards. We recommend verifying with suppliers that any new technology meets that HIPAA/HITECH standards for secure data transmission are met. Security of an ingestible biosensor system begins with the RFID pills. Set to specific bands of communications, RFID signals can be intercepted during transmission to the receiver, or as the receiver sends a signal to an online server [20]. Multiple security encryptions are available to prevent a potential attacker from intercepting an RFID or SMS signal [21, 22]. Partnerships with industry and clinical data security experts can help minimize signal interception so that a patient’s privacy is protected. Biometric passcodes, alteration of signal characteristics to minimize the distance over which data packets describing ingestion are transmitted, and design of behavioral interventions that protect confidentiality are among the multiple potential solutions to optimize privacy as ingestible biosensors enter clinical use.
Potential Complications of Ingestible Biosensors for Medication Adherence
Adverse events with ingestible biosensors include potential retention of RFID tags and alteration to medication dissolution. Similar to capsule endoscopy pills, RFID-labeled biosensors have epoxy-encapsulated metal components that do not degrade during transit through the gut. To date, clinical trials using digital pills have not reported retention in the gastrointestinal tract of ingested biosensors [8, 19]. By way of comparison, capsule endoscopy pills (31 × 11 mm) are far larger than the nondegradable metal component of ingestible biosensors (1 × 1 × 0.3 mm) [23]. Greater than a decade’s experience with capsule endoscopy has demonstrated a less than 2 % retention rate [16, 23, 24]. The observation from capsule endoscopy studies is that symptoms of bowel obstruction and pain are associated with a bowel lumen of less than 5 mm in diameter [25]. Retention of nondegradable metal components of ingestible biosensors may occur, but their small size is unlikely to lead to clinically significant outcomes.
Incorporation of a RFID tag into a gelatin capsule can theoretically alter the dissolution of the therapeutic medication encapsulated within it. A gelatin capsule can act as a barrier, preventing access to the gastric environment that dissolves and releases the drug. Activation of the RFID tag emits an ultra high-frequency (UHF) signal that may alter the molecular structure of drugs [26]. Several lines of evidence suggest that an RFID-tagged gelatin capsule overlying a medication will not alter the pharmacokinetics, pharmacodynamics, or structure of that drug. First, gelatin capsules, because of their rapid and complete dissolution in aqueous media, are commonly used to formulate a wide variety of prescription and over-the-counter medications. Second, gelatin capsules have been used to improve palatability of medications, such as saquinavir and l-thyroxine, without discernible change in drug concentration [27, 28]. Third, reformulation of medications including antiretroviral and hypothyroid drugs into gelatin capsules produced no change in pharmacodynamic parameters, including Cmax and area-under-the-curve [27–29]. Fourth, in vitro experiments exposing insulin and chemotherapeutic agents to active RFID signals failed to change the structure or composition of drug after nuclear magnetic resonance and reverse-phase high-performance liquid chromatography [30, 31].
Designing Devices for Maximal Success
Securing success with an ingestible biosensor requires that each component—an ingestible biosensor, RFID relay, and the patient/clinician interface—will be accepted and used effectively [32]. Factors for success may include palatability of the digital pill, design of the relay device, and quality of the interaction between the interface and users. Patients should play a key role in design and development of ingestible biosensor-based technology systems. Patients’ willingness to swallow labeled pills, wear a relay device and respond to interventions by their care provider, is paramount to using digital pills as a direct measure of medication adherence [33, 34]. Continuous evaluation of an ingestible biosensor system through iterative field testing, with refinements in technology based on user input, will help inform ideal design of components of the system. Design of the RFID relay device is critical to improving usability of the system as a whole—an obtrusive, unattractive device may hinder acceptability, lead to premature disuse of the adherence system, and produce unusable or invalid data. A cumbersome relay device that requires frequent charging, or that is physically uncomfortable or stigmatizing will inhibit use of the technology and prevent potential ingestion events from being recorded. Worn devices that are particularly noticeable may result in unwanted attention in social situations, resulting in emotional or social discomfort.
Interface architecture is critical for integrating an ingestible biosensor into a network of wearable biometric devices [12]. An interface of an ingestible biosensor should be designed so that biometric data from a body-sensing network can be interpreted, and a real-time, meaningful intervention delivered in the context of an ingestion event or nonevent. Interventions can be pushed to patients through utilizing short message service (SMS) or multimedia messaging service (MMS) that exist on the control channel of smartphones and cellular phones. Due to the ubiquity of smartphones in the United States, development of an app as an intervention can also be considered; apps, however, must be updated with each iteration of smartphone-operating system, a shortcoming that contributes to striking increases in programming and upkeep costs [35]. We believe utilizing existing MMS messaging is a nonobtrusive and cost-efficient response. Allowing device interactivity without having to activate a mobile app also saves valuable battery life when compared to MMS, and may promote user acceptance of an intervention [36].
Ingestible Biosensors to Monitor Medication Adherence
Ingestible biosensors offer several opportunities to improve medication adherence across various disciplines. Enhanced medication adherence using an ingestible biosensor system could limit progression of chronic diseases such as HIV, reduce hospital readmissions in patients with congestive heart failure, or ensure compliance with substance abuse treatment programs in the outpatient setting. Ingestible biosensors can accomplish these effects in several ways. First, ingestible biosensors provide direct evidence of medication ingestion. Importantly, data regarding ingestion is obtained in real time. Existing methods to assess medication adherence generally infer rather than measure medication ingestion [4, 37, 38]. By labeling medication with a biosensor, direct documentation of ingestion occurs, eliminating recall bias or distortion. Second, ingestible biosensors allow longitudinal measures of adherence and could provide data on adherence patterns. Current medication adherence techniques assess ingestion events over the course of weeks or months [39]. Such methods miss nascent periods of nonadherence that may not be recognized with current medication adherence strategies [40]. Because of this imprecision in current approaches to adherence monitoring, interventions for nonadherence may not be delivered until a patient has been noncompliant for an extended period of time—after behaviors associated with nonadherence have become conditioned and an undesired clinical outcome has already occurred. An ingestible biosensor, with its real-time ingestion detection ability, can identify short adherence gaps, delivering interventions at times of nonadherence before such behaviors become problematic.
Third, ingestible biosensors can detect incipient changes in adherence behavior in real time. When linked to user descriptions of activity surrounding the taking of medicines, ingestible biosensor systems can elucidate the behavioral contexts of adherence and nonadherence. Immediate feedback tailored to the setting of a missed dose may improve the relevance and applicability of adherence interventions.
Fourth, ingestible biosensors provide individualized, real-time performance feedback that can effect behavioral change. Performance feedback is important in maintaining adherence. Data obtained through an ingestible biosensor can allow precise feedback on adherence performance that can be rewarding for individuals with good adherence, and enlightening for those with suboptimal adherence. These insights into individual adherence can affect behavioral changes and provide vital clinical information for caretakers.
For a toxicologist, the introduction of ingestible biosensors to measure medication adherence in a variety of prescription and nonprescription drugs may help determine the history of ingestion in a poisoned patient. The agents a patient ingested, as well as the time of ingestion, are critical pieces of information frequently unavailable to toxicologists evaluating a poisoned patient. Mainstream acceptance and integration of ingestible biosensors with a universal cloud storage and interpretation system can allow a toxicologist prompt access to ingestion data in the setting of an overdose, and allow for rapid bedside treatment, and administration of appropriate antidotes in an evidence based manner. Medications that have potential for toxicity in their early initiation phases can be monitored by a toxicologist with ingestion data integrated with a physical exam and serum drug levels to help tailor therapy.
Additionally, toxicologists involved in substance abuse treatment centers can utilize an ingestible biosensor system to help improve adherence to treatments aimed at sobriety. Combined with global positioning system (GPS) data, medication adherence or nonadherence data can provide important data regarding geographical locations that may contribute to episodes of medication adherence. Real-time ingestion adherence and nonadherence patterns provide information to treating toxicologists regarding potential triggers that may cause a patient to relapse, allowing a deliverable interaction prior to repeat drug use. Interventions from an ingestible biosensor system can provide directed interventions at critical times of nonadherence to help patients maintain sobriety.
Conclusion
Ingestible biosensors comprising a RFID-tagged digital pill containing a target medication provides a new opportunity to evaluate medication adherence, respond to nonadherence, and improve health. Iterative technology development with improved wireless transmission and patient-centered interfaces will allow delivery of real-time interventions that can help patients adhere to medication regimens. Further refinement of a comprehensive device, recorder and interface should include input from users to maximize their acceptability and adoption by patients. An effortless, unobtrusive ingestible biosensor can provide real-time ingestion data that can be used to tailor individualized, live interventions to improve medication adherence.
Acknowledgments
Dr. J. Castillo-Mancilla is supported by the National Institutes of Health 5K23AI104315-02. Dr. C. Darling is supported by the National Heart, Lung, and Blood Institute 5K23HL101991-05. Dr. K. Horvath is supported by the National Institutes of Health 5R34DA033833-04 and 1R34MH105202-01. Dr. P. Hibberd is supported by the National Institutes of Health 5K24AT003683-09. Dr. E. W. Boyer is supported by the National Institutes of Health 1K24DA037109.
Footnotes
Mr. E. Buffkin is President and Chief Operating Officer for eTect BIO.
Contributor Information
Peter R. Chai, Phone: 508-344-7577, Email: peter.chai@umassmemorial.org
Jose Castillo-Mancilla, Phone: (303) 724-4934.
Eric Buffkin, Phone: 352-367-8328.
Chad Darling, Phone: 508-334-7577.
Rochelle K. Rosen, Phone: 401-793-8182
Keith J. Horvath, Phone: 612-626-1799
Edwin D. Boudreaux, Phone: 508-334-3817
Gregory K. Robbins, Phone: 617-724-0076
Patricia L. Hibberd, Phone: 617-643-8640
Edward W. Boyer, Phone: 508-344-7577
References
- 1.Ickovics JR, Meisler AW. Adherence in AIDS clinical trials: a framework for clinical research and clinical care. J Clin Epidemiol. 1997;50(4):385–91. doi: 10.1016/S0895-4356(97)00041-3. [DOI] [PubMed] [Google Scholar]
- 2.Institute NEH. Thinking outside the pillbox: a system‐wide approach to improving patient medication adherence for chronic disease. New England Healthcare Institute Research Brief: New England Healthcare Institute 2009.
- 3.Miller LG, Liu H, Hays RD, Golin CE, Beck CK, Asch SM, et al. How well do clinicians estimate patients’ adherence to combination antiretroviral therapy? J Gen Intern Med. 2002;17(1):1–11. doi: 10.1046/j.1525-1497.2002.09004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 5.Rudd P, Byyny RL, Zachary V, LoVerde ME, Mitchell WD, Titus C, et al. Pill count measures of compliance in a drug trial: variability and suitability. Am J Hypertens. 1988;1(3 Pt 1):309–12. doi: 10.1093/ajh/1.3.309. [DOI] [PubMed] [Google Scholar]
- 6.DiCarlo L, Moon G, Intondi A, Duck R, Frank J, Hafazi H, et al. A digital health solution for using and managing medications: wirelessly observed therapy. IEEE Pulse. 2012;3(5):23–6. doi: 10.1109/MPUL.2012.2205777. [DOI] [PubMed] [Google Scholar]
- 7.BIO E. Etect. http://etectbio.com. January 2015.
- 8.Kane JM, Perlis RH, DiCarlo LA, Au‐Yeung K, Duong J, Petrides G. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74(6):e533–40. doi: 10.4088/JCP.12m08222. [DOI] [PubMed] [Google Scholar]
- 9.Boyer EW, Smelson D, Fletcher R, Ziedonis D, Picard RW. Wireless technologies, ubiquitous computing and mobile health: application to drug abuse treatment and compliance with HIV therapies. J Med Toxicol Off J Am Coll Med Toxicol. 2010;6(2):212–6. doi: 10.1007/s13181-010-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horvath KJ, Carrico AW, Simoni J, Boyer EW, Amico KR, Petroll AE. Engagement in HIV medical care and technology use among stimulant‐using and nonstimulant‐using men who have sex with men. AIDS Res Treat. 2013;2013:121352. doi: 10.1155/2013/121352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore JO, Boyer EW, Safren S, Robbins GK, Boudreaux ED, Rosen R, et al. Designing interventions to overcome poor numeracy and improve medication adherence in chronic illness, including HIV/AIDS. J Med Toxicol Off J Am Coll Med Toxicol. 2011;7(2):133–8. doi: 10.1007/s13181-011-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen RK, Ranney M, Boyer EW. Formative research for mHealth HIV adherence: the iHAART app. Annual Hawaii International Conference on System Sciences. Hawaii: IEEE & Computer Society Press; 2015. pp. 2779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuce MR, Dissanayake T. Easy-to-swallow wireless telemetry. Microwave Mag IEEE. 2012;13(6):90–101. doi: 10.1109/MMM.2012.2205833. [DOI] [Google Scholar]
- 14.Frehill P, Chambers D, Rotariu C. Using Zigbee to integrate medical devices. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2007; 2007: 6718–21. doi: 10.1109/iembs.2007.4353902. [DOI] [PubMed]
- 15.Gomez C, Oller J, Paradells J. Overview and evaluation of bluetooth low energy: an emerging low-power wireless technology. Sensors. 2012;12:11734–53. doi: 10.3390/s120911734. [DOI] [Google Scholar]
- 16.Li F, Leighton JA, Sharma VK. Capsule endoscopy: a comprehensive review. Minerva Gastroenterol Dietol. 2007;53(3):257–72. [PubMed] [Google Scholar]
- 17.Ou JZ, Yao CK, Rotbart A, Muir JG, Gibson PR, Kalantar-Zadeh K. Human intestinal gas measurement systems: in vitro fermentation and gas capsules. Trends Biotechnol. 2015 doi: 10.1016/j.tibtech.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Adib F, Mao H, Kabelac Z, Katabi D, Miller RC. Smart homes that monitor breathing and heart rate. Seoul: CHI 2015 Crossings; 2015. [Google Scholar]
- 19.Belknap R, Weis S, Brookens A, Au-Yeung KY, Moon G, DiCarlo L, et al. Feasibility of an ingestible sensor‐based system for monitoring adherence to tuberculosis therapy. PLoS One. 2013;8(1):e53373. doi: 10.1371/journal.pone.0053373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar P, Lee HJ. Security issues in healthcare applications using wireless medical sensor networks: a survey. Sensors (Basel, Switzerland) 2012;12(1):55–91. doi: 10.3390/s120100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He D, Chan S, Tang S. A novel and lightweight system to secure wireless medical sensor networks. IEEE J Biomed Health Inform. 2014;18(1):316–26. doi: 10.1109/JBHI.2013.2268897. [DOI] [PubMed] [Google Scholar]
- 22.He D, Chen C, Chan S, Bu J, Zhang P. Secure and lightweight network admission and transmission protocol for body sensor networks. IEEE J Biomed Health Informatics. 2013;17(3):664–74. doi: 10.1109/JBHI.2012.2235180. [DOI] [PubMed] [Google Scholar]
- 23.Kovac C. “Video pill” may supplement standard endoscopy. BMJ (Clin Res ed) 2001;323(7309):358. doi: 10.1136/bmj.323.7309.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li F, Gurudu SR, De Petris G, Sharma VK, Shiff AD, Heigh RI, et al. Retention of the capsule endoscope: a single-center experience of 1000 capsule endoscopy procedures. Gastrointest Endosc. 2008;68(1):174–80. doi: 10.1016/j.gie.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 25.Helander HF, Fandriks L. Surface area of the digestive tract—revisited. Scand J Gastroenterol. 2014;49(6):681–9. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- 26.Acierno R, Carata E, DePascali S, Fanizzi F, Maffia M, Mainetti L, et al. Potential effects of RFID systems on biotechnology insulin preparation: a study using HPLC and NMR spectroscopy. IEEE/ICME Conference on Complex Medical Engineering; Gold Coast, Australia: IEEE; 2010. p. 198–203.
- 27.Perry CM, Noble S. Saquinavir soft‐gel capsule formulation. A review of its use in patients with HIV infection. Drugs. 1998;55(3):461–86. doi: 10.2165/00003495-199855030-00014. [DOI] [PubMed] [Google Scholar]
- 28.Vita R, Fallahi P, Antonelli A, Benvenga S. The administration of L‐thyroxine as soft gel capsule or liquid solution. Expert Opin Drug Deliv. 2014;11(7):1103–11. doi: 10.1517/17425247.2014.918101. [DOI] [PubMed] [Google Scholar]
- 29.Klein CE, Chiu YL, Awni W, Zhu T, Heuser RS, Doan T, et al. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft-gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. J Acquir Imm Def Syndr (1999) 2007;44(4):401–10. doi: 10.1097/QAI.0b013e31803133c5. [DOI] [PubMed] [Google Scholar]
- 30.Acierno R, DePascali S, Fanizzi F, Maffia M, Mainetti L, Patrono L, et al. Investigating potential effects of RFID systems on the molecular structure of the human insulin. 5th Cairo International Biomedical Engineering Conference; 2010; Cairo: IEEE; 2010.
- 31.Kozakova S, Gonec R. RFID technology in preparation and administration of cytostatic infusions, deploying RFID—challenges, solutions, and open issues. InTech. 2011. http://www.intechopen.com/books/deploying‐rfid‐challenges‐solutions‐and‐open‐issues/rfid-technology-in‐preparation‐and‐administration‐of‐cytostatic‐infusions. Accessed May 11, 2015.
- 32.Birnbaum F, Lewis D, Rosen RK, Ranney ML. Patient engagement and the design of digital health. Acad Emerg Med Off J Soc Acad Emerg Med. 2015;22(6):754–6. doi: 10.1111/acem.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Royce W, editor. Managing the development of large software systems. IEEE WESCON; 1970: Proceedings of IEEE WESCON.
- 34.Whitworth E, Biddle R. The social nature of agile teams. Agile Conference IEEE; 2007. p. 26–36.
- 35.Ranney ML, Choo EK, Wang Y, Baum A, Clark MA, Mello MJ. Emergency department patients’ preferences for technology‐based behavioral interventions. Ann Emerg Med. 2012;60(2):218–27.e48. doi: 10.1016/j.annemergmed.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Carroll A, G H. An analysis of power consumption in a smartphone. USENIX Annual Technical Conference; 2010; Boston: Proceedings of the 2010 USENIX Annual Technical Conference; 2010.
- 37.Grossberg R, Zhang Y, Gross R. A time‐to‐prescription‐refill measure of antiretroviral adherence predicted changes in viral load in HIV. J Clin Epidemiol. 2004;57(10):1107–10. doi: 10.1016/j.jclinepi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Haynes RB, McDonald H, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications. The Cochrane database of systematic reviews. 2002;(2):Cd000011. doi:10.1002/14651858.cd000011. [DOI] [PubMed]
- 39.Castillo‐Mancilla JR, Searls K, Caraway P, Zheng JH, Gardner EM, Predhomme J, et al. Short communication: tenofovir diphosphate in dried blood spots as an objective measure of adherence in HIV‐infected women. AIDS Res Hum Retrovir. 2015;31(4):428–32. doi: 10.1089/aid.2014.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Organization WH. Adherence to long‐term therapies: evidence for action. Geneva: World Health Organization; 2003. [Google Scholar]