Abstract
Background. The clinical and microbiological characteristics of nontyphoidal Salmonella (NTS) meningitis in South Africa, where human immunodeficiency virus (HIV) prevalence is high (approximately 15% in persons ≥15 years of age), were reviewed.
Methods. From 2003 through 2013, 278 cases were identified through national laboratory-based surveillance. Clinical information (age, sex, outcome, Glasgow Coma Scale [GCS], and HIV status) was ascertained at selected sites. Isolates were serotyped; susceptibility testing and multilocus sequence typing on Salmonella enterica serovar Typhimurium isolates was performed. Multivariable logistic regression was used to determine factors associated with mortality outcome, using Stata software, version 13.
Results. Where age was ascertained, 139 of 256 (54.3%) patients were <15 years. Males represented 151 of 267 (56.6%). Mortality outcome was recorded for 112 of 146 (76.7%) enhanced surveillance patients; 53 of 112 (47.3%) died. Death was associated with GCS ≤13 (adjusted odds ratio [OR], 18.7; 95% confidence interval [CI], 3.0–118.5; P = .002) on multivariable analysis. Where data were available, all 45 patients aged >15 years were HIV infected, compared with 24 of 46 (52.2%) patients aged <5 years. Neonates were less likely to be HIV infected than infants aged 2–12 months (OR, 4.8; 95% CI, 1.1–21.1; P = .039).
Salmonella Typhimurium represented 106 of 238 (44.5%) serotyped isolates: 65 of 95 (68.4%) were ST313 vs ST19, respectively, and significantly associated with HIV-infected patients (P = .03) and multidrug resistance (OR, 6.6; 95% CI, 2.5–17.2; P < .001).
Conclusions. NTS meningitis in South Africa is highly associated with HIV in adults, with neonates (irrespective of HIV status), and with Salmonella Typhimurium ST313. GCS is the best predictor of mortality: early diagnosis and treatment are critical. Focused prevention requires further studies to understand the sources and transmission routes.
Keywords: Salmonella, meningitis, HIV, Salmonella Typhimurium ST313
Bacterial meningitis in Africa remains an important disease with a high associated mortality [1–7]. Meningitis in human immunodeficiency virus (HIV)–infected persons is frequently associated with cryptococcosis, tuberculosis, and Streptococcus pneumoniae, Haemophilus influenzae type b (Hib), and Neisseria meningitidis [3, 4, 8–12]. Numerous other pathogens have been described as a cause of meningitis among HIV-infected persons [1, 7, 9, 12, 13]. Nontyphoidal Salmonella (NTS) is emerging as a significant meningeal pathogen among HIV-infected persons, following the decline in incidence of S. pneumoniae and Hib with the introduction of pneumococcal conjugate and Hib conjugate vaccines [1, 6, 9, 12–14]. In previous series, in adults from South African institutions, NTS meningitis represented 16% of acute bacterial meningitis cases among HIV-infected patients [7]. An estimated 8% of all meningitis cases for which a microbiological diagnosis was made was attributed to acute bacterial meningitis [15]. NTS meningitis was not reported in pediatric series [16, 17].
The association between HIV and invasive NTS infections, including meningitis, was described early in the AIDS epidemic [18]. Case reports of adults with NTS meningitis frequently describe an association with HIV [7, 18–20], although other immunosuppressive conditions have been described [21–23]. Molyneux et al described NTS meningitis in a cohort of 105 Malawian children, aged between 2 months and 16 years, over a 10-year period [2]. Approximately half were HIV infected, and 12.4% were infected with malaria; mortality rates were >50% [2]. There are rare reports of NTS meningitis in previously healthy individuals [24], suggesting that comorbidity is common, but not an absolute prerequisite to infection.
A meta-analysis of published African studies suggests that Salmonella bacteremia accounts for 21.4% of all bacteremias [25], with an incidence rate of 227 per 100 000 [26]. In contrast, Salmonella meningitis accounted for <10% of all-cause meningitis (incidence rate of 20/100 000) in Malawian patients in 2012 [6], suggesting approximately 1% of NTS bacteremias results in meningitis. Salmonella enterica serovars Typhimurium and Enteritidis account for 65.2% and 33.1% of all invasive NTS infections, respectively [25], and similar observations have been made regarding the frequency with which these serovars occur in NTS meningitis [2]. In the past 30 years, the emergence of invasive Salmonella Typhimurium ST313, a sequence type associated with the African AIDS epidemic, has been highlighted [27, 28].
We describe the clinical and microbiological data associated with a series of patients presenting with NTS meningitis in South Africa, a country that is largely malaria-free, with high HIV prevalence affecting approximately 15% of the population ≥15 years of age [29], to better understand the association with HIV, potential predisposing conditions, and the role of Salmonella Typhimurium ST313.
METHODS
Case Definition
National active laboratory-based surveillance for invasive salmonellosis, defined as the isolation of NTS from a normally sterile body site, including Salmonella meningitis, was performed by the Centre for Enteric Diseases (CED) of the National Institute for Communicable Diseases in South Africa from 2003 through 2013, as previously described [30]. A case of NTS meningitis was defined as any patient from whom NTS was isolated from cerebrospinal fluid (CSF). A nosocomial infection was defined as NTS meningitis infection in a patient in whom the diagnosis of meningitis was made ≥48 hours after the patient had been admitted to a medical or long-term-care facility. We used the annualized South African population from 2003 to 2013, which increased from 45.80 million to 52.98 million over the period, to calculate incidence (http://www.statssa.gov.za/publications).
All diagnostic microbiology laboratories in South Africa were requested to submit Salmonella isolates from patients fulfilling the case definition, for serotyping and susceptibility testing, supplemented by audits from 2005 to identify isolates not received by the reference laboratory. Additional clinical information was collected on cases at 24 sentinel hospitals in 9 provinces through the Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa network, a national surveillance system sharing resources to monitor diseases of public health importance. This included data on HIV status, other immunosuppressive conditions, admission and discharge dates, antibiotic exposure, timing of meningitis diagnosis (number of hours after patient admission), and disease outcome.
Laboratory Characterization
All Salmonella isolates received were serotyped by CED according to established protocols (Mast Group, Merseyside, United Kingdom; Bio-Rad, Marnes-la-Coquette, France; Remel, Kent, United Kingdom; Statens Serum Institut, Copenhagen, Denmark). Minimum inhibitory concentrations (MICs) were determined for the following antimicrobials: ampicillin, chloramphenicol, trimethoprim-sulfamethoxazole (cotrimoxazole), tetracycline, ciprofloxacin, ceftriaxone, and ceftazidime, using Etest strips according to the manufacturer's instructions (bioMérieux, Marcy-l’Étoile, France). Multidrug resistance was defined as resistance to ≥3 of these antimicrobials. Production of extended-spectrum β-lactamase was tested for using the Mast Laboratories double disk method, according to the manufacturer's instructions (Mast Diagnostics, Bootie, England).
Genotypic Characterization of Salmonella Typhimurium
Genotyping of Salmonella Typhimurium isolates was performed using multilocus sequence typing (MLST), as described at the Salmonella MLST database (http://mlst.warwick.ac.uk/mlst/dbs/Senterica), including DNA sequencing analysis of the following 7 housekeeping genes: aroC, dnaN, hemD, hisD, purE, sucA, and thrA. DNA sequencing was performed using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, California) and an Applied Biosystems 3500 Genetic Analyzer. DNA sequences were collated and analyzed using the DNASTAR Lasergene (version 8.0) software (DNASTAR, Inc, Madison, Wisconsin), followed by analysis at the Salmonella MLST database, where allele numbers and a MLST sequence type (ST) were assigned.
Ethical Approval
Ethical approval for this study was granted by the Human Research Ethics Committee of the University of the Witwatersrand (M110601).
Statistical Analysis
Variables analyzed included age, sex, HIV status, Glasgow Coma Scale (GCS), nosocomial infection, cotrimoxazole prophylaxis, use of antiretrovirals (ARVs), CD4+ count, other comorbid conditions, isolation of Salmonella Typhimurium or multidrug-resistant Salmonella, and Salmonella sequence type. Clinically relevant groupings were created for continuous variables, to aid interpretation of results. Univariate and multivariate logistic regression were used to determine factors associated with mortality. Multivariate analysis using a manual forward stepwise progression was used, with a cutoff of P < .1 for the univariate analysis, dropping nonsignificant factors. Model fit was assessed by using the Hosmer–Lemeshow goodness-of-fit test. Analysis was performed using Stata version 13 (StataCorp, College Station, Texas). Two-sided P values of <.05 were considered significant throughout. Due to the nature of the study, important variables had missing data. For both univariate and multivariate analyses, a complete case analysis was conducted in which patients with missing data were excluded from the analysis.
RESULTS
Cumulative Data
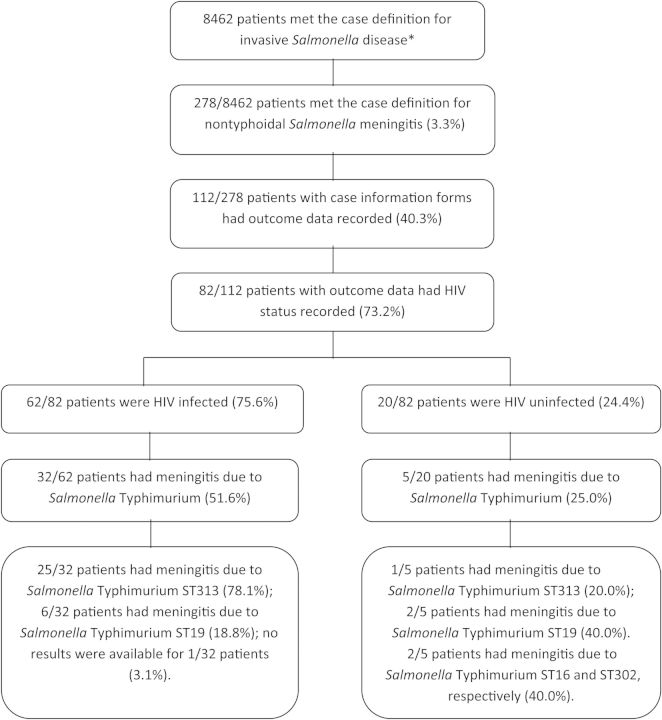
Figure 1 summarizes case numbers, including relevant clinical and microbiological data pertaining to these. We identified a total of 278 cases of laboratory-confirmed NTS meningitis in South African hospitals from 2003 through 2013, representing 3.3% of all invasive salmonellosis (Figure 1). Bimodal peaks of disease incidence were observed in children <5 years of age and adults >15 years of age (Figure 2A). NTS was isolated from the CSF only in 196 (70.5%) patients; NTS was additionally isolated from blood, stool, or other body sites of 82 (29.5%) patients. The average incidence of NTS meningitis during the period was 5 per 10 000 000 per year. Clinical data are summarized in Table 1.
Figure 1.
Patients with nontyphoidal Salmonella (NTS) meningitis identified in South Africa, 2003–2013. *Invasive disease was defined as the isolation of NTS from a normally sterile body site. Abbreviations: HIV, human immunodeficiency virus; ST, sequence type.
Figure 2.
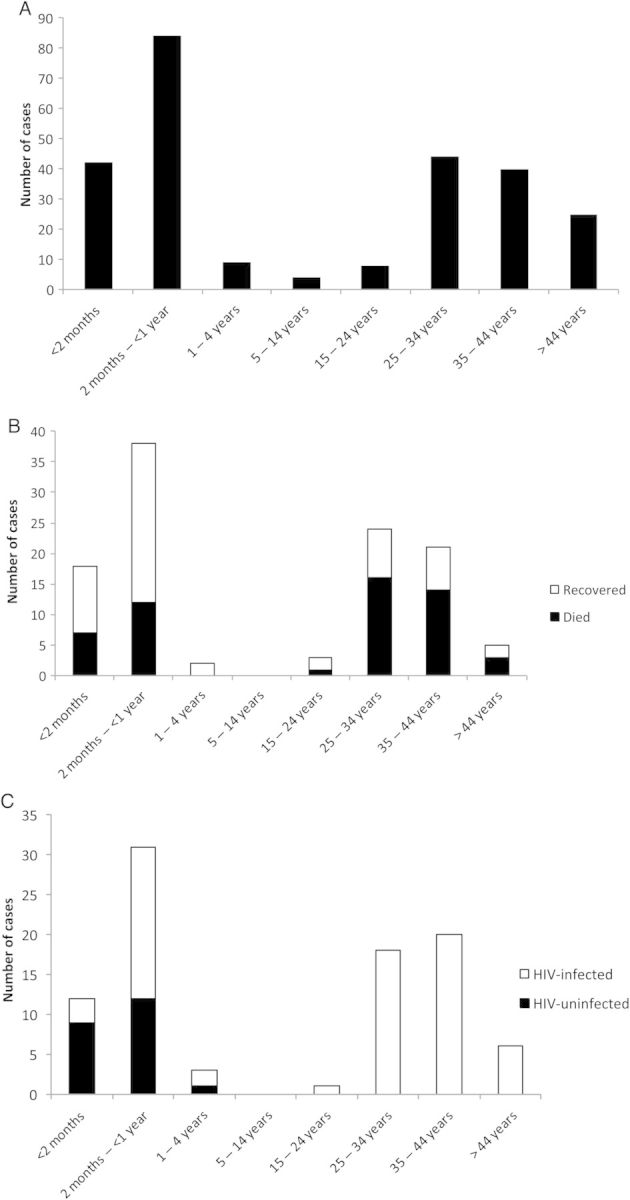
A, Number of cases of nontyphoidal Salmonella (NTS) meningitis (n = 256) by age group, 2003–2013. B, Number of cases of NTS meningitis (n = 111) by age group and mortality, 2003–2013. One patient who recovered did not have an age recorded. C, Number of cases of NTS meningitis (n = 91) by age group and human immunodeficiency virus (HIV) status, 2003–2013. One HIV-infected patient did not have an age recorded.
Table 1.
Clinical Characteristics Associated With Patients With Salmonella Meningitis in South Africa, 2003–2013
| Characteristic | Outcome |
Total,a No. (%) | P Value | |
|---|---|---|---|---|
| Survived, No. (%) | Died, No. (%) | |||
| Data from all cases | 278 (100) | |||
| Age | ||||
| <2 mo | 11 (26.2) | 7 (16.7) | 42 (16.4) | … |
| 2 mo to <1 y | 26 (30.9) | 12 (14.3) | 84 (32.8) | .5 |
| 1–4 y | 2 (22.2) | 0 (0.0) | 9 (3.5) | .2 |
| 5–14 y | … | … | 4 (1.6) | … |
| 15–24 y | 2 (25.0) | 1 (12.5) | 8 (3.1) | .9 |
| 25–34 y | 8 (18.2) | 16 (36.4) | 44 (17.2) | .07 |
| 35–44 y | 7 (17.5) | 14 (35.0) | 40 (15.6) | .08 |
| >44 y | 2 (8.0) | 3 (12.0) | 25 (9.8) | .4 |
| Sex | ||||
| Female | 20 (17.2) | 26 (22.4) | 116 (43.4) | … |
| Male | 37 (24.5) | 26 (17.2) | 151 (56.6) | .1 |
| Sentinel site data | 59 (52.7) | 53 (47.3) | 112 (52.5) | |
| HIV status | ||||
| Uninfected | 17 (77.3) | 3 (13.6) | 22 (23.9) | … |
| Infected | 32 (45.7) | 30 (42.9) | 70 (76.1) | .008 |
| Glasgow Coma Scale | ||||
| ≤13 | 2 (20.0) | 8 (80.0) | 10 (23.3) | … |
| >13 | 27 (81.8) | 5 (15.2) | 33 (76.7) | .3 |
| Nosocomial infection | ||||
| No | 52 (51.5) | 45 (44.6) | 101 (88.6) | … |
| Yes | 6 (46.2) | 6 (46.2) | 13 (11.4) | .8 |
| Cotrimoxazole prophylaxis | ||||
| No | 40 (65.6) | 20 (32.8) | 61 (75.3) | … |
| Yes | 10 (50.0) | 9 (45.0) | 20 (24.7) | .3 |
| Antiretrovirals | ||||
| No | 31 (57.4) | 22 (40.7) | 54 (78.3) | … |
| Yes | 9 (60.0) | 5 (33.3) | 15 (21.7) | .7 |
| CD4 count, cells/µL | ||||
| ≤200 | 10 (34.5) | 16 (55.2) | 29 (87.9) | … |
| >200 | 2 (50.0) | 2 (50.0) | 4 (12.1) | .7 |
| Other comorbidity | ||||
| No | 40 (50.6) | 29 (25.3) | 79 (63.7) | … |
| Yes | 19 (42.2) | 24 (53.3) | 45 (36.3) | .2 |
| Salmonella serotype | ||||
| Enteritidis | 21 (55.3) | 12 (31.6) | 38 (26.0) | … |
| Typhimurium | 17 (27.4) | 29 (46.8) | 62 (42.5) | .02 |
| Other | 21 (45.7) | 12 (26.1) | 46 (31.5) | .2 |
| Salmonella multidrug resistance | ||||
| No | 40 (46.5) | 27 (31.4) | 86 (63.7) | … |
| Yes | 16 (32.7) | 22 (44.9) | 49 (36.3) | .08 |
| Salmonella Typhimurium sequence type | ||||
| ST19 | 6 (35.3) | 4 (23.5) | 17 (30.9) | … |
| ST313 | 8 (21.1) | 23 (60.5) | 38 (69.1) | .05 |
Abbreviations: HIV, human immunodeficiency virus; ST, sequence type.
a Total includes those patients for whom outcome was unknown.
Clinical Data
Additional clinical information was available from 146 (52.5%) patients with NTS meningitis: 112 had a known outcome (76.7%) (Figure 1), and 111 (99.1%) and 82 (73.2%) had an available age and HIV status, respectively. Sex was recorded in 267 patients: 151 of 267 (56.6%) were male. Table 2 summarizes clinical risk factors for mortality. Nineteen of 58 (32.8%) children aged <15 years died, compared with 34 of 53 (64.2%) patients aged ≥15 years (P = .001; Table 2, Figure 2B). HIV status was recorded for 92 of 146 (63.0%) patients; 70 (76.1%) were HIV infected (Figure 2C). Among patients with recorded age and HIV status, all 45 patients aged ≥15 years were HIV infected, compared with 24 of 46 (52.2%) patients aged <5 years (Figure 2C). Children aged between 5 and 15 years had no outcome or HIV data recorded. Nine of 11 (81.9%) infants aged >1 year for whom data were available had a history of HIV exposure at birth.
Table 2.
Univariate and Multivariate Analysis of Risk Factors Associated With Mortality in Patients With Salmonella Meningitis in South Africa, 2003–2013
| Characteristic | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | P Value | AOR | (95% CI) | P Value | |
| Age | ||||||
| <15 y | 1 | … | … | 1 | … | … |
| ≥15 y | 3.7 | (1.7–8.1) | .001 | 2.3 | (.4–12.1) | .338 |
| Sex | ||||||
| Male | 1 | … | … | |||
| Female | 1.9 | (.9–4.0) | .117 | |||
| HIV status | ||||||
| Uninfected | 1 | … | … | 1 | … | … |
| Infected | 5.3 | (1.4–20.0) | .013 | 0.9 | (.1–15.7) | .987 |
| Glasgow Coma Scale | ||||||
| ≤13 | 1 | … | … | 1 | … | … |
| >13 | 21.6 | (3.5–133.3) | .001 | 18.7 | (3.0–118.5) | .002 |
| Nosocomial infection | ||||||
| No | 1 | … | … | |||
| Yes | 1.1 | (.3–3.7) | .844 | |||
| Cotrimoxazole prophylaxis | ||||||
| No | 1 | … | … | |||
| Yes | 1.8 | (.6–5.1) | .272 | |||
| Antiretrovirals | ||||||
| No | 1 | … | … | |||
| Yes | 0.8 | (.2–2.7) | .695 | |||
| CD4+ count, cells/µL | ||||||
| ≤200 | 1 | … | … | |||
| >200 | 0.6 | (.8–5.2) | .663 | |||
| Other comorbid conditions | ||||||
| No | 1 | … | … | |||
| Yes | 1.7 | (.8–4.0) | .157 | |||
| Salmonella serotype | ||||||
| Other serotypes | 1 | … | … | 1 | … | … |
| Typhimurium | 3.0 | (1.3–6.5) | .006 | 0.6 | (.1–4.8) | .659 |
| Salmonella multidrug resistance | ||||||
| No | 1 | … | … | 1 | … | … |
| Yes | 2.0 | (.9–4.6) | .084 | 0.6 | (.1–5.5) | .648 |
| Salmonella Typhimurium sequence type | ||||||
| ST313 | 1 | … | … | 1 | … | … |
| ST19 | 0.2 | (.05–1.04) | .056 | 1.0 | (.04–23.0) | .994 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; ST, sequence type.
Neonates were significantly less likely to be HIV infected than infants aged between 2 months and 1 year (3/12 [25.0%] vs 19/31 [61.3%]; odds ratio [OR], 4.8; 95% confidence interval [CI], 1.1–21.1; P = .039); however, there was no difference in mortality between the 2 groups (7/18 [38.9%] vs 12/38 [31.6%]; OR, 0.7; 95% CI, .2–2.3; P = .590). Nosocomial NTS meningitis was diagnosed in 13 of 118 (11.0%) patients for whom admission dates were available.
The length of hospital stay varied between 0 and 77 days (median of 12 days). Cases were 7 times more likely to die within the initial 10 days of their hospital stay (OR, 7.1; 95% CI, 3.1–16.4; P < .001). There was no significant association between nosocomial vs community-acquired infections and mortality (Table 2).
CD4+ cell counts were available for 33 of 146 (22.6%) patients. A CD4+ count <200 cells/µL was not significantly associated with mortality (Table 2). Whether the HIV-infected patients received ARVs was recorded for 41 patients with NTS meningitis; 10 (24.4%) received ARVs at the time of diagnosis of NTS meningitis. There was no significant effect on the mortality between HIV-infected persons on ARVs and those not on ARVs (P = .7; Table 2).
The GCS was recorded for 43 patients: 7 of 43 (16.3%) had a GCS ≤13. For the 35 (81.4%) patients with GCS recorded for whom HIV status was known, 9 of 10 HIV-uninfected patients had a GCS >13 (90.0%), compared with 18 of 25 (72.0%) HIV-infected patients. Multivariable analysis confirmed that mortality was associated with a GCS ≤13 (adjusted OR, 18.7; 95% CI, 3.0–118.5; P = .002; Table 2). The Hosmer–Lemeshow goodness-of-fit test confirmed the fitness of the model (χ2 = 0.04, P = .8411).
Comorbidities potentially associated with NTS meningitis were recorded in 45 of 124 (36.2%) patients. Laboratory-confirmed cryptococcal meningitis was diagnosed in 3 of 45 (6.7%); 5 of 45 (11.1%) had a history of head injury; and 29 of 45 (64.4%) were receiving therapy for tuberculosis or had a history of active tuberculosis, but the site of tuberculosis infection was not stated (ie, pulmonary vs extrapulmonary or central nervous system infection). One HIV-uninfected 3-month-old infant receiving treatment for tuberculosis had a GCS of 1. No patients were coinfected with other etiological agents of acute bacterial meningitis (S. pneumoniae, N. meningitidis, or Hib). None of the patients had malaria.
In addition, 81 of 146 (55.5%) patients had data on cotrimoxazole prophylaxis. Cotrimoxazole prophylaxis was not significantly associated with outcome (P = .3; Table 2). Individual patient records suggested that all patients received appropriate antimicrobial therapy for NTS meningitis on admission or were changed to appropriate antimicrobial therapy once susceptibility data were available for the Salmonella isolated from the patients’ CSF.
Microbiological Data
Microbiological characterization was undertaken on 247 of 278 (88.8%) isolates. Salmonella Typhimurium predominated (Table 3).
Table 3.
Commonest Salmonella Serotypes, 2003–2013, and Antimicrobial Resistance Profiles (n = 247) Associated With Salmonella Meningitis in South Africa
| Serotype (No. Tested) | Selected Antimicrobials Tested Against Strains of Nontyphoidal Salmonella |
|||||
|---|---|---|---|---|---|---|
| Ampicillina | Chloramphenicola | TMP-SMXa | Tetracyclinea | Ciprofloxacina | ESBL Productiona,b | |
| Typhimurium (104) | 72 (69.2) | 39 (37.5) | 67 (64.4) | 47 (45.1) | 30 (28.8) | 9 (8.6) |
| Enteritidis (66) | 5 (7.6) | 3 (435) | 3 (4.5) | 8 (12.1) | 14 (21.2) | 1 (1.5) |
| Isangi (15) | 14 (93.3) | 13 (86.7) | 14 (93.3) | 15 (100.0) | 9 (60.0) | 12 (80.0) |
| Virchow (10) | 9 (90.0) | 7 (70.0) | 8 (80.0) | 8 (80.0) | 0 (0.0) | 8 (80.0) |
| Dublin (10) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other (42) | 10 (23.8) | 9 (21.4) | 12 (28.6) | 17 (40.5) | 3 (7.1) | 6 (14.3) |
Data are presented as No. (%). Complete antimicrobial resistance data were not available for all serotypes tested.
Abbreviations: ESBL, extended-spectrum β-lactamase; TMP-SMX, trimethoprim-sulfamethoxazole.
a No. of intermediate and fully resistant isolates.
b Production of extended-spectrum β-lactamase.
Patient outcome information was available for 111 of 247 (44.9%) cases with NTS serovar and antimicrobial susceptibility data. Patients infected with Salmonella Typhimurium were more likely to die compared with other serovars on univariate analysis (P = .006); this was not significant on multivariable analysis (Table 2). Death rates were not significantly higher in those patients who had multidrug-resistant isolates.
Where HIV status was known, 39 of 70 (55.7%) HIV-infected patients had meningitis due to Salmonella Typhimurium, compared with 31 of 70 (44.3%) HIV-infected patients with NTS meningitis due to other serovars, although this was not significant (P = .08).
Multidrug resistance (defined above) was detected in 103 of 247 (41.7%) of the isolates; 56 of 247 (22.7%) were resistant or intermediately resistant to ciprofloxacin (Table 3). Salmonella enterica serovar Isangi and serovar Virchow isolates were more likely to be extended-spectrum β-lactamase producers than other serovars (20/30 [66.7%] for Salmonella Isangi and Salmonella Virchow vs 17/247 [6.9%] for other serovars; OR, 46.1; 95% CI, 15.4–138.3). Salmonella Typhimurium was more likely to be multidrug resistant than other serovars (62/104 [59.6%] vs 34/143 [23.8%] for other serovars; OR, 4.7; 95% CI, 2.7–8.2; P < .001).
MLST of Salmonella Typhimurium
Ninety-seven of 104 Salmonella Typhimurium isolates (93.3%) were MLST subtyped (Table 4). There was a trend toward association of outcome with meningitis due to Salmonella Typhimurium ST313 on univariate analysis (P = .056; Table 2). HIV-infected patients were significantly more likely to be infected by Salmonella Typhimurium ST313 (P = .03) compared with other sequence types (Table 4). Salmonella Typhimurium ST16 and ST302 were isolated from HIV-uninfected patients.
Table 4.
Association of Salmonella Typhimurium Multilocus Sequence Type, Human Immunodeficiency Virus Infection, and Multidrug Resistance (Resistance to ≥3 Antimicrobials)
| Salmonella Typhimurium Sequence Type (n = 97) | HIV-Uninfected Patients (n = 7) | HIV-Infected Patients (n = 38) | Nonresistant Isolates or Isolates With Limited Resistance | Multidrug-Resistant Isolatesa |
|---|---|---|---|---|
| 16 (n = 1) | 1 (100.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) |
| 19 (n = 30) | 4 (28.6) | 10 (71.4) | 21 (70.0) | 9 (30.0) |
| 302 (n = 1) | 1 (100.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) |
| 313 (n = 65) | 1 (3.4) | 28 (96.6) | 17 (26.1) | 48 (73.8) |
Data are presented as No. (%).
Abbreviation: HIV, human immunodeficiency virus.
a Includes intermediate resistance.
Multidrug resistance was significantly associated with Salmonella Typhimurium ST313 (48/65 [73.8%]) compared with Salmonella Typhimurium ST19 (9/30 [30.0%]) (OR, 6.6; 95% CI, 2.5–17.2; P < .001).
DISCUSSION
To our knowledge, this is the largest series of Salmonella meningitis described. Molyneux et al [2] described a large series in children aged 2 months to 16 years in Malawi. Predisposing conditions in Malawi included HIV infection and malaria [2]. An earlier publication has highlighted NTS as a cause of meningitis in HIV-infected South African adult patients and described the associated characteristics [7], NTS meningitis representing approximately 1.3% of all-cause meningitis in this age group [15]. Previously, we have compared South African and Malawian data, reviewing the role of NTS in invasive disease in these 2 countries. The countries both showed a bimodal age distribution, with disease occurring primarily in young children and adults aged 20–50 years, confirming the importance of these ages in association with invasive disease due to NTS [31].
Our report again highlights the vulnerability of children aged <5 years to NTS meningitis. All the adults in our series and 58% of children <5 years of age were HIV infected, although none had malaria. A similar distribution in patients’ ages occurs with invasive shigellosis in South Africa, with excessive case numbers occurring in the very young and a second peak from early adulthood [30].
In addition to our series, the strong association of NTS meningitis with HIV infection in adults is suggested by case reports in the literature [18–20, 32]. Predisposing conditions, which we did not observe, include autoimmune conditions or other coinfections [21–23]; rarely, patients may have no predisposing conditions [24].
In the follow-up of the Malawian pediatric meningitis cases, impaired consciousness was significant in the outcome of cases of NTS meningitis [1]. We had similar findings in adults and children. Patients presenting with a GCS ≤13 were at a greater risk of death, irrespective of HIV status. Of note, none of the HIV-uninfected patients who had a recorded GCS >13 died. Previous reports from South Africa on meningitis due to N. meningitidis and S. pneumoniae emphasize the significance of severity of illness at presentation [3, 4].
Death rates in children in our series were lower than those described in the Malawian series [1, 2]; almost half of the deaths were in adults. Wall et al reviewed meningitis due to all causes in adolescents and adults in Malawi and similarly found that GCS was the strongest independent predictor of mortality, using a cutoff of 11 rather than 13 [5].
Irrespective of age, HIV infection was a major contributing factor for death in the univariate model. In South Africa, excessive deaths due to meningococcal and pneumococcal meningitis in association with HIV infection have been reported [3, 4], emphasizing the vulnerability of these patients to severe infections. In Malawian adults, mortality due to bacterial meningitis increased over time [5, 6]: we did not follow patients after discharge, but longer hospital stay was associated with improved survival in our series.
Antiretroviral therapy did not impact mortality in this study, stressing the importance of early diagnosis and treatment of NTS meningitis. Our patients also received appropriate antimicrobial therapy; incorrect treatment did not play a major role in the outcome. We postulated that other comorbidities may have contributed to the development of NTS meningitis, but data were too scanty to develop definitive conclusions.
Neonates and young children are particularly vulnerable groups warranting further consideration. We did not have access to information posthospitalization, but the Malawian study suggested that infants and young children who recover from the acute infection are susceptible to neurological sequelae [1, 2]. Although mortality was independent of HIV status in our series, a greater proportion of infants who acquired NTS meningitis were HIV exposed at birth. Absence of maternal immunity in HIV-infected mothers may increase the risk in infants to acquiring NTS meningitis, which should be included in the differential diagnosis of causes of neonatal and infant meningitis in settings of high HIV seroprevalence [13, 33–36]. Previously, we described the role of childhood Shigella infections predisposing HIV-infected women to invasive shigellosis [30]. In this instance, the reverse appears to be true: Maternal HIV infection may predispose neonates to invasive salmonellosis, confirming the importance of maternal health and a competent immune system in decreasing infant mortality in South Africa [37].
In immunosuppressed patients, whether due to HIV infection or extreme youth, innate characteristics of Salmonella may predispose the organism to invading the central nervous system. Salmonella Typhimurium has the ability to adhere to, penetrate, and invade the brain microvascular endothelium, in association with a proinflammatory immune response [38]. Our understanding of the organism may need to be altered to adapt to new management paradigms; following the introduction of new vaccines to prevent childhood meningitis, new pathogens associated with meningitis may be seen to emerge [9, 12, 13].
Salmonella serovar was a better predictor of outcome than multidrug resistance: Salmonella Typhimurium was more highly associated with death. Salmonella Typhimurium ST313 is well associated with HIV [27]; we found that the organism contributes significantly to NTS meningitis in HIV-infected patients. Besides ST313, representing 67% of typed Salmonella Typhimurium isolates, ST19 represented 31% of typed isolates; ST19 is commonly described worldwide, including from South Africa [39]. A stronger association between HIV infection and Salmonella meningitis due to Salmonella Typhimurium ST313, compared with Salmonella Typhimurium ST19, was noted. In our previous report of predominantly noninvasive Salmonella Typhimurium ST19 infection, most of the patients were HIV uninfected [39].
To prevent NTS meningitis infections, further studies are needed regarding the source of infections in South Africa. We demonstrated that nosocomial NTS meningitis was rare; infections were likely community acquired. Salmonella are ubiquitous, and the association between foodborne disease transmission and human-to-human transmission with invasive disease is recognized [24, 40, 41]; although transmission is often assumed to be foodborne, actual routes are not always clear. Attention should be paid to preventing mother-to-child transmission, including maternal screening for fecal pathogens, and ensuring maternal health in a potentially disadvantaged subset of HIV-infected individuals [37, 42–45].
This study had limitations. Clinical data were collected at selected sites only and may not be relevant to all the cases, and clinical data were often incomplete. Not all patients at enhanced sites had outcome data, HIV results, CD4+ counts, and access to ARV treatment. Data on tuberculous meningitis, prior treatment for cryptococcal meningitis, or acute bacterial meningitis were not collected. Insufficient data were collected on maternal HIV status in pediatric cases, and this impact could not be fully assessed. Fecal cultures were not performed on the mothers of infants in association with meningitis; we cannot comment on whether maternal carriage of NTS contributed to infection in infants. Incomplete clinical data meant that the ubiquity and consequences of Salmonella Typhimurium ST313 in NTS meningitis could not be fully elucidated. We elected not to do imputations for the missing data, believing that on univariate analysis at least, sufficient association was shown between outcome, age, HIV status, GCS, Salmonella Typhimurium, and Salmonella Typhimurium sequence types to highlight critical factors associated with Salmonella meningitis. Considering the high proportion of missing data in our data set, imputation (especially of binary data) could lead to biased inference. The complete case analysis that was conducted in this study assumes that data are missing completely at random. Our data may not be missing completely at random—but rather missing at random where relevant information regarding outcome, HIV result, or consent at a sentinel site was not collected—or not missing at random: Sentinel hospitals are typically referral hospitals where HIV-infected persons may preferentially present. We do believe we identified the majority of NTS meningitis cases over the period; typically in South Africa, patients with suspected meningitis will have lumbar punctures performed and CSF samples submitted to the laboratory for culture.
In conclusion, we describe a national series of laboratory-confirmed meningitis cases due to NTS over an 11-year period, highlighting the importance of disease severity as measured by GCS, HIV status in adults, and infection in neonates and infants, and the association of outcome and HIV status with specific serovars and sequence types. Early diagnosis and appropriate therapy may decrease death rates, and optimizing maternal health may lower case numbers in neonates and infants. Better understanding of the role of Salmonella Typhimurium, specifically Salmonella Typhimurium ST313, may also assist in controlling invasive disease due to NTS.
Notes
Acknowledgments. We thank our many partners in GERMS-SA, National Institute for Communicable Diseases (NICD), a division of the National Health Laboratory Service (NHLS), for submission of isolates to the Centre for Enteric Diseases. GERMS-SA includes Carel Haummann, Patricia Hanise, Pieter Ekermans; Sandeep Vasaikar (Eastern Cape); Anwar Hoosen, Dominique Goedhals, Justyna Wojno, Madeleine Pieters (Free State); Alan Karstaedt, Caroline Maluleka, Charl Verwey, Charles Feldman, Jeannette Wadula, Kathy Lindeque, Maphoshane Nchabeleng, Norma Bosman, Ranmini Kularatne, Ruth Lekalakala, Sharona Seetharam, Theunis Avenant, Nicolette du Plessis, Trusha Nana, Vindana Chibabhai (Gauteng); Asmeeta Burra, Constant Kapongo, Fathima Naby, Halima Dawood, Koleka Mlisana, Lisha Sookan, Praksha Ramjathan, Prasha Mahabeer, Romola Naidoo, Sumayya Haffejee, Yacoob Coovadia (Kwa-Zulu Natal); Andries Dreyer, Ken Hamese (Limpopo); Greta Hoyland, Jacob Lebudi (Mpumalanga); Dhamiran Naidoo, Eunice Weenink; Riezaah Abrahams (Northern Cape); Ebrahim Variava, Eduard Silberbauer (North West); Catherine Samuel, Preneshni Naicker (Western Cape); Adrian Brink, Charlotte Sriruttan, Inge Zietsman, Maria Botha, Peter Smith, Xoliswa Poswa (AMPATH); Chetna Govind, Keshree Pillay, Suzy Budavari, (LANCET); Marthinus Senekal (PathCare); Cynthia Whitney, Stephanie Schrag, Jennifer Verani (Centers for Disease Control and Prevention [CDC]); Ananta Nanoo, Anne von Gottberg, Cecilia Miller, Cheryl Cohen, Claire von Mollendorf, Languta Sibiya, Linda de Gouveia, Linda Erasmus, Mmakgomo Rakhudu, Marshagne Smith, Melony Fortuin-de Smidt, Nazir Ismail, Nelesh Govender, Nevashan Govender, Nireshni Naidoo, Olga Perovic, Ruth Mpembe, Sarona Lengana, Sonwabo Lindani, Susan Meiring, Vanessa Quan (NICD).
Author contributions. All authors contributed to the writing of this manuscript and reviewed the final content. K. H. K., F. J. A., and K. P. K. designed the study. K. H. K., A. S., and P. C.-G. developed the database. A. S., A. M. S., H. I., and N. P. T. were responsible for the laboratory characterization of Salmonella isolates. K. H. K., A. M., and P. C.-G. completed the data analysis.
Disclaimer. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the NICD/NHLS or the CDC.
Financial support. This publication was made possible by a grant from the Bill & Melinda Gates Foundation (OPP1125993). This research has been supported by the NICD/NHLS and the President's Emergency Plan for AIDS Relief through the CDC (5U2GPS001328) and, in part, for 2003–2006 by funds from the US Agency for International Development Antimicrobial Resistance Initiative, transferred via a cooperative agreement (number U60/CCU022088) from the CDC. For 2007–2009, it was supported by the CDC, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Global AIDS Program Cooperative Agreement (U62/PSO022901). K. H. K., A. S., A. M. S., H. I., and N. P. T. are permanent employees of the NHLS and receive no additional funding from other institutions resulting in a conflict of interest. A. M. is employed through the Global Disease Detection Program of the CDC, Pretoria, South Africa. P. C.-G. is funded through 5U2GPS001328, F. J. A. is staff at the CDC, and K. P. K. is employed by the Bill & Melinda Gates Foundation.
Supplement sponsorship. This article appeared as part of the supplement “Invasive Salmonella Disease in Africa,” sponsored by the University of Otago.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa (GERMS-SA), Carel Haummann, Patricia Hanise, Pieter Ekermans, Sandeep Vasaikar, Anwar Hoosen, Dominique Goedhals, Justyna Wojno, Madeleine Pieters, Alan Karstaedt, Caroline Maluleka, Charl Verwey, Charles Feldman, Jeannette Wadula, Kathy Lindeque, Maphoshane Nchabeleng, Norma Bosman, Ranmini Kularatne, Ruth Lekalakala, Sharona Seetharam, Theunis Avenant, Nicolette du Plessis, Trusha Nana, Vindana Chibabhai, Asmeeta Burra, Constant Kapongo, Fathima Naby, Halima Dawood, Koleka Mlisana, Lisha Sookan, Praksha Ramjathan, Prasha Mahabeer, Romola Naidoo, Sumayya Haffejee, Yacoob Coovadia, Andries Dreyer, Ken Hamese, Greta Hoyland, Jacob Lebudi, Dhamiran Naidoo, Eunice Weenink, Riezaah Abrahams, Ebrahim Variava, Eduard Silberbauer, Catherine Samuel, Preneshni Naicker, Adrian Brink, Charlotte Sriruttan, Inge Zietsman, Maria Botha, Peter Smith, Xoliswa Poswa, Chetna Govind, Keshree Pillay, Suzy Budavari, Marthinus Senekal, Cynthia Whitney, Stephanie Schrag, Jennifer Verani, Ananta Nanoo, Anne von Gottberg, Cecilia Miller, Cheryl Cohen, Claire von Mollendorf, Languta Sibiya, Linda de Gouveia, Linda Erasmus, Mmakgomo Rakhudu, Marshagne Smith, Melony Fortuin-de Smidt, Nazir Ismail, Nelesh Govender, Nevashan Govender, Nireshni Naidoo, Olga Perovic, Ruth Mpembe, Sarona Lengana, Sonwabo Lindani, Susan Meiring, and Vanessa Quan
References
- 1.McCormick DW, Wilson ML, Mankhambo L, et al. Risk factors for death and severe sequelae in Malawian children with bacterial meningitis, 1997–2010. Pediatr Infect Dis J 2013; 32:e54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molyneux EM, Mankhambo LA, Phiri A, et al. The outcome of non-typhoidal Salmonella meningitis in Malawian children, 1997–2006. Ann Trop Paediatr 2009; 29:13–22. [DOI] [PubMed] [Google Scholar]
- 3.Cohen C, Singh E, Wu HM, et al. Increased incidence of meningococcal disease in HIV-infected individuals associated with higher case-fatality ratios in South Africa. AIDS 2010; 24:1351–60. [DOI] [PubMed] [Google Scholar]
- 4.Nyasulu P, Cohen C, de Gouveia L, Feldman C, Klugman KP, von Gottberg A. Increased risk of death in human immunodeficiency virus-infected children with pneumococcal meningitis in South Africa, 2003–2005. Pediatr Infect Dis J 2011; 30:1075–80. [DOI] [PubMed] [Google Scholar]
- 5.Wall EC, Cartwright K, Scarborough M, et al. High mortality among adolescents and adults with bacterial meningitis in sub-Saharan Africa: an analysis of 715 cases from Malawi. PLoS One 2013; 8:e69783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wall EC, Everett DB, Mukaka M, et al. Bacterial meningitis in Malawian adults, adolescents, and children during the era of antiretroviral scale-up and Haemophilus influenzae type b vaccination, 2000–2012. Clin Infect Dis 2014; 58:e137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teckie G, Karstaedt A. Spontaneous adult gram-negative bacillary meningitis in Soweto, South Africa. Int J Infect Dis 2015; 30:38–40. [DOI] [PubMed] [Google Scholar]
- 8.Bogaerts J, Rouvroy D, Taelman H, et al. AIDS-associated cryptococcal meningitis in Rwanda (1983–1992): epidemiologic and diagnostic features. J Infect 1999; 39:32–7. [DOI] [PubMed] [Google Scholar]
- 9.Bottomley MJ, Serruto D, Safadi MA, Klugman KP. Future challenges in the elimination of bacterial meningitis. Vaccine 2012; 30(suppl 2):B78–86. [DOI] [PubMed] [Google Scholar]
- 10.Asselman V, Thienemann F, Pepper DJ, et al. Central nervous system disorders after starting antiretroviral therapy in South Africa. AIDS 2010; 24:2871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marais S, Meintjes G, Pepper DJ, et al. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis 2013; 56:450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iriso R, Ocakacon R, Acayo JA, Mawanda MA, Kisayke A. Bacterial meningitis following introduction of Hib conjugate vaccine in northern Uganda. Ann Trop Paediatr 2008; 28:211–6. [DOI] [PubMed] [Google Scholar]
- 13.Nansera D, Max I, Annet K, Gessner BD. Bacterial meningitis among children under the age of 2 years in a high human immunodeficiency virus prevalence area after Haemophilus influenzae type b vaccine introduction. J Paediatr Child Health 2012; 48:324–8. [DOI] [PubMed] [Google Scholar]
- 14.von Gottberg A, Cohen C, Whitelaw A, et al. Invasive disease due to Haemophilus influenzae serotype b ten years after routine vaccination, South Africa, 2003–2009. Vaccine 2012; 30:565–71. [DOI] [PubMed] [Google Scholar]
- 15.Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis 2010; 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madhi SA, Madhi A, Petersen K, Khoosal M, Klugman KP. Impact of human immunodeficiency virus type 1 infection on the epidemiology and outcome of bacterial meningitis in South African children. Int J Infect Dis 2001; 5:119–25. [DOI] [PubMed] [Google Scholar]
- 17.Wolzak NK, Cooke ML, Orth H, van Toorn R. The changing profile of pediatric meningitis at a referral centre in Cape Town, South Africa. J Trop Pediatr 2012; 58:491–5. [DOI] [PubMed] [Google Scholar]
- 18.Fraimow HS, Wormser GP, Coburn KD, Small CB. Salmonella meningitis and infection with HIV. AIDS 1990; 4:1271–3. [DOI] [PubMed] [Google Scholar]
- 19.Belloso WH, Romano M, Greco GS, et al. Recurrent meningitis and subarachnoid hemorrhage due to Salmonella in an HIV+ patient: case report and mini-review of the literature. Open AIDS J 2011; 5:62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonard MK, Murrow JR, Jurado R, Gaynes R. Salmonella meningitis in adults infected with HIV: case report and review of the literature. Am J Med Sci 2002; 323:266–8. [DOI] [PubMed] [Google Scholar]
- 21.Al-Aani FK, Abusalah S, Al-Aqeedi R, Ibrahim A. Salmonella meningitis in an adult with type B viral hepatitis and an incidental schwannoma. BMJ Case Rep 2009; doi:10.1136/bcr.11.2008.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerona JG, Navarra SV. Salmonella infections in patients with systemic lupus erythematosus: a case series. Int J Rheum Dis 2009; 12:319–23. [DOI] [PubMed] [Google Scholar]
- 23.Vargas PJ, King G, Navarra SV. Central nervous system infections in Filipino patients with systemic lupus erythematosus. Int J Rheum Dis 2009; 12:234–8. [DOI] [PubMed] [Google Scholar]
- 24.OhAiseadha CO, Dunne OM, Desmond F, O'Connor M. Salmonella meningitis and septicaemia in an non-immunocompromised adult, associated with a cluster of Salmonella Enteritidis PT 14b, Ireland, November 2009. Euro Surveill 2010; 15:19489. [PubMed] [Google Scholar]
- 25.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:417–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal Salmonella disease, 2010(1). Emerg Infect Dis 2015; 21:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okoro CK, Kingsley RA, Connor TR, et al. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet 2012; 44:1215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paglietti B, Falchi G, Mason P, et al. Diversity among human non-typhoidal salmonellae isolates from Zimbabwe. Trans R Soc Trop Med Hyg 2013; 107:487–92. [DOI] [PubMed] [Google Scholar]
- 29.National Department of Health. 2011 national antenatal sentinel HIV & syphilis prevalence survey, 2012. Available at: http://www.health.gov.za/index.php/2014-03-17-09-09-38/reports/. Accessed 21 August 2015.
- 30.Keddy KH, Sooka A, Crowther-Gibson P, et al. Systemic shigellosis in South Africa. Clin Infect Dis 2012; 54:1448–54. [DOI] [PubMed] [Google Scholar]
- 31.Feasey NA, Archer BN, Heyderman RS, et al. Typhoid fever and invasive nontyphoid salmonellosis, Malawi and South Africa. Emerg Infect Dis 2010; 16:1448–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez A, Teira R, Varona M, Gonzalez de ES, Santamaria JM. Recurrent Salmonella enteritidis meningitis in a patient with AIDS. Scand J Infect Dis 1995; 27:177–8. [DOI] [PubMed] [Google Scholar]
- 33.Anil M, Helvaci M, Ozkalay N, et al. Salmonella Typhimurium outbreak in a neonatal unit in Turkey. Indian J Pediatr 2009; 76:629–33. [DOI] [PubMed] [Google Scholar]
- 34.Cooke FJ, Ginwalla S, Hampton MD, et al. Report of neonatal meningitis due to Salmonella enterica serotype Agona and review of breast milk-associated neonatal Salmonella infections. J Clin Microbiol 2009; 47:3045–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukerji A, Sulowski C, Friedman JN, Opavsky MA. Salmonella Poona meningitis and mastitis causing neonatal meningitis. Pediatr Infect Dis J 2009; 28:1141–2. [DOI] [PubMed] [Google Scholar]
- 36.Wu HM, Huang WY, Lee ML, Yang AD, Chaou KP, Hsieh LY. Clinical features, acute complications, and outcome of Salmonella meningitis in children under one year of age in Taiwan. BMC Infect Dis 2011; 11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ndirangu J, Newell ML, Thorne C, Bland R. Treating HIV-infected mothers reduces under 5 years of age mortality rates to levels seen in children of HIV-uninfected mothers in rural South Africa. Antivir Ther 2012; 17:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Sorge NM, Zialcita PA, Browne SH, Quach D, Guiney DG, Doran KS. Penetration and activation of brain endothelium by Salmonella enterica serovar Typhimurium. J Infect Dis 2011; 203:401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith AM, Mthanthi MA, Haumann C, et al. Nosocomial outbreak of Salmonella Typhimurium primarily affecting a pediatric ward, South Africa, 2012. J Clin Microbiol 2014; 52:627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altekruse S, Hyman F, Klontz K, Timbo B, Tollefson L. Foodborne bacterial infections in individuals with the human immunodeficiency virus. South Med J 1994; 87:169–73. [DOI] [PubMed] [Google Scholar]
- 41.Keddy KH, Dwarika S, Crowther P, et al. Genotypic and demographic characterization of invasive isolates of Salmonella Typhimurium in HIV co-infected patients in South Africa. J Infect Dev Ctries 2009; 3:585–92. [DOI] [PubMed] [Google Scholar]
- 42.Bonnet F, Lewden C, May T, et al. Opportunistic infections as causes of death in HIV-infected patients in the HAART era in France. Scand J Infect Dis 2005; 37:482–7. [DOI] [PubMed] [Google Scholar]
- 43.Brooks JT, Kaplan JE, Holmes KK, Benson C, Pau A, Masur H. HIV-associated opportunistic infections—going, going, but not gone: the continued need for prevention and treatment guidelines. Clin Infect Dis 2009; 48:609–11. [DOI] [PubMed] [Google Scholar]
- 44.May MT, Sterne JA, Costagliola D, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet 2006; 368:451–8. [DOI] [PubMed] [Google Scholar]
- 45.May MT, Hogg RS, Justice AC, et al. Heterogeneity in outcomes of treated HIV-positive patients in Europe and North America: relation with patient and cohort characteristics. Int J Epidemiol 2012; 41:1807–20. [DOI] [PMC free article] [PubMed] [Google Scholar]