Abstract
Background
Lead encephalopathy is a severe manifestation of lead poisoning that can present with altered mental status and seizures and has been associated with illicit moonshine consumption. Lead encephalopathy has traditionally been treated using dimercaprol (British anti-Lewisite, BAL) and calcium disodium ethylenediaminetetraacetic acid (CaNa2EDTA).
Case Report
We describe a patient with lead encephalopathy related to lead-contaminated moonshine consumption, who was treated using dimercaptosuccinic acid (DMSA) due to a national shortage of CaNa2EDTA. A 66-year-old woman presented to a hospital with headache, irritability, and altered mental status. On hospital day 16, she was found to have a whole blood lead concentration of 148.2 μg/dL and a 24-h urine lead concentration of 232 μg/day. Due to a national shortage of CaNa2EDTA, the patient was given one dose of BAL and then started on DMSA via nasogastric tube. She dramatically improved over 4 days and was subsequently transitioned to oral DMSA and outpatient treatment. One day prior to discharge, her whole blood lead concentration was 47.2 μg/dL and her mental status was normal. DMSA was used in lieu of CaNa2EDTA to treat the patient with lead encephalopathy. The patient subsequently experienced clinical improvement and declining whole blood level concentrations.
Conclusion
Further prospective studies are needed to compare the efficacy of DMSA versus CaNa2EDTA in patients with lead encephalopathy.
Keywords: Lead, Succimer, DMSA, Encephalopathy, Moonshine
Background
Lead poisoning remains a prevalent yet largely preventable disease in the USA and throughout the world, despite considerable efforts to decrease exposure. Pediatric lead screening, awareness, remediation of lead-based paint in houses, removal of lead from gasoline, and occupational regulations have all led to a decreased burden of disease; however, exposure and severe toxicity still occur.
Consumption of lead-contaminated moonshine remains a source of lead exposure. A nationwide study from 1995 to 2001 looking at 115 samples of moonshine confiscated by the Alcohol, Tobacco, and Firearms (ATF) agency noted approximately 28.7 % of all samples had lead concentrations above 300 μg/L [1]. The Food and Drug Administration (FDA) has established concentrations of lead in wine of 300 μg/L and juice of 50 μg/L as thresholds for concern regarding potential health hazards. Established regulatory limits for lead concentrations in drinking water are much lower with the Environmental Protection Agency (EPA) and FDA utilizing values of 15 and 5 μg/L for public water supplies and bottled water, respectively. Furthermore, FDA lists a permissible daily exposure of lead of 5 μg/day (which would be expected to result in a blood lead measurement of 1–2 μg/dL in children using EPA modeling methodology) [2].
Individuals who consume lead-contaminated moonshine can present to healthcare facilities with signs of lead toxicity including encephalopathy. Treatment of acute lead encephalopathy in adults has traditionally relied on the use of dimercaprol (British anti-Lewisite, BAL) and edetate calcium disodium (CaNa2EDTA). We present a case of a patient with lead encephalopathy who was treated with dimercaptosuccinic acid (DMSA) due to a national shortage of CaNa2EDTA.
Case Report
A 66-year-old woman with a history of diabetes mellitus type-two presented to an emergency department after a new-onset generalized tonic-clonic seizure. She was also found to be anemic with a hemoglobin of 7.5 g/dL and hematocrit of 24.3 %. No etiology was found for her seizures or anemia and she was discharged home. Two weeks later, she presented to another emergency department with complaints of worsening headache, weakness, and confusion. Her vital signs were P—87 beats per minute, R—16 breaths per minute, BP—134/87 mmHg, SpO2—100 % on RA, and T—36.2 °C tympanic. Cranial nerve examination, cerebellar testing, sensory examination, and mental status were normal, though she had 4/5 bilateral upper and lower extremity muscle strength. Laboratory testing was notable for hemoglobin 7.4 g/dL, hematocrit 24.5 %, MCV 76.1 fL, potassium 2.8 mmol/L, bicarbonate 20 mmol/L, and anion gap 17. Her liver and renal function were normal, though her urinalysis was notable for specific gravity of 1.014, ketones >150 mg/dL, and urobilinogen 4 mg/dL. CT scan of the brain showed diffuse effacement of the sulci with preservation of the gray-white differentiation, which was consistent with diffuse cerebral edema.
The patient had worsening mental status and required intubation and mechanical ventilation on hospital day (HD) 2. She had extensive testing for infectious, autoimmune, and other metabolic processes, but no etiology was found. She was extubated on HD 11 though her altered mental status and agitation persisted. The regional poison center was contacted and recommended testing for heavy metals. Blood was drawn on HD 14 and 2 days later, her whole blood lead concentration returned at 148.2 μg/dL. Twenty-four-hour urine lead was also elevated at 232 μg/day. Treatment with dimercaprol (British anti-Lewsite, BAL) and CaNa2EDTA was recommended.
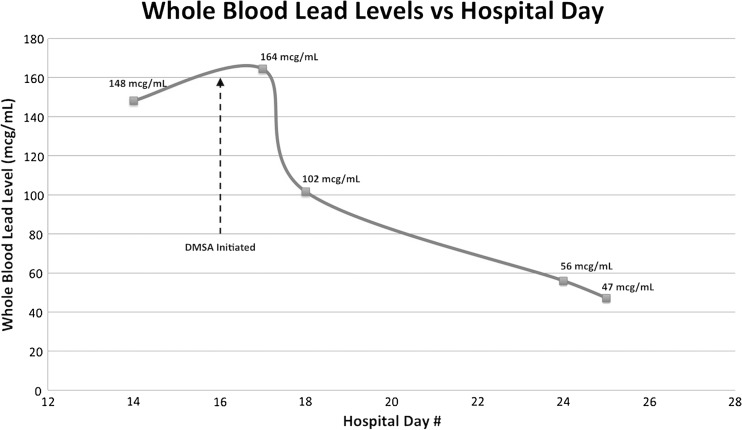
The patient was given one dose of 200 mg of intramuscular BAL (4 mg/kg) in anticipation of initiating CaNa2EDTA 4 h later. Despite numerous attempts to secure CaNa2EDTA, it was not available from any facility in the region. A decision was made on HD 16 to initiate treatment with DMSA as an alternative and 500 mg (10 mg/kg/dose, maximum of 500 mg/dose) of crushed DMSA was administered via nasogastric tube every 8 h. Given DMSA is a water-soluble dithiol analogue of BAL, BAL administration was stopped. By HD 20, she was back to her baseline mental status and her upper and lower muscle strength returned to normal. She had mild left upper and lower extremity decreased sensation to light touch. She then revealed she had been drinking illicit moonshine with her roommate for several years. She was discharged home on HD 26 with persistent left-sided decreased light touch. She completed the full regimen of 500 mg DMSA thrice daily for 5 days then twice daily for 14 days after discharge. Her whole blood lead concentration response to treatment is demonstrated in Fig. 1, which shows a very rapid decline in whole blood levels immediately after initiating DMSA followed by a continued downward trend. The initial increase in whole blood lead concentrations immediately after initiation of DMSA may reflect mobilization of lead from soft tissue and bone prior to significant renal elimination. No additional testing was possible due to lack of patient follow-up after hospital discharge.
Fig. 1.
Whole blood lead levels versus hospital stay
Testing of the moonshine acquired from the patient’s home using inductively coupled plasma/mass spectroscopy (ICP/MS by NMS Labs, Willow Grove, PA) revealed a lead concentration of 15,000 μg/L—50 times the threshold set by the FDA’s highest threshold for possible adverse health effects.
Discussion
A 2011 prevalence study reported 11 % of all FDA-approved medications were in shortage, with injectable medication compromising 76.5 % of these drugs [3]. As pharmacies limit or eliminate par levels for specific medications based on factors such as cost and frequency of use, clinicians can expect to be faced with alternative treatment decisions. The American College of Medical Toxicology and other organizations have advocated strategies to address antidote shortages and minimize their impact [4]. Actions such as safely extending expiration dates, maintaining regional lists of antidotes, and creating formal antidote-sharing agreements may be helpful. However, our case highlights the challenges of a drug that is produced by only one manufacturer with an interruption in drug manufacturing.
Currently, CaNa2EDTA is the only FDA-approved treatment for acute lead encephalopathy in both children and adults. CaNa2EDTA chelates lead by exchanging its central calcium ion with a lead ion in the blood. It can cross the blood-brain barrier but still is found in much higher concentrations (ratios typically 20:1) in the blood versus CSF [5]. Although CSF penetration of CaNa2EDTA is beneficial, there is an initial risk of redistribution of lead into the CSF with CaNa2EDTA. Therefore, CaNa2EDTA administration is often preceded by a dose of intramuscular BAL to limit this redistribution into the CNS [6]. BAL and CaNa2EDTA are given intramuscularly and intravenously, respectively, due to poor oral absorption and thus have been the preferred agents in encephalopathic patients who may be at risk for aspiration with oral medication use.
DMSA is an oral chelating agent that was originally FDA-approved for treatment of lead toxicity in children with whole blood concentrations over 45 μg/dL [7]. DMSA is a stable water-soluble derivative of dimercaprol (BAL) that chelates lead, iron, mercury, zinc, nickel, arsenic, and cadmium [8]. In contrast with CaNa2EDTA, DMSA does not cross the blood-brain barrier and thus removal of lead from the CNS likely occurs due to the development of a concentration gradient of lead between the CNS and blood [8]. Despite these differences in routes of administration and ability to penetrate the blood-brain barrier, there are several case reports that appear to demonstrate the efficacy of DMSA in reducing whole blood lead concentrations. The majority of these studies focus on mild-to-moderately elevated concentrations in the absence of symptoms consistent with lead encephalopathy. However, two human case series using DMSA as a primary treatment modality in patients with lead encephalopathy have been published [9, 10].
Fournier et al. described three patients with lead encephalopathy and whole blood lead concentrations as high as 34.3 μmol/L (710 μg/dL) who were noted to have rapid improvement in symptoms and whole blood lead concentrations in response to DMSA monotherapy [10]. All three patients had increases in urinary lead excretion of 3.75–11.3 times that of pretreatment concentrations. Moderate decreases in whole blood lead concentrations were noted over a 5–15-day period during treatment with DMSA.
Thurtle et al. reported on the treatment of 1307 lead poisoned Nigerian children younger than 5 years of age with venous blood lead concentrations >45 μg/dL [8]. Thirty-six percent of the children had venous blood lead concentrations >80 μg/dL while 6 % of the cohort had venous blood lead concentrations >120 μg/dL at the initiation of treatment. DMSA was the only treatment used for 1301 of the 1307 patients. Fourteen of the children included in the study were obtunded and encephalopathic and received DMSA chelation doses via nasogastric tube. The total case fatality rate for all patients treated with at least one dose of DMSA was 0.6 % (8/1301) among all patients with lead encephalopathy and 21.4 % (3/14) in obtunded children that required dosing via nasogastric tube. Both case fatality rates were markedly below the 48 % mortality rate of probable or suspected cases prior to intervention with DMSA [9].
Additionally, animal studies in rats with severe lead toxicity demonstrated significant reduction in whole blood lead concentrations with either DMSA or CaNa2EDTA treatment with no statistical difference between near molar-equivalent doses of the two [11].
Our report adds to the small number of reported cases in which DMSA was used to treat lead encephalopathy in the absence of CaNa2EDTA. There are numerous limitations in making any distinct conclusions regarding efficacy of DMSA in this setting. The largest limitation is absence of urinary lead concentration measurements after initiation of DMSA. Specifically, 24-h urine measurements would have been a more accurate measurement of response to chelation compared to whole blood concentrations given the renal elimination of chelated lead. Whole blood lead concentrations often do not precisely correlate with body lead burden due to variability in both elimination and distribution of lead into and out of the vascular compartment. Differences in mechanism and site of action would possibly predict a lower efficacy of DMSA versus CaNa2EDTA to chelate lead from the CNS, though this difference was not directly tested and cannot be inferred in this case report.
Further studies comparing the effectiveness and safety of DMSA versus CaNa2EDTA in patients with lead encephalopathy are needed. Specifically, serial 24-h urine measurements to assess increased lead excretion along with longer observation periods in a larger cohort of patients would help to more directly compare these two agents.
Conclusion
We present a case of lead encephalopathy that demonstrated rapid clinical improvement and decreased whole blood lead concentrations after receiving DMSA via nasogastric tube. Though DMSA cannot be recommended as a first-line treatment for lead encephalopathy at this time, it should be considered in cases in which CaNa2EDTA is unavailable due to drug shortages, there are contraindications to CaNa2EDTA, or when an oral chelating agent would be preferred.
Acknowledgments
Sources of Funding for Project
There are no sources of funding.
Conflict of Interest
The authors declare that they have no competing interests.
Footnotes
Previous Presentation of Data at Meetings or in Abstract Form
This case was presented as an abstract at NACCT Conference held in New Orleans, LA in October, 2014.
References
- 1.Morgan BW, Parramore CS, Ethridge M. Lead contaminated moonshine: a report of bureau of alcohol, tobacco and firearms analyzed samples. Vet Hum Toxicol. 2004;46:89–90. [PubMed] [Google Scholar]
- 2.Guideline for Elemental Impurities Q3D. Int. Conf. Harmon. Tech. Requir. Regist. Pharm. Hum. Use. 2013. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm371025.pdf.
- 3.Le P, Seoane-Vazquez E, Rodriguez-Monguio R, Fox ER, Szeinbach SL, Dunehew AR, et al. The prevalence of pharmaceutical shortages in the United States. J Generic Med. 2011;8:210–8. doi: 10.1177/1741134311428105. [DOI] [Google Scholar]
- 4.ACMT Antidote shortages in the USA: impact and response. J Med Toxicol. 2015;11:144–6. doi: 10.1007/s13181-013-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foreman H, Trujillo T. The metabolism of C14 labeled ethylenediaminetetraacetic acid in human beings. J Lab Clin Med. 1954;43:566. [PubMed] [Google Scholar]
- 6.Chisolm J. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73:1–38. doi: 10.1016/S0022-3476(68)80036-8. [DOI] [PubMed] [Google Scholar]
- 7.Mann KV, Travers JD. Succimer, an oral lead chelator. Clin Pharmacol. 1991;10:914–22. [PubMed] [Google Scholar]
- 8.Bradberry S, Vale A. Dimercaptosuccinic acid (succimer; DMSA) in inorganic lead poisoning. Clin Toxicol. 2009;47:617–31. doi: 10.1080/15563650903174828. [DOI] [PubMed] [Google Scholar]
- 9.Thurtle N, Greig J, Cooney L, Amitai Y, Ariti C, Brown MJ, et al. Description of 3,180 courses of chelation with dimercaptosuccinic acid in children ≤5 y with severe lead poisoning in Zamfara, Northern Nigeria: a retrospective analysis of programme data. PLoS Med [Internet]. 2014 [cited 2014 Oct 9];11:e1001739. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25291378. [DOI] [PMC free article] [PubMed]
- 10.Fournier L, Thomas G, Garnier R, Buisine A, Houze P, Pradier F, et al. 2,3-Dimercaptosuccinic acid treatment of heavy metal poisoning in humans. Med Toxicol Adverse Drug Exp [Internet]. 1986;3:499–504. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2851085. [DOI] [PubMed]
- 11.Bradberry S, Vale A. A comparison of sodium calcium edetate (edetate calcium disodium) and succimer (DMSA) in the treatment of inorganic lead poisoning. Clin Toxicol [Internet]. 2009 [cited 2014 May 2];47:841–58. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19852620. [DOI] [PubMed]