Abstract
Objectives
In the Fluid and Catheter Treatment Trial (FACTT) of the National Institutes of Health Acute Respiratory Distress Syndrome Network, a conservative fluid protocol (FACTT Conservative) resulted in a lower cumulative fluid balance and better outcomes than a liberal fluid protocol (FACTT Liberal). Subsequent Acute Respiratory Distress Syndrome Network studies used a simplified conservative fluid protocol (FACTT Lite). The objective of this study was to compare the performance of FACTT Lite, FACTT Conservative, and FACTT Liberal protocols.
Design
Retrospective comparison of FACTT Lite, FACTT Conservative, and FACTT Liberal. Primary outcome was cumulative fluid balance over 7 days. Secondary outcomes were 60-day adjusted mortality and ventilator-free days through day 28. Safety outcomes were prevalence of acute kidney injury and new shock.
Setting
ICUs of Acute Respiratory Distress Syndrome Network participating hospitals.
Patients
Five hundred three subjects managed with FACTT Conservative, 497 subjects managed with FACTT Liberal, and 1,124 subjects managed with FACTT Lite.
Interventions
Fluid management by protocol.
Measurements and Main Results
Cumulative fluid balance was 1,918 ± 323 mL in FACTT Lite, −136 ±491 mL in FACTT Conservative, and 6,992 ± 502 mL in FACTT Liberal (p < 0.001). Mortality was not different between groups (24% in FACTT Lite, 25% in FACTT Conservative and Liberal, p = 0.84). Ventilator-free days in FACTT Lite (14.9 ±0.3) were equivalent to FACTT Conservative (14.6±0.5) (p = 0.61) and greater than in FACTT Liberal (12.1 ±0.5, p < 0.001 vs Lite). Acute kidney injury prevalence was 58% in FACTT Lite and 57% in FACTT Conservative (p = 0.72). Prevalence of new shock in FACTT Lite (9%) was lower than in FACTT Conservative (13%) (p = 0.007 vs Lite) and similar to FACTT Liberal (11%) (p = 0.18 vs Lite).
Conclusions
FACTT Lite had a greater cumulative fluid balance than FACTT Conservative but had equivalent clinical and safety outcomes. FACTT Lite is an alternative to FACTT Conservative for fluid management in Acute Respiratory Distress Syndrome.
Keywords: acute kidney injury, adult respiratory distress syndrome, clinical protocols, critical illness, fluid therapy, shock
Conservative fluid management improves ventilator-free days and oxygenation in patients with the Acute Respiratory Distress Syndrome (ARDS). In the Fluid and Catheter Treatment Trial (FACTT) of the National Institutes of Health, National Heart Lung and Blood Institute, ARDS Network (NIH/NHLBI ARDS Network), patients were randomized and managed with either a conservative fluid protocol (FACTT Conservative) or a liberal fluid protocol (FACTT Liberal) (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/B130) (1). Both FACTT Conservative and FACTT Liberal protocols provided instructions for subjects with a mean arterial pressure greater than 60 mm Hg who had not received vasopressors for at least 12 hours. FACTT Conservative targeted a central venous pressure (CVP) of less than 4 mm Hg or a pulmonary artery occlusion pressure (PAOP) of less than 8 mm Hg, whereas FACTT Liberal targeted a CVP of 10–14 mm Hg or PAOP of 14–18 mm Hg. Management with the FACTT Conservative protocol resulted in a significantly lower cumulative fluid balance over 7 days. While there was no difference in 60-day mortality, the FACTT Conservative group had more ventilator-free days and an improved oxygenation index and lung injury score (1).
The FACTT Conservative and Liberal protocols are complex. They provide instructions determined by CVP or PAOP, urinary output, and an effective or ineffective circulation (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/B130). For subjects not in shock, there are a total of 18 different protocol cells with instructions that include dobutamine infusion, fluid bolus, or furosemide administration. There are no protocol-directed instructions for management of shock.
Fluid management was an important cointervention in the NIH/NHLBI ARDS Network studies following FACTT (2–4). The ARDS Network investigators developed a simplified conservative fluid protocol, FACTT Lite. FACTT Lite excluded instructions for ineffective circulation because the clinical examination findings of ineffective circulation did not correlate with cardiac index (5), and the instructions in the FACTT Conservative protocol for management of ineffective circulation were rarely encountered. FACTT Lite (Table 1) provides three possible instructions determined by the CVP and urine output: furosemide administration, fluid bolus, or no intervention. Similar to the original FACTT Conservative protocol, FACTT Lite contains instructions to withhold furosemide until the subject has achieved at least 12 hours of a mean arterial pressure greater than 60 mm Hg off vasopressors. Fluid management of subjects in shock was left to the discretion of the clinical team. Although the FACTT Lite protocol has been used in subsequent ARDS Network studies (2–4), its performance has never been formally evaluated.
Table 1. Simplified Conservative Fluid Management Protocol (Fluid and Catheter Treatment Trial Lite).
| Central Venous Pressure (Recommended) | Pulmonary Artery Occlusion Pressure (Optional) | Mean Arterial Pressure ≥ 60 mm Hg and Off Vasopressors ≥ 12 Hr | |
|---|---|---|---|
| Urine Output < 0.5 mL/kg/hr | Urine Output ≥ 0.5 mL/kg/hr | ||
| > 8 | > 12 | Furosemidea; reassess in 1 hr | Furosemidea; reassess in 4 hr |
| 4–8 | 8–12 | Give fluid bolus; reassess in 1 hr | Furosemidea; reassess in 4 hr |
| < 4 | < 8 | Give fluid bolus; reassess in 1 hr | No intervention; reassess in 4 hr |
recommended furosemide dosing = begin with 20 mg bolus or 3 mg/hr infusion or last known effective dose. Double each subsequent dose until goal achieved (oliguria reversal or intravascular pressure target) or maximum infusion rate of 24 mg/hr or 160 mg bolus reached. Do not exceed 620mg/d. Also, if patient has heart failure, consider treatment with dobutamine.
This protocol was initiated within 4hr of randomization in enrolled patients and continued until unassisted breathing or study day 7, whichever occurred first.
- Discontinue maintenance fluids.
- Continue medications and nutrition
- Manage electrolytes and blood products per usual practice.
- For shock, use any combination of fluid boluses (recommended fluid bolus = 15 mL/kg crystalloid [round to nearest 250 mL] or 1 unit packed red cells or 25 g albumin) and vasopressor(s) to achieve mean arterial pressure ≥ 60 mm Hg as fast as possible. Wean vasopressors as quickly as tolerated beginning 4 hr after blood pressure has stabilized.
- Withhold diuretic therapy in renal failure (defined as dialysis dependence, oliguria with serum creatinine > 3mg/dL, or oliguria with serum creatinine 0–3 with urinary indices indicative of acute renal failure) and until 12 hr after last fluid bolus or vasopressor given.
We retrospectively compared the performance of FACTT Lite with FACTT Conservative and FACTT Liberal. We hypothesized that the FACTT Lite protocol would be equivalent to FACTT Conservative, and more favorable than FACTT Liberal, with respect to cumulative fluid balance over 7 days, number of ventilator-free days, 60-day mortality, and prevalence of new onset shock and acute kidney injury.
Materials and Methods
We identified three cohorts for study: 1) subjects randomized to FACTT Conservative; 2) subjects randomized to FACTT Liberal; and 3) subjects enrolled in two later ARDS Network studies that used FACTT Lite (2–4). We excluded patients on chronic dialysis from the FACTT Lite cohort to match the exclusions of the original FACTT study. Presence of a central venous catheter was an inclusion criterion for FACTT and for the studies included in the FACTT Lite cohort.
Comparison of subjects in the FACTT Conservative and Liberal cohorts was previously reported (1) as part of the FACTT study that enrolled 1,000 subjects from June 2000 to October 2005, with a 60-day mortality of 25.2%. The ARDS Network studies that used FACTT Lite for cointervention control included a clinical trial of an aerosolized β2-agonist for treatment of acute lung injury (2) that enrolled 282 subjects from August 2007 to July 2008. The FACTT Lite cohort included 236 subjects from that study, with a 60-day mortality of 22%. The second study included in the FACTT Lite cohort was the Early Versus Delayed Enteral Feeding (3) and Omega-3 Fatty Acid/Antioxidant Supplementation for ARDS (4) study that enrolled 1,000 subjects between January 2008 and March 2011. The FACTT Lite cohort included 888 subjects from that study with a 60-day mortality of 22.2%.
We obtained data for each group of subjects from prospectively completed case report forms that included age, sex, enrollment Acute Physiology and Chronic Health Evaluation (APACHE) III score (6), baseline oxygenation variables (after randomization but before study interventions), and baseline CVP. We also collected data on daily fluid intake and output, creatinine, and receipt of vasopressors at baseline and for study days 1–7. Fluid intake included the total volume of crystalloid solutions, colloids, blood products, and enteral feeding solutions. Fluid output included urine, stool, gastric drainage, and any other fluid output.
The primary outcome was cumulative fluid balance over 7 days. In a sensitivity analysis, we excluded subjects in shock at baseline. We defined shock as receiving vasopressors to support blood pressure. Secondary outcomes included daily fluid balance, mean daily furosemide dose, ventilator-free days, and ICU-free days. Ventilator-free days are days alive and free from mechanical ventilation through day 28 (7). ICU-free days are days alive and out of the ICU through day 28. Safety outcomes were the prevalence of acute kidney injury and new onset shock during the study. We also reported 60-day mortality adjusted for age and APACHE III score in order to control for differences in severity of illness among the different studies (8).
We defined acute kidney injury as an increase in serum creatinine of 50% or an absolute increase of more than 0.3 mg/dL over a 48-hour window during study days 1–7 (9, 10). Because of the significant difference in fluid balance between FACTT Conservative and FACTT Liberal, we used a creatinine adjusted for fluid balance to define acute kidney injury, similar to prior analyses of acute kidney injury in FACTT (9). For FACTT Lite, baseline creatinine was the lowest value from the 24 hours before randomization. To calculate adjusted creatinine, we estimated the volume of distribution for creatinine on the day of randomization, which is equal to total body water and assumed to be 60% of the subject's total body weight at the time of randomization (11). For each study day, we calculated cumulative on-study fluid balance using the 24-hour fluid intake and output. Then, we calculated adjusted creatinine = (measured serum creatinine) × (1 + [on-study cumulative net fluid balance/total body water]).
We prospectively collected daily furosemide dosing for the FACTT Conservative and Liberal groups during the FACTT study. For the FACTT Lite cohort, we retrospectively collected furosemide doses from 18 hospitals in 10 of the 12 ARDS Network sites. In a randomly selected subgroup of subjects who were on mechanical ventilation, had a systolic blood pressure greater than or equal to 90 mm Hg, were not on a vasopressor, and had received furosemide, we compared mean daily dose of furosemide administered among the three cohorts. We stratified sampling by sites. We calculated the number of FACTT Lite subjects needed from each site according to a 2.9:1 sampling ratio of 2.9 FACTT Conservative and Liberal subjects to each FACTT Lite subject in order to achieve adequate power. We then randomly sampled daily furosemide doses for that weighted sample of ARDS Network subjects from each participating site.
The Intermountain Healthcare Institutional Review Board, Salt Lake City, Utah, approved a waiver of informed consent for this retrospective study using data from already completed ARDS Network clinical trials and retrospectively collected furosemide dosing data for the FACTT Lite cohort.
Statistical Analysis
Comparisons of continuous variables, such as fluid balance, between FACTT Lite and FACTT Conservative or FACTT Liberal groups were made with a t test. Comparison of continuous variables among all groups was made with a one-way analysis of variance. Comparisons of proportions among FACTT Lite and FACTT Conservative or FACTT Liberal groups were made using a chi-square test. Data are reported as mean ± sem unless otherwise indicated. A two-tailed α of 0.05 was used as the threshold for determining statistical significance. To account for baseline differences influencing 60-day mortality among the cohorts, we used a forward stepwise regression model to select independent variables important in predicting 60-day mortality (similar to an approach used in a prior ARDS Network study) (8). The identified variables (age and severity of illness) were then used in a bivariate prediction model to generate adjusted 60-day mortality outcomes.
Results
Demographics of the study populations are displayed in Table 2. We analyzed 1,124 subjects from the ARDS Network studies managed with the FACTT Lite protocol. We excluded 40 subjects on chronic dialysis from ARDS Network studies using FACTT Lite. We analyzed a total of 497 subjects in the FACTT Liberal group and 503 subjects in the FACTT Conservative group. Subjects in the FACTT Lite group were slightly older, had a higher baseline creatinine and Pao2/Fio2 ratio, and were more often in shock than subjects in the FACTT Conservative or FACTT Liberal groups.
Table 2. baseline Descriptive Statistics by Fluid Management Strategy.
| Characteristic | FACTT Lite (n = 1,124) | FACTT Conservative (n = 503) | FACTT Liberal (n = 497) | p Lite Versus Conservative | p Lite Versus Liberal |
|---|---|---|---|---|---|
| Age (yr) | 51.8 ± 0.5 | 50.1 ± 0.7 | 49.5 ± 0.7 | 0.045 | 0.007 |
|
| |||||
| Male (%) | 583 (52) | 263 (52) | 271 (55) | 0.88 | 0.32 |
|
| |||||
| Acute Physiology and Chronic Health Evaluation III score | 91.0 ± 0.8 | 93.1 ± 1.4 | 95.2 ± 1.4 | 0.18 | 0.007 |
|
| |||||
| In shock at baseline (% requiring vasopressors) | 550 (49) | 159 (31) | 171 (35) | < 0.001 | < 0.001 |
|
| |||||
| Baseline creatinine (mg/dL) | 1.6 ± 0.04 | 1.3 ± 0.04 | 1.2 ± 0.04 | < 0.001 | 0.002 |
|
| |||||
| Central venous pressure (mm Hg) | 11.7 ± 0.2 | 11.9 ± 0.3 | 12.2 ± 0.3 | 0.628 | 0.132 |
|
| |||||
| Pao2/Fio2 | 144 ± 2 | 130 ± 3 | 129 ± 3 | 0.0002 | < 0.0001 |
|
| |||||
| Fluid balance (mL) 24 hr prior to randomization | 2,580 ± 95 | 2,655 ± 156 | 2,875 ± 166 | < 0.001 | < 0.001 |
| Primary cause of acute lung injury (%) | < 0.001 | < 0.001 | |||
| Pneumonia | 668 (59) | 231 (46) | 240 (48) | ||
| Sepsis | 184 (16) | 110 (22) | 123 (25) | ||
| Aspiration | 132 (12) | 82 (16) | 67 (13) | ||
| Trauma | 58 (5) | 38 (8) | 36 (7) | ||
| Multiple transfusion | 20 (2) | 7 (1) | 2 (0) | ||
| Other | 62 (6) | 35 (7) | 29 (7) | ||
|
| |||||
| ICU type (%) | 0.001 | < 0.001 | |||
| Medical | 677 (60) | 333 (66) | 330 (66) | ||
| Surgical | 63 (6) | 50 (10) | 50 (10) | ||
| Medical and surgical | 242 (22) | 74 (15) | 68 (14) | ||
| Other | 142 (12) | 46 (9) | 49 (10) | ||
FACTT = Fluid and Catheter Treatment Trial.
Values are reported as mean ± sem or n (%) as appropriate.
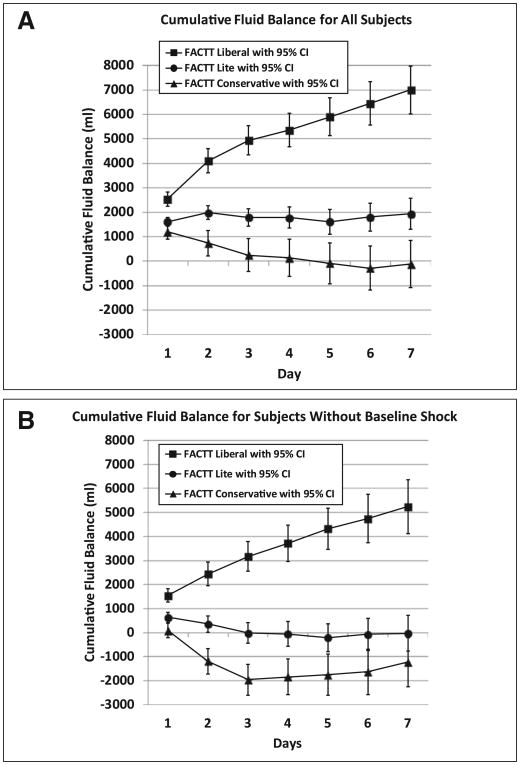
Cumulative fluid balance over 7 days in the FACTT Lite group was 1,918 ± 323 mL, in the FACTT Conservative group was −136 ± 491 mL (p < 0.001 compared to FACTT Lite), and in the FACTT Liberal group was 6,992 ± 502 mL (p < 0.001 compared to FACTT Lite) (Fig. 1A). When subjects without baseline shock were analyzed (Fig. 1B), cumulative fluid balance over 7 days in the nonshock FACTT Lite group was −38 ± 375 mL, in the nonshock FACTT Conservative group was −1,240 ± 523 mL (p = 0.06 compared to FACTT Lite), and in the nonshock FACTT Liberal group was 5,235 ± 569 mL (p < 0.001 compared to FACTT Lite).
Figure 1.
A, Cumulative fluid balance for all subjects: Cumulative fluid balance over study days 1 to 7 for the three cohorts. The conservative and liberal fluid protocol groups are from the Fluid and Catheter Treatment Trial (FACTT) of the Acute Respiratory Distress Syndrome (ARDS) Network and are referred to as “FACTT Conservative” and “FACTT Liberal.” The FACTT Lite group is from subsequent ARDS Network studies that used a simplified fluid conservative protocol. Both the FACTT Conservative and FACTT Lite groups had significantly less cumulative fluid balance compared with the FACTT Liberal group (p < 0.001). The FACTT Lite group had significantly greater cumulative fluid balance over 7 days compared with the FACTT Conservative group (p < 0.001). The error bars indicate 95% CIs for cumulative fluid balance on each study day for each group. The number of subjects included in the analysis for each day for the FACTT Conservative and Liberal groups was previously reported (1). The number of subjects included in the analysis for each day for the FACTT Lite group is as follows: n = 1,120 (day 1), n = 1,102 (day 2), n = 1,069 (day 3), n = 1,008 (day 4), n = 921 (day 5), n = 846 (day 6), and n = 773 (day 7). B, Cumulative fluid balance for subjects without baseline shock: Cumulative fluid balance over study days 1 to 7 is shown for subjects without baseline shock in each of the groups. Both the FACTT Conservative and FACTT Lite groups without baseline shock had significantly less cumulative fluid balance compared with the FACTT Liberal group without baseline shock (p < 0.001). The FACTT Lite group had a similar cumulative fluid balance over 7 days compared with the FACTT Conservative group (p = 0.06). The error bars indicate 95% CIs for cumulative fluid balance on each study day for each group.
Both the FACTT Lite and FACTT Conservative groups had significantly lower daily fluid balance than FACTT Liberal (p < 0.001 on all days). The FACTT Lite group had a higher daily fluid balance on day 1 (1,587 ± 88 mL) and day 2 (394 ± 79) than the FACTT Conservative group (day 1 = 1,187 ± 151 mL, p = 0.016, and day 2 = −376 ± 161 mL, p < 0.001 compared to FACTT Lite), but a similar daily fluid balance on days 3–7 (p > 0.05). We observed similar findings in the subjects without baseline shock.
Cumulative fluid intake over 7 days in the FACTT Lite group (22,232 ± 309 mL) was lower than in the FACTT Conservative group (24,086 ± 496 mL) (p = 0.001 compared to FACTT Lite) and FACTT Liberal group (28,482 ± 572 mL) (p < 0.001 compared to FACTT Lite). Cumulative fluid output over 7 days in the FACTT Lite group (20,533 ± 295 mL) was lower than in the FACTT Conservative group (24,187 ± 505 mL) (p < 0.001 compared to FACTT Lite) and similar to the Liberal group (21,463 ± 568 mL) (p = 0.11 compared to FACTT Lite).
After adjustment for age and APACHE III score, 60-day mortality was similar between groups (p = 0.84) (Table 3). The FACTT Lite and FACTT Conservative groups had similar ventilator-free days (p = 0.61), and FACTT Lite had higher ventilator-free days than FACTT Liberal (p < 0.001) (Table 3). Similarly, FACTT Lite had the same ICU-free days as FACTT Conservative and more than FACTT Liberal (p < 0.001). Prevalence of new onset shock was lower in the FACTT Lite group than in the FACTT Conservative group (p = 0.007), but similar to the FACTT Liberal group (p = 0.18) (Table 3).
Table 3. Outcomes by Fluid Management Strategy.
| Outcome | FACTT Lite (n = 1,124) (%) | FACTT Conservative (n = 503) (%) | FACTT Liberal (n = 497) (%) | p Lite Versus Conservative | p Lite Versus Liberal |
|---|---|---|---|---|---|
| Ventilator-free days | 14.9 ± 0.3 | 14.6 ± 0.5 | 12.1 ± 0.5 | 0.61 | < 0.001 |
| ICU-free days | 14.4 ± 0.3 | 13.4 ± 0.4 | 11.2 ± 0.4 | 0.054 | < 0.001 |
| 60-day mortality | 249 (22) | 128 (25) | 124 (28) | 0.15 | 0.007 |
| Adjusted 60-day mortalitya | 272 (24) | 123 (25) | 127 (25) | 0.91 | 0.56 |
| New onset shockb | 104 (9) | 67 (13) | 55 (11) | 0.007 | 0.18 |
| Acute kidney injury before adjustment for fluid balance | 653 (58) | 288 (57) | 253 (51) | 0.72 | 0.006 |
| Acute kidney injury after adjustment for fluid balance | 631 (56) | 290 (58) | 328 (66) | 0.60 | < 0.001 |
FACTT = Fluid and Catheter Treatment Trial.
Adjusted for age and Acute Physiology and Chronic Health Evaluation III score.
Defined as on vasopressors at some point between day 1 and day 7, but not at baseline.
Values are reported as mean ± sem or n (%) as appropriate.
The prevalence of acute kidney injury was similar in the FACTT Lite group and FACTT Conservative groups (p = 0.72) irrespective of adjustment for fluid balance (Table 3). Both the FACTT Lite and FACTT Conservative groups had higher rates of acute kidney injury than the FACTT Liberal group before adjustment for fluid balance, but lower rates than FACTT Liberal after adjustment for fluid balance.
Mean daily furosemide dose differed among cohorts (Table 4). FACTT Lite mean daily furosemide dose was lower than FACTT Conservative on all days except day 4. FACTT Lite mean daily furosemide dose was greater than FACTT Liberal on all days except day 6.
Table 4. Mean Furosemide Dose per Study Day among Patients Receiving Furosemide.
| Study Day | FACTT Lite Mean Furosemie Dose in mg and No. of Subjects (n) | FACTT Conservative Mean Furosemide Dose in mg and No. of Subjects (n) | FACTT Liberal Mean Furosemide Dose in mg and No. of Subjects (n) | p Lite Versus Conservative | p Lite Versus Liberal |
|---|---|---|---|---|---|
| 1 | 72 ± 16 (49) | 140 ± 10 (229) | 28 ± 4 (238) | 0.004 | < 0.001 |
| 2 | 85 ± 16 (52) | 156 ± 11 (240) | 25 ± 3 (275) | 0.004 | < 0.001 |
| 3 | 64 ± 10 (54) | 145 ± 12 (220) | 22 ± 3 (290) | 0.001 | < 0.001 |
| 4 | 81 ± 20 (48) | 130 ± 12 (198) | 23 ± 3 (266) | 0.064 | < 0.001 |
| 5 | 46 ± 11 (48) | 143 ± 14(175) | 23 ± 4 (260) | < 0.001 | 0.022 |
| 6 | 31 ± 10 (40) | 138 ± 15 (148) | 17 ± 3 (230) | < 0.001 | 0.093 |
| 7 | 34 ± 13 (30) | 90 ± 13 (112) | 13 ± 2 (215) | 0.032 | 0.002 |
FACTT = Fluid and Catheter Treatment Trial.
Discussion
Fluid management with FACTT Lite resulted in a significantly greater cumulative fluid balance by 2,054 mL over 7 days than FACTT Conservative, but a significantly lower cumulative fluid balance by 5,074 mL over 7 days than FACTT Liberal. In subjects without baseline shock, in whom the fluid protocol was applied throughout the duration of the study, management with FACTT Lite resulted in an equivalent cumulative fluid balance to FACTT Conservative. FACTT Lite had similar clinical outcomes of ventilator-free days, ICU-free days, and mortality as FACTT Conservative and significantly greater ventilator-free days and ICU-free days than FACTT Liberal.
Evaluation of daily fluid balance provides insight into the differences in cumulative fluid balance. FACTT Lite daily fluid balance was significantly greater on days 1 and 2 than FACTT Conservative but was the same on days 3–7. Greater baseline shock in the FACTT Lite group does not explain the observed increase in fluid balance on days 1 and 2 because similar results were observed in subjects without baseline shock. One possible explanation for these findings is lower clinician compliance with FACTT Lite than with FACTT Conservative during the first 2 study days.
At the time that the FACTT study was performed, the FACTT Liberal fluid strategy represented the usual prior practice. Cumulative fluid balance in the FACTT Liberal group was similar to cumulative fluid balance in prior ARDS Network trials (1) when the approach to fluid management was not specified (8, 12). The FACTT Lite fluid protocol therefore results in a significantly lower cumulative fluid balance than the historical usual fluid management of ARDS patients prior to the FACTT study.
FACTT Lite had equivalent or better safety outcome variables than FACTT Conservative. Prevalence of acute kidney injury was similar in the FACTT Lite and FACTT conservative groups before and after adjustment for fluid balance. New onset shock during the study was lower in the FACTT Lite group than in the FACTT conservative group. A less aggressive diuresis in the first 2 days might explain the lower prevalence of new onset shock in the FACTT Lite group compared with the FACTT conservative group.
Although we did not specifically measure protocol compliance with FACTT Lite, comparison of daily mean furosemide dose between groups may act as a surrogate for protocol compliance. FACTT Lite was designed to capture the most commonly applied instructions from FACTT Conservative. FACTT Lite and FACTT Conservative should yield a similar mean daily furosemide dose, and both should have a significantly greater daily furosemide dose than FACTT Liberal. The FACTT Lite protocol had a higher mean daily furosemide dose than FACTT Liberal but less than FACTT Conservative. This may be due to 1) a lower compliance with the FACTT Lite protocol than with FACTT Conservative; or 2) a less aggressive diuresis in FACTT Lite than FACTT Conservative; or 3) a lower requirement for diuresis. FACTT Lite had significantly lower 7-day cumulative fluid intake than FACTT Conservative or FACTT Liberal. Less fluid infusion in the FACTT Lite cohort would require less diuresis, and a lower daily furosemide dose, to achieve the same fluid balance as FACTT Conservative. FACTT Lite did have the same daily fluid balance as FACTT Conservative on study days 3–7, but not study days 1–2.
A previous study from the ARDS Network (9) showed that adjusting creatinine for fluid balance impacts acute kidney injury ascertainment. Prevalence of acute kidney injury in FACTT Lite mirrors that observed in FACTT Conservative. Although acute kidney injury prevalence was higher in both FACTT Lite and FACTT Conservative than in FACTT Liberal before adjustment for fluid balance, prevalence after adjustment for fluid balance was lower in both FACTT Lite and FACTT Conservative. Prevalence of new acute renal failure requiring dialysis while on study would have been an ideal renal outcome variable to include in this study, but those data are not available for the FACTT Lite cohort.
Our study had several limitations. The comparison was retrospective. FACTT Lite was used in ARDS Network clinical trials after the FACTT study and secular changes could have played a role. FACTT Lite was a cointervention control rather than a primary intervention in contrast to FACTT Conservative and FACTT Liberal that were compared prospectively in a randomized clinical trial that was previously reported (1). As a primary intervention, protocol compliance with FACTT Conservative and FACTT Liberal was emphasized in the FACTT study. As a cointervention control in ARDS Network studies (2–4), FACTT Lite protocol compliance was not rigorously monitored, and we do not have precise data about compliance.
The FACTT Conservative, Liberal, and Lite protocols do not stipulate management during shock. Adequate initial fluid resuscitation of patients in septic shock decreases mortality (13, 14). Initial resuscitation of shock followed by a conservative late fluid management strategy is associated with low mortality (15). An optimal fluid management protocol for patients with ARDS would include specific protocol-directed resuscitation for shock combined with a conservative fluid management strategy once shock has resolved. Further studies could evaluate the combination of the FACTT Lite protocol with specific protocol-directed shock resuscitation.
FACTT Lite was designed as an easier protocol to implement in the ICU than FACTT Conservative. By eliminating the categories of ineffective circulation as defined by clinical examination findings (5) and condensing the CVP ranges from four to three, the FACTT Lite protocol had fewer rows, columns, and cells dictating instructions. FACTT Lite was implemented as a cointervention control in ARDS Network studies in more than 40 participating hospitals, about one third of which did not participate in the FACTT study and had never used the fluid conservative protocol. Only limited onsite education regarding the FACTT Lite protocol was available to physicians and nurses by local ARDS Network staff. Given these limitations, FACTT Lite had to be easily understood and implemented by physician and nursing staff in the ICU.
Conclusions
Although the FACTT Lite protocol had a greater cumulative fluid balance than FACTT Conservative, the results of our study indicate that the FACTT Lite protocol is safe and has equivalent ventilator-free days, ICU-free days, acute kidney injury, and adjusted 60-day mortality to FACTT Conservative. FACTT Conservative has improved ventilator-free days, ICU-free days, and prevalence of acute kidney injury than FACTT Liberal. FACTT Lite can be used as a simplified and safe alternative to FACTT Conservative for the management of fluid balance in patients with ARDS.
Supplementary Material
Acknowledgments
Supported, in part, by the National Institutes of Health, National Heart Lung and Blood Institute, Acute Respiratory Distress Syndrome Network contracts (HHSN268200536171C, HHSN268200536165C, and HHSN268200536179C).
Dr. Grissom lectured for the Society of Critical Care Medicine (honorarium for lecturing at Fundamentals of Critical Care Ultrasound Courses) and received support for article research from the National Institutes of Health (NIH). His institution received grant support from the NIH National Heart Lung and Blood Institute (NHLBI) Acute Respiratory Distress Syndrome (ARDS) Network and Prevention and Early Treatment of Acute Lung Injury Network. Dr. Brown received support for article research from the NIH; served as board member for Vecna Technologies (medical advisor to robotics/informatics company); lectured for the Society of Critical Care Medicine (Critical Care Ultrasound courses as faculty/co-chair); received support from SBP World Technologies (Dr. Brown is cofounder of an air pollution mitigation company); and received royalties from Oxford University Press (academic history book). His institution has a patent with Intermountain Healthcare (Dr. Brown assigned a patent-pending airway device to Intermountain) and received grant support from National Institutes of General Medical Sciences (K23 award; K23GM094465) and from the Intermountain Research and Medical Foundation (various investigator-initiated research studies). Dr. Liu served as board member for Astute Biomedical (Clinical Events Adjudication Committee), Complexa (Scientific Advisory Board), and Cytopheryx (Data Safety Monitoring Board); consulted for Chemocentryx and Abbvie; provided expert testimony for the Law Offices of Randy Moore; has stock options with Amgen; received support for travel from the American Thoracic Society and the American Society of Nephrology; received support from Abbott and CMIC (gifts of reagents for biomarker assays); and received support for article research from the NIH. Dr. Liu and her institution received grant support from the NIH. Dr. Schoenfeld received support for article research from the NIH. His institution received grant support from the NHLBI. Dr. Tidswell received support for travel from the NHLBI, received royalties from University California, San Francisco and received support for article research from the NIH. His institution received grant support from the NHLBI. Dr. Hite consulted for the NIH and Cumberland Pharmaceuticals (Safety Monitoring Boards), received grant support from the NIH (studies focused on ARDS, sepsis, and pneumonia), and received support for article research from the NIH. His institution received grant support from the NIH. Dr. Rock received support from Hospira (supplied dexmedetomidine for the Delirium clinical trial), Covidean (supplied bispectral index monitors for the Dexlirium trial), and CAS Medical Systems (supplied tissue oximeter monitors for the Dexlirium trial) and received support for article research from the NIH. His institution received grant support from the NIH (support for ARDS Network trials: Fluid and Catheter Treatment Trial, Statins for Acutely Injured Lungs from Sepsis [SAILS], Early Versus Delayed Enteral Feeding, Albuterol for Treatment of Acute Lung Injury and for support for NIH-sponsored clinical trials: Modifying the Impact of ICU-Induced Neurologic Dysfunction-USA; Dexlirium; and Red Cell Storage Duration Study), support for travel from the NIH, and provision of materials/assistance (for the NIH-sponsored ARDS Network trial called “SAILS,” AstraZeneca supplied study drugs and the resources to measure blood levels of rosuvas-tatin). Dr. Miller received support for travel. Dr. Morris received support for article research from the NIH. His institution received grant support, support for travel, and provision of assistance/materials from the NIH ARDS Network contract
Appendix 1. The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network
University of Washington, Harborview—L. Hudson*, S. Gundel, C. Hough, M. Neff, K. Sims, A. Ungar, T. Watkins; Baystate Medical Center—J. Steingrub*, M. Tidswell, E. Braden, L. DeSouza, J. Germain, C. Kardos, D. Kelley, L. Kozikowski, S. Ouellette; Baylor College of Medicine—K. Guntupalli, V. Bandi, C. Pope, C. Ross; Johns Hopkins University—R. Brower*, H. Fessler, D. Hager, P. Mendez-Tellez, D. Needham, K. Oakjones; Johns Hopkins Bayview Medical Center—J. Sevransky, A. Workneh; University of Maryland—C. Shanholtz, D. Herr, H. Howes, G. Netzer, P. Rock, A. Sampaio, J. Titus; Union Memorial Hospital—P. Sloane, T. Beck, H. Highfield, S. King; Washington Hospital Center—B. Lee, N. Bolouri; Cleveland Clinic Foundation—H. P. Wiedemann*, R. W. Ashton, D. A. Culver, T. Frederick, J. A. Guzman, J. J. Komara Jr, A. J. Reddy; University Hospitals of Cleveland—R. Hejal, M. Andrews, D. Haney; MetroHealth Medical Center—A. F. Connors, S. Lasalvia, J. D. Thornton, E. L. Warren; University of Colorado Hospital, Aurora—M. Moss*, E. L. Burnham, L. Gray, J. Maloney, M. Mealer; Denver Health Medical Center—I. Douglas, K. Over-dier, K. Thompson, R. Wolken; Rose Medical Center—S. Frankel, J. McKeehan; Swedish Medical Center—M. L. Warner; Saint Anthony's Hospital—T. Bost, C. Higgins, K. Hodgin; Duke University—N. MacIntyre*, L. Brown, C. Cox, M. Gentile, J. Govert, N. Knudsen; University of North Carolina—S. Carson, L. Chang, S. Choudhury, W. Hall, J. Lanier; Vanderbilt University—A. P. Wheeler*, G. R. Bernard, M. Hays, S. Mogan, T. Rice; Wake Forest University—R. D. Hite*, K. Bender, A. Harvey, P. E. Morris, Mary Ragusky; Moses Cone Memorial Hospital—P. Wright, S. Groce, J. McLean, A. Overton; University of Virginia—J. Truwit, K. Enfield, M. Marshall; LDS Hospital and Intermountain Medical Center—A. Morris*, A. Austin, S. Barney, S. Brown, J. Ferguson, H. Gallo, T. Graydon, C. Grissom, E. Hirshberg, A. Jephson, N. Kumar, R. Miller, D. Murphy, J. Orme, A. Stowe, L. Struck, F. Thomas, D. Ward, L. Weaver; LDS Hospital—P. Bailey, W. Beninati, L. Bezdijan, T. Clemmer, S. Rimkus, R. Tanaka; McKay-Dee Hospital—C. Lawton, D. Hanselman; Utah Valley Regional Medical Center—K. Sundar, W. Alward, C. Bishop, D. Eckley, T. Hill, B. Jensen, K. Ludwig, D. Nielsen, M. Pearce; University of California, San Francisco—M. A. Matthay*, C. Calfee, B. Daniel, M. Eisner, O. Garcia, K. Kordesch, K. Liu, N. Shum, H. Zhou; University of California, San Francisco, Fresno— M. W. Peterson, J. Blaauw, K. Van Gundy; University of California Davis—T. Albertson, B. Morrissey, E. Vlastelin; Mayo Foundation—R. Hubmayr*, D. Brown, M. Dubin, E. Festic, O. Gajic, R. Hinds, S. Holets, D. Kor, A. Lee, M. Passe, G. Simpson, J. Wright; Louisiana State University Health Sciences Center, New Orleans—B. deBoisblanc*, A. Antoine, D. Charbonnet, J. Hunt, P. Lauto, A. Marr, G. Meyaski, C. Romaine, R. Tejedor; Earl K. Long Medical Center, Baton Rouge General Medical Center Mid-City and Baton Rouge General Medical Center Bluebonnet—S. Brierre, J. Byrne, T. Jagneaux, C. LeBlanc, K. Moreau, C. Thomas; Ochsner Clinic Foundation—S. Jain, D. Taylor, L. Seoane; Our Lady of the Lake Medical Center—C. Hebert, J. Thompson; Tulane Medical Center—F. Simeone, J. Fearon; Clinical Coordinating Center (Massachusetts General Hospital and Harvard Medical School)—D. Schoenfeld*, M. Guha, E. Hammond, N. Lavery, P. Lazar, R. Morse, C. Old-mixon, N. Ringwood, E. Smoot, B. T. Thompson, R. Wilson; National Heart, Lung and Blood Institute—A. Harabin, S. Bredow, M. Waclawiw, G. Weinmann
*Denotes Principal Investigator.
Footnotes
See also p. 477
The members of the National Institutes of Health/National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome Network are listed in Appendix 1.
Institutions where this work was performed: Data analysis and article preparation were performed at Intermountain Medical Center, Murray, Utah, with input from coauthors at institutions participating in the National Institutes of Health, National Heart Lung and Blood Institute, Acute Respiratory Distress Syndrome Network (ARDS Network). Primary data collection during prospective clinical trials of the ARDS Network occurred at participating hospitals. Data from the primary ARDS Network studies were obtained from the Clinical Coordinating Center at Massachusetts General Hospital in Boston.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (http://journals.lww.com/ccmjournal).
The remaining authors have disclosed that they do not have any potential conflicts of interest.
For information regarding this article: colin.grissom@imail.org
References
- 1.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 2.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184:561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury: The EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice TW, Wheeler AP, Thompson BT, et al. NIH NHLBI Acute Respiratory Distress Syndrome Network of Investigators; NHLBI ARDS Clinical Trials Network. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grissom CK, Morris AH, Lanken PN, et al. National Institutes of Health/National Heart, Lung and Blood Institute Acute Respiratory Distress. Association of physical examination with pulmonary artery catheter parameters in acute lung injury. Crit Care Med. 2009;37:2720–2726. doi: 10.1097/ccm.0b013e3181a59532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 7.Schoenfeld DA ARDS Network. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 8.The National Heart, Lung, and Blood Institute, Acute Respiratory Distress Syndrome Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 9.Liu KD, Thompson BT, Ancukiewicz M, et al. National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Acute kidney injury in patients with acute lung injury: Impact of fluid accumulation on classifcation of acute kidney injury and associated outcomes. Crit Care Med. 2011;39:2665–2671. doi: 10.1097/CCM.0b013e318228234b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macedo E, Bouchard J, Soroko SH, et al. Program to Improve Care in Acute Renal Disease Study. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14:R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 13.Rivers E, Nguyen B, Havstad S, et al. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 14.ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.