Abstract
Chronic rhinosinusitis (CRS) is a heterogeneous disease characterized by local inflammation of the upper airways and sinuses which persists for at least 12 weeks. CRS can be divided into two phenotypes dependent on the presence of nasal polyps (NPs); CRS with NPs (CRSwNP) and CRS without NPs (CRSsNP). Immunological patterns in the two diseases are known to be different. Inflammation in CRSsNP is rarely investigated and limited studies show that CRSsNP is characterized by type 1 inflammation. Inflammation in CRSwNP is well investigated and CRSwNP in Western countries shows type 2 inflammation and eosinophilia in NPs. In contrast, mixed inflammatory patterns are found in CRSwNP in Asia and the ratio of eosinophilic NPs and non-eosinophilic NPs is almost 50:50 in these countries. Inflammation in eosinophilic NPs is mainly controlled by type 2 cytokines, IL-5 and IL-13, which can be produced from several immune cells including Th2 cells, mast cells and group 2 innate lymphoid cells (ILC2s) that are all elevated in eosinophilic NPs. IL-5 strongly induces eosinophilia. IL-13 activates macrophages, B cells and epithelial cells to induce recruitment of eosinophils and Th2 cells, IgE mediated reactions and remodeling. Epithelial derived cytokines, TSLP, IL-33 and IL-1 can directly and indirectly control type 2 cytokine production from these cells in eosinophilic NPs. Recent clinical trials showed the beneficial effect on eosinophilic NPs and/or asthma by monoclonal antibodies against IL-5, IL-4Rα, IgE and TSLP suggesting that they can be therapeutic targets for eosinophilic CRSwNP.
Keywords: Chronic rhinosinusitis, Eosinophilia, Nasal polyps, TSLP, Type 2 inflammation
Introduction
Chronic rhinosinusitis (CRS) is a heterogeneous disease characterized by local inflammation of the upper airways and sinuses that persists for at least 12 weeks. It is a significant cause of morbidity in adults across the world, affecting over 10 million Americans.1–4 CRS is frequently divided into two groups based on the presence or absence of nasal polyps (NPs): CRS with NPs (CRSwNP) and CRS without NPs (CRSsNP). Inflammatory patterns in the two diseases are known to be different. Initial studies indicated that CRSsNP is characterized by type 1 inflammation and CRSwNP by type 2 inflammation.4–7 However, recent evidence suggests that this theory may not fit in Asia where mixed inflammatory patterns can frequently be found in Asian patients in contrast to those in Western countries. This review article will focus on the pathogenic role of immune cells in CRS and will clarify the current knowledge of inflammatory patterns in CRSsNP and CRSwNP in the Western and Asian populations.
CRS is also known to be characterized by tissue remodeling. However, the pattern of remodeling between CRSsNP and CRSwNP is different.8–11 Importantly, this variation is controlled by distinct inflammatory patterns, known to be different in the two diseases. Since these patterns are controlled by immune signaling, we will introduce remodeling as an example of differences between CRSsNP and CRSwNP that is controlled by immune signaling and then introduce the inflammatory patterns in CRS in the following sections.
Remodeling
Tissue remodeling in CRS includes changes to the tissue structure and the deposition of extracellular matrix (ECM).12,13 Several factors that have been implicated in remodeling including matrix metal-loproteinase (MMP) and TGF-β are well invested molecules in CRS. MMPs are a family of zinc-dependent proteolytic enzymes that degrade various components of ECM and mediate remodeling in both physiological and pathological processes.14 Several groups have investigated a subset of MMPs in CRS and shown that MMP-7 and MMP-9 were elevated in both CRSsNP and CRSwNP.9,10,15 This suggests that MMP-7 and MMP-9 might be remodeling factors common to both forms of CRS. TGF-β mediates anti-inflammatory effects by acting on many types of immune cells.16 TGF-β also affects fibrosis by increasing deposition of ECM such as collagen, fibronectin and proteoglycan.17 TGF-β has three isoforms, TGF-β1, TGF-β2 and TGF-β3. Van Bruaene et al. reported in the Belgium population that TGF-β1, TGF-β2, and collagen were elevated in CRSsNP.8 The same group also investigated the presence of TGF-β in the Chinese population. Li et al. reported that TGF-β1 and collagen were elevated in CRSsNP.9 In contrast, Shi et al. found in the Chinese population that TGF-β1 was reduced but TGF-β2 and collagen were elevated in CRSsNP.10 Additionally, Sejima et al. reported that TGF-β was elevated in CRSsNP in the Japanese population although they did not state the isoform.18 Although there is some discrepancy, these studies suggest that TGF-β and collagen are elevated in CRSsNP across the world and may contribute to the remodeling process in this form of the disease.
As mentioned above, the pattern of remodeling in CRSwNP is known to be different compared to CRSsNP. Indeed, collagen was reduced in CRSwNP and TGF-β was either reduced or no difference compared to control subjects.8–11 In contrast to CRSsNP, the fact that fibrin deposition in the submucosa is especially high in NPs might represent a key event in the tissue remodeling process during CRSwNP.11,19 Takabayashi et al. recently reported that cross-linked fibrin was elevated and tissue plasminogen activator (t-PA) was reduced in NPs.11 The t-PA converts plasminogen to plasmin which promotes fibrin degradation.11,20 Importantly, type 2 cytokines, IL-4 and IL-13 which are elevated in NPs, reduced the expression of t-PA in epithelial cells.11 In a separate study, Takabayashi et al. reported that factor XIII-A (FXIIIA) was significantly elevated in NPs and it was produced by M2 macrophages (also known as alternatively activated macrophages).19 FXIIIA is a coagulation factor that can induce fibrin deposition by cross-linking fibrin and via the antifibrinolytic effect by mediating the binding of α2-plasmin inhibitor to fibrin.19,21,22 This data suggests that overexpression of FXIIIA and reduction of t-PA may facilitate the excessive deposition of fibrin in NPs via induction of fibrin cross-linking and the inhibition of fibrinolysis (Fig. 1). Very importantly, these factors are tightly controlled by the type 2 inflammatory milieu. Therefore they may not be a good fit to describe the mechanism of remodeling in non-eosinophilic Asian NPs (see following sections), which requires further study.
Fig. 1.
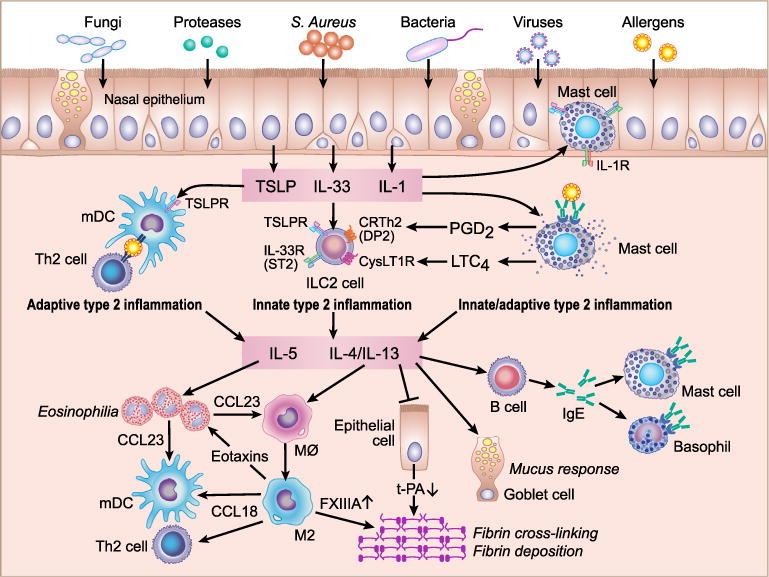
Potential mechanism for amplification of type 2 inflammation in eosinophilic CRSwNP. Several factors including fungi, proteases, S. aureus, bacteria, viruses and allergens can activate nasal epithelial cells to induce the production of epithelial derived cytokines TSLP, IL-33 and IL-1. Epithelial derived cytokines activate three types of immune cells in NPs. TSLP stimulates mDCs to induce naive CD4+ T cell differentiation into Th2 cells which then induce adaptive type 2 inflammation. TSLP and IL-33 stimulate ILC2s to induce the production of type 2 cytokines (innate type 2 inflammation). Mast cells are accumulated in the epithelium and are also present in the mucosal area in NPs. TSLP, IL-33 and IL-1 stimulate epithelial and mucosal mast cells to produce IL-5 and IL-13 (innate type 2 inflammation). Ag/IgE/IgER complexes on mast cells induce degranulation and thereafter mast cells produce IL-5 and IL-13 (adaptive type 2 inflammation). Production of PGD2 and LTC4 from mast cells also stimulates ILC2s. IL-5 promotes eosinophilia and eosinophils produce CCL23 to recruit macrophages and mDC. IL-4/13 activates macrophages, B cells and epithelial cells to induce eosinophil and Th2 cell recruitment, remodeling and IgE mediated reactions. M2 macrophages produce eotaxins and CCL18 to recruit eosinophils, mDCs and Th2 cells.
As we mention above, CRSsNP and CRSwNP are controlled by distinct inflammatory signals from various immune cells. Indeed, remodeling in CRSsNP might be controlled by TGF-β signaling. In contrast, remodeling in CRSwNP is regulated by type 2 inflammation. In addition, CRSwNP can be further divided into two phenotypes based on inflammatory patterns. Starting in the next section, we will describe the inflammatory patterns in CRSsNP and CRSwNP separately and also focus on the difference between the two types of CRSwNP; eosinophilic and non-eosinophilic.
Inflammatory patterns in CRSsNP
The mechanism of inflammation in CRSsNP is poorly understood. Van Zele et al. initially discovered that the type 1 cytokine IFN-γ was significantly elevated in CRSsNP in Belgium.6 Several groups confirmed the elevation of IFN-γ in CRSsNP in Belgium, China and Korea.23–25 In contrast, other groups could not find elevation of IFN-γ in CRSsNP in Japanese, Chinese and American populations.7,18,26 Another CRSsNP dependent cytokine could be TGF-β. Van Bruaene et al. discovered that TGF-β was predominantly elevated in CRSsNP and several groups confirmed the elevation of TGF-β in CRSsNP in Asia although there is some discrepancy (see remodeling section).8–10,18 Since CRSsNP is a heterogeneous disease and is diagnosed by the phenotypic absence of NPs, the sample population may strongly affect the result. Future studies may require sub-classification of CRSsNP and investigation of the inflammatory patterns in each group.
Eosinophilic and non-eosinophilic CRSwNP
CRSwNP is well known to be characterized by type 2 inflammation and eosinophilia compared to CRSsNP or control sinus mucosa. However, the inflammation in CRSwNP varies based on race and regional differences. Several studies have indicated that 70–90% of CRSwNP in Europe and the United States showed eosinophilia in NPs.4, 6, 27–32 We also have found intense infiltration of eosinophils in NPs. Our data showed that eosin+ and EG2+ eosinophils were highly accumulated in the lamina propria as well as in the epithelium of NPs (Fig. 2A, B). However, this does not hold true in East Asia including China, Korea and Japan. Zhang et al. reported that the levels of eosinophilia in NPs were significantly weaker in south China than in Belgium.31 Cao et al. reported that more than 50% of CRSwNP presented non-eosinophilic inflammation in China.24 Eosinophilic NPs have also been found in less than 50% of patients in Japan and Korea.33–35 Although these studies suggest that eosinophilic NPs in East Asia are less frequent, some studies in China showed a higher frequency of eosinophilia in NPs. Indeed, Wen et al. used a larger cohort of patients with CRSwNP in China and found that 76.5% had eosinophilia in NPs.36 The discrepancy in these results could be explained by the fact that every researcher used a different estimation system to determine the eosinophilia. Indeed, Cao et al., Wen et al. and Kim et al. randomly collected 10 high power field (HPF) images and determined eosinophilia.24,36,37 However, Cao et al. used 10% eosinophils in HPF, Wen et al. used 8 eosinophils/HPF and Kim et al. used 5 eosinophils/HPF as cutoff values.24,36,37 In another case, Ikeda et al. and Yoshimura et al. collected three HPFs containing the greatest degree of cellular infiltration and determined the eosinophilia by cutoff values of 100 eosinophils/HPF and 15% eosinophils in HPF, respectively.33,35 These conflicting results highlight the need for internationally recognized guidelines for determination of eosinophilic and non-eosinophilic NPs in CRSwNP. On balance, eosinophilic NPs are a common phenotype in Europe and the United States, however in East Asia, eosinophilia is less frequent and about half of CRSwNP is characterized by non-eosinophilic NPs. Recent studies indicate that the prevalence of eosinophilic NPs is increasing in Asia. Kim et al. evaluated eosinophilia in NPs obtained from 1993–1994 and 2010–2011 and found that the prevalence of eosinophilic NPs increased from 24.0% to 50.9% in Korea.37 Katotomichelakis et al. evaluated NPs at two time points, 1999 and 2011, in Thailand, and found that the average number of eosinophils/HPF changed to 35 from 5.38 It is possible that eosinophilic NPs may continue to increase in Asia in the future.
Fig. 2.
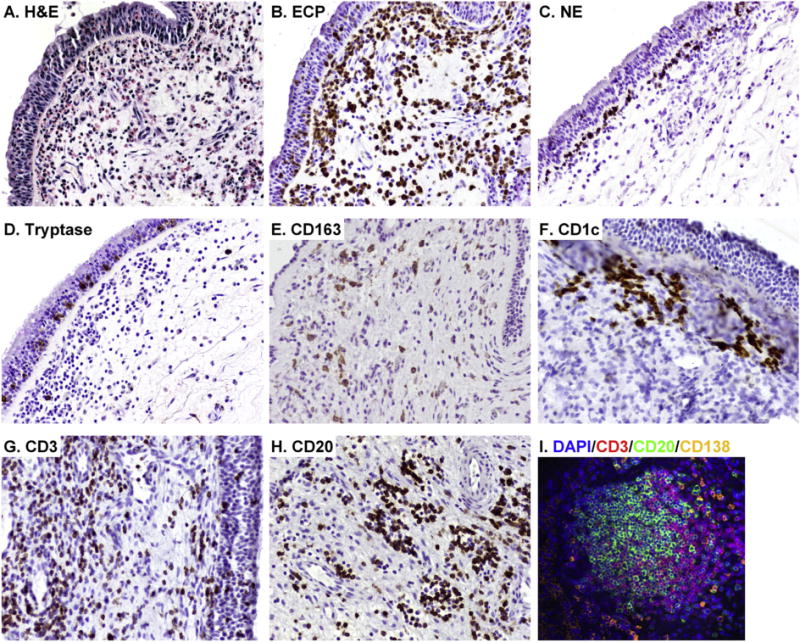
Increased presence of immune cells in NPs. Representative H&E staining in NPs is shown in A. Representative immunostainings were performed with anti-ECP mAb (EG2) for eosinophils (B), anti-neutrophil elastase (NE) mAb for neutrophils (C), anti-tryptase mAb for mast cells (D), anti-CD163 mAb for M2 macrophages (E), anti-CD1c mAb for mDC1s (F), anti-CD3 mAb for T cells (G) and anti-CD20 mAb for B cells (H). Immunofluorescence assay was performed with anti-CD3 mAb (red fluorescence), anti-CD20 mAb (green fluorescence) and anti-CD138 mAb (orange fluorescence) for plasma cells (I), Nuclei were counterstained with 4′6-diamidino-2- phenylindole (DAPI; blue fluorescence). Magnification; ×200.
In contrast to eosinophilic inflammation, inflammatory patterns in non-eosinophilic NPs are poorly understood. Non-eosinophilic NPs can be divided into several phenotypes and neutrophilia is the best characterized phenotype in non-eosinophilic NPs. In China, Wen et al. showed that 46.0% of NPs had neutrophilia and 35.8% had a mixed phenotype with eosinophilia.36 Ikeda et al. showed 20% of NPs had neutrophilia in Japan.33 In contrast, in the western country of Germany, Polzehl et al. showed that neutrophils were detected in early stage NPs but they were not elevated compared to control nasal mucosa.30 Van Zele et al. showed that neutrophils were significantly elevated in NPs in Belgium, although the degree of neutrophilia was significantly weaker than eosinophilia in those NPs.6 We also detected neutrophils and a subset of NPs showed elevation of neutrophils mixed with eosinophilia in the US (Fig. 2C, unpublished observation). This evidence indicates that neutrophilic CRSwNP is more commonly found in Asia. In contrast, neutrophil infiltration can be found in Europe and the US and it is generally mixed with eosinophilia. However, the degree of neutrophilia is weaker than eosinophilia.
Inflammatory patterns in eosinophilic CRSwNP
Since CRSwNP is characterized by eosinophilia in western countries, many researchers have investigated the role of eosinophil activation and survival factors in NPs. Hamilos et al. and others initially discovered that IL-5 was elevated in allergic NPs.39 Simon et al. and others confirmed the elevation of IL-5 in NPs and identified that IL-5 was a major eosinophil survival factor in NPs.40 After that, many investigators confirmed the elevation and importance of IL-5 in eosinophilic NPs across the world.6,7,18,23–25,29,31 Since IL-5 is a key factor of eosinophilia, Gevaert et al. performed clinical trials of anti-IL-5 in severe cases of NPs. Both mepolizumab and reslizumab, which are humanized anti-human IL-5 monoclonal antibodies, result in a significant reduction of NP size in 50–60% patients and IL-5 levels in nasal secretion can be predictive of response to anti-IL-5 therapy.41,42
In addition to activation and survival, eosinophil recruitment into tissue should be another key event to induce eosinophilia. Chemokines are a large group of proteins that participate in recruitment of inflammatory cells into tissue sites by binding G protein-coupled receptors on their target cells. Among them, chemokine receptor CCR3 is predominantly expressed on eosinophils and 5 chemokines, MCP-4 (CCL13), RANTES (CCL5), eotaxin-1 (CCL11), eotaxin-2 (CCL24) and eotaxin-3 (CCL26), are well known to recruit eosinophils via CCR3. Several researchers investigated these chemokines in eosinophilic CRSwNP. The importance of RANTES in NPs is still not clear and several groups showed that RANTES was not elevated in eosinophilic NPs.43 Although MCP-4 is a potent chemoattractant, the presence of MCP-4 in eosinophilic NPs is not well studied and only a few studies show the elevation of MCP-4 in NPs.44,45 In contrast, many researchers reported the importance of eotaxins in NPs. Ponath et al. cloned human eotaxin-1 as an eosinophil chemoattractant factor and found it in eosinophilic NPs.46 Many researchers then investigated individual eotaxins in eosinophilic NPs and found that they were elevated. Importantly, Olze et al. Yao et al. and Takabayashi et al. investigated three eotaxins and found that eostaxin-1, -2 and -3 were all elevated in eosinophilic NPs.47–49 Furthermore, several investigators found a correlation between eotaxins and tissue eosinophilia in CRSwNP.47 These results indicate that eotaxins are key chemotactic factors for eosinophils in eosinophilic NPs.
Type 2 inflammation and epithelial cytokines in eosinophilic CRSwNP
Type 2 cytokines including IL-4, IL-5 and IL-13 are believed to control the inflammation in eosinophilic CRSwNP. As described above, IL-5 is a key activation and survival factor for eosinophils in NPs. In contrast, IL-4 and IL-13 are key factors that control mucus production in epithelial cells and IgE responses in B cells and plasma cells (Fig. 1). IL-4 and IL-13 also significantly contribute to several key inflammatory events in NPs including the activation of macrophages and remodeling. Very recently, a Phase 2a proof-of-concept study of dupilumab, which is a fully human monoclonal antibody against the α-subunit of the IL-4 receptor that blocks both IL-4 and IL-13 signaling, showed improvement of NP score as well as patient-reported symptom scores in moderate-to-severe CRSwNP (http://newsroom.regeneron.com/releasedetail.cfm?ReleaseID=873630). This reinforces the importance of type 2 inflammation in the pathogenesis of eosinophilic CRSwNP.
Type 2 cytokines, IL-4, IL-5 and IL-13, can be produced from several immune cells including Th2 cells, mast cells and group 2 innate lymphoid cells (ILC2s). Type 2 cytokines can be induced by both adaptive and innate signaling in these cell types. Recent evidence suggests that newly identified epithelial-derived cytokines, IL-25, IL-33 and thymic stromal lymphopoietin (TSLP), help to shape the local activation of type 2 immunity and exaggerated expression of these cytokines induces type 2 inflammatory diseases including atopic dermatitis and bronchial asthma.50–53 These epithelial-derived cytokines can have effects on both innate and adaptive type 2 immunity. Therefore many investigators have started to look at these epithelial cytokines in CRS.
IL-25 and IL-33 mainly contribute to innate type 2 inflammatory responses.54,55 IL-25 is a member of the IL-17 cytokine family. Unlike other IL-17 family cytokines, IL-25 promotes type 2 inflammation including eosinophilia even in Rag2 deficient mice.55 IL-25R is expressed on memory Th2 cells, basophils and ILC2s and IL-25 enhances type 2 cytokine production in these cells.56 In the case of CRS, only a few groups have investigated the presence of IL-25. Lam et al. reported that IL-25 mRNA was significantly elevated in ethmoid sinuses of CRSwNP compared to controls and CRSsNP.57 In contrast, Miljkovic et al. reported that IL-25 mRNA was significantly decreased in NPs compared to ethmoid sinuses of controls and CRSsNP.58 Our data showed that expression of mRNA for IL-25 was very low in sinuses and the level of IL-25 mRNA was similar between NPs and control sinus tissues (unpublished observations). In addition, no one has yet shown the presence of IL-25 protein in CRS. On balance, IL-25 may not be a key regulator of type 2 inflammation in CRS. However, further study will be required to investigate the presence of IL-25 protein in eosinophilic NPs.
IL-33 is the latest member of the IL-1 cytokine family that induces type 2 inflammation.59 The production of IL-33 is known to be controlled by innate immune signaling including via P2 purinergic receptors.60 IL-33 signals through a heterodimeric receptor complex consisting of IL-1RL1 (ST2) and IL-1RAcP. IL-33R is expressed on memory Th2 cells, basophils, mast cells, NKT cells and ILC2s.56 IL-33 induces type 2 inflammation in Rag deficient mice suggesting that IL-33 mainly contributes to innate type 2 inflammation.54 In the case of CRS, several groups reported the presence of IL-33 in CRS. Shaw et al. Lam et al. and Miljkovic et al. reported that IL-33 mRNA was highly expressed in nasal mucosa but was not elevated in NPs or other inflamed areas of the sinuses in CRSwNP.57,58,61 Baba et al. also found that the concentration of IL-33 protein was not significantly different between eosinophilic NPs and control sinus tissue although it was highly detected.62 These results indicate that IL-33 may contribute to the inflammation in CRS but is not elevated in CRSwNP.
TSLP is an IL-7 like cytokine that induces type 2 inflammation. Several animal studies indicated that TSLP may contribute to both innate and adaptive type 2 inflammation.63,64 The production of TSLP is known to be controlled by both innate and adaptive immune signaling including via activation of toll like receptors and cytokine receptors.51,52,65,66 In contrast to IL-25 and IL-33, TSLP was reproducibly reported to have a pathogenic role in CRSwNP. Indeed, several groups reported that TSLP mRNA was significantly elevated in eosinophilic NPs in the US, Canada, China, Japan and Italy.7,67–71 Although it is notably difficult to detect TSLP protein in clinical samples by commercially available detection systems, Nagarkar et al. and Allakhverdi et al. found that TSLP activity was significantly elevated in NPs compared to control sinus tissue.7,67 This indicates that TSLP protein is elevated in NPs. Interestingly, Nagarkar et al. discovered cleavage products of TSLP when TSLP protein was incubated with NP tissue extracts.7 More importantly, they also found that the cleaved product might have higher activity than full-length TSLP.7 These results suggest that regulation of induction and posttranslational modifications in tissue control local TSLP-mediated type 2 inflammation. Very importantly, a recent clinical trial showed that AMG 157 which is a fully human anti-TSLP monoclonal antibody reduced allergen-induced early and late asthmatic responses in patients with mild allergic asthma.72 This indicates that TSLP is a key factor and an important therapeutic target in asthma. Since TSLP may also control type 2 inflammation in eosinophilic NPs, anti-TSLP may become a therapeutic agent for CRSwNP in the future.
Based on the literature, TSLP may be a key controller of type 2 immunity in eosinophilic NPs. IL-33 may also be involved in the inflammation although it is not elevated in CRS. Starting in the next section, we will describe the presence of type 2 cytokine producing cells and the potential role of epithelial-derived cytokines on type 2 inflammation in eosinophilic NPs.
T cells and dendritic cells
T cells are known to be elevated in eosinophilic NPs and T cell accumulation can be found in both mucosal and epithelial areas of NPs (Fig. 2G). Th2 cells are known as the primary source of type 2 cytokines. Recently, two separate groups have provided an in-depth analysis of Th cell subsets found in NPs. Derycke et al. reported that NPs were characterized by mixed T helper cells and only Th2 cells were significantly elevated in NPs in Belgium.73 Shi et al. reported that increased Th2 cells were found only in eosinophilic NPs although Th1 cells and Th17 cells were elevated in both eosinophilic and non-eosinophilic NPs in China.74 These results suggest that Th2 cells are highly elevated in eosinophilic CRSwNP across the world.
Although TSLP directly promoted Th2 differentiation from naïve T cells in the absence of antigen presenting cells (APC) in mice,75,76 TSLP dependent-Th2 differentiation required APC in humans.77,78 TSLP-R was not expressed on naïve T cells and TSLP did not directly induce Th2 cytokine production in naïve T cells in humans, although expression of TSLP-R was weakly induced by TCR-dependent activation on naïve T cells and by allergen plus TSLP on a subset of Th2 cells in atopic subjects.79–81 Therefore TSLP dependent-Th2 differentiation is believed to be controlled by dendritic cells (DCs) in humans.
Dendritic cells are known to be important in skewing Th responses in the mucosa. Human DCs can be divided into two major subsets, myeloid DCs (mDCs) and plasmacytoid DCs (pDCs).82 Myeloid DCs act as strong antigen presenting cells and one of their key functions is to control polarization of T helper cells. In contrast, pDCs are less effective at antigen presentation than mDCs but strongly promote antiviral immunity. Recently, three groups identified DC subsets in NPs and found that mDCs were significantly elevated in NPs in the US, Belgium and China.74,83,84 Myeloid DCs can be classified into two subsets in humans, mDC1 and mDC2, which can be differentiated by cell surface expression of CD1c and CD141, respectively. Poposki et al. and Pezato et al. further differentiated mDC phenotypes and found that both mDC1s and mDC2s were significantly elevated in NPs although mDC2s were a minor population of mDCs in NPs.83,84 Importantly, Yerkovich et al. reported that mDC2s were significantly elevated in patients with atopic asthma, and found that mDC2s strongly induced a Th2-polarized response although the mechanisms were not clear.85 This suggests that the elevation of mDC2s in NPs may contribute to the local Th2-polarized environment. However, the mechanism needs further investigation.
In contrast to mDC2, the mechanisms of mDC1-related Th2 polarization are partially identified. Shi et al. evaluated DC subsets from NPs of eosinophilic and non-eosinophilic patients. They found that mDC1s isolated from either eosinophilic or non-eosinophilic NPs could skew naïve T cells toward Th1 and Th17 phenotypes, but only DCs from eosinophilic NPs were able to skew naïve T cells toward a Th2 phenotype.74 They also investigated the mechanism of Th2 skewing by mDC1s from eosinophilic NPs. They found that the frequency of mDC1s expressing either OX40 ligand (OX40L) or programmed death ligand-1 (PD-L1) was significantly increased in eosinophilic NPs.74 Furthermore, they also found that blockade of either OX40L or PD-L1 suppressed the skewing of Th cells to produce type 2 cytokines.74 TSLP-R is known to be highly expressed on mDC1s and TSLP-stimulated mDCs induce naive CD4+ T cells to differentiate into Th2 cells.78 Importantly, this response is known to be via OX40L and TSLP induces expression of OX40L on mDCs.78 This evidence indicates that mDC1s in eosinophilic NPs may be primed by TSLP and thereafter control Th2 responses in NPs. Since TSLP is known to strongly induce the production of chemokines, TARC, MDC and eotaxins, in mDC,78 mDC1s in eosinophilic NPs may also contribute to the recruitment of Th2 cells and eosinophils into NPs.
Since epithelial cells are one of the main sources of TSLP, we initially hypothesized that mDC1s might be accumulated in the epithelium of NPs, yet we found that accumulation of mDC1s can be detected in lamina propria but not in epithelium.83 However, CD1c+ mDC1s were frequently found just below the surface of the epithelium in NPs (Fig. 2F). This suggests that it may be easy for mDC1s to interact with epithelial cells and epithelial cell derived cytokines including TSLP in NPs. This evidence suggests that TSLP may control adaptive type 2 inflammation through activation of mDCs and induction of Th2 differentiation (Fig. 1).
The mechanism of accumulation of mDCs in eosinophilic NPs is not clear. We recently reported that the chemokines CCL18 and CCL23 were significantly elevated in NPs.86,87 CCL23 is known to recruit mDCs via the receptor CCR1.88 We also found that levels of CCL23 significantly correlated with CCR1 and CD1c in NP tissues.87 CCL18 is known to be chemotactic for immature mDC1.89 These results suggest that overproduction of CCL18 and CCL23 may contribute to the accumulation of mDC1 in NPs. CCL18 is also known to recruit Th2 cells via CCR8.90 Interestingly, we also identified that CCL18 and CCL23 were mainly produced by M2 macrophages and eosinophils, respectively.86,87 CCL23 also potently recruits CCR1+ monocytes and macrophages.88 Since eosinophilic NPs are in a type 2 microenvironment, recruited macrophages can be differentiated into the M2 phenotype by type 2 cytokines. CD163+ M2 macrophages are known to be accumulated in NPs (Fig. 2E).19,87,91 They are known to produce eosinophil chemokines including eotaxins and MCP-4.92 This data indicates that production of CCL23 from eosinophils, recruitment of macrophages, differentiation into M2 macrophages and production of eosinophil chemokines from M2 macrophages may constitute a positive feedback loop to amplify the eosinophilia in NPs (Fig. 1).
Mast cells
Mast cells play key roles in host defense, homeostasis, tissue repair, and mechanisms of allergic inflammation. Mast cells express the high-affinity IgE receptor, FcɛRI. Upon cross-linking of the FcɛRI via IgE-antigen, mast cells release several pre-stored mediators, including histamine and proteases and synthesize many mediators including prostaglandins (PGs), cysteinyl leukotrienes (CysLTs) and type 2 cytokines especially IL-5 and IL-13. Recent reports suggest that a subset of patients with asthma and eosinophilic esophagitis that displayed a type 2 cytokine high-phenotype have elevated mast cells.93–96 Interestingly, mast cells primary accumulated in the epithelium.93,94 In addition, intraepithelial mast cells in these patients showed a mast cell-tryptase (MC-T) but not mast cell-tryptase/chymase (MC-TC) phenotype.95,96 In the case of CRS, our group found that mast cells were significantly elevated in mucosal and glandular epithelium but not within the lamina propria in NPs (Fig. 2D).48 Interestingly, mast cell phenotypes were different in mucosal and glandular epithelium. Takabayashi et al. found that mucosal epithelial mast cells showed an MC-T phenotype. In contrast, glandular epithelial mast cells showed an MC-TC phenotypes in the US.48 Cao et al. also found significant elevation of MC-T in the mucosal epithelium of eosinophilic NPs in China.97 These results suggest that mast cells are highly elevated in the epithelium of eosinophilic CRSwNP across the world. However, functional differences between MC-T and MC-TC in the pathogenesis of CRS will need to be further investigated.
Mast cells are known to express IL-1R, IL-33R (ST2) and TSLP-R on the cell surface and to respond to these ligands. Although TSLP alone isn’t sufficient to stimulate mast cells, TSLP synergizes with IL-1 and IL-33; potently activating mast cells to produce type 2 cytokines.98,99 As described, mast cell numbers were increased only within the NP epithelium in patients with eosinophilic CRSwNP.48 However, the specific role of the epithelium in the direct activation of mast cells was not clear. We hypothesized that epithelial cells can produce a combination of cytokines that directly activates intraepithelial mast cells to produce type 2 cytokines. We therefore investigated the effect of viruses, TLR ligands and cytokines on the interaction of mast cells and epithelial cells in vitro. We found that airway epithelial cells directly promoted production of type 2 cytokines in mast cells during viral infection through the production of IL-1 and TSLP and that this occurred only in a type 2 microenvironment.99 We initially expected that IL-33 released from epithelial cells contributes to this reaction. However, we could not detect IL-33 protein in poly(I:C) stimulated or influenza infected epithelial cells and ST2-Fc fusion protein could not block this response.99 This suggests that intraepithelial mast cells in eosinophilic NPs may contribute to the production of type 2 cytokines via interaction with epithelial cytokines TSLP and IL-1 in response to pathogens on epithelial cells. Interestingly, Iikura et al. found that IL-1 and IL-33 enhanced IgE receptor mediated type 2 cytokine production in mast cells.100 Since mast cells produce type 2 cytokines through epithelial-derived cytokines and IgE-mediated reactions, mast cells may contribute to both innate and adaptive type 2 inflammation in eosinophilic NPs (Fig. 1).
ILC2
The last population of type 2 cytokine producing cells to discuss is innate lymphoid cells (ILCs). ILCs are a recently identified minor population of immune cells that lack antigen receptors and lineage (Lin) markers. ILCs are activated by innate immune signaling including pathogens and innate cytokines. Like T helper cells, ILCs can be classified into 3 subsets, ILC1, ILC2 and ILC3 dependent on the production of cytokines.101,102 Among them, ILC2s produce large quantities of type 2 cytokines (including IL-5 and IL-13). The first evidence for ILC2-like cells was reported by Fort et al. in 2001.103 An ILC2 population was identified by several groups in 2010 in mice and variously named nuocytes, natural helper cells, innate helper 2 cells and others.104–106 In 2013, Spits et al. organized the nomenclature of the ILC family members and defined group 2 ILCs (ILC2s) as ILCs that produce type 2 cytokines and are dependent on GATA3 and RORα for their development and function.101
Human ILC2s were discovered by Mjösberg et al. in 2011 and were defined as CD45+, Lin−, CD127+, CRTH2+ cells.107 Importantly, they also identified an ILC2 population in NPs of Dutch patients and found that ILC2s were significantly elevated in NPs compared to control sinus mucosa.107 Several groups have now confirmed the elevation of ILC2 in NPs in the US and Australia.58,61,108 However, in my knowledge, the presence of ILC2s in eosinophilic NPs in the Asian population has yet not been investigated. Future study is required to identify ILC2s in the Asian population and investigate whether ILC2s are significantly elevated in eosinophilic NPs compared to non-eosinophilic NPs and control sinus mucosa.
ILC2s are known to express IL-33R and TSLP-R on the cell surface. IL-33 and TSLP are known to induce type 2 cytokine production in ILC2s, although the effect by individual cytokines is minimal. However, the combination of TSLP and IL-33 synergistically enhances the production of type 2 cytokines including IL-5 and IL-13 in human ILC2s isolated from peripheral blood and NPs.109,110 As described above, TSLP is significantly upregulated in eosinophilic NPs.7,71 IL-33 is present but not elevated in NPs.61,62 This suggests that ILC2s may contribute to the local innate type 2 inflammation in eosinophilic NPs by responding to TSLP and IL-33 with the production of type 2 cytokines.
CRTH2 is now known to be one of the key markers of human ILC2. CRTH2 is a chemokine receptor homologous molecule and is known as the PGD2 receptor 2 (DP2). PGD2 is known to recruit Th2, eosinophils and basophils via CRTH2.111 Chang et al. recently discovered that PGD2 also induced chemotaxis of human ILC2s.112 Xue et al. found that PGD2 dependent migration of ILC2 was via CRTH2.113 Importantly, Xue et al. also found that PGD2 strongly induced the production of type 2 cytokines including IL-4, IL-5 and IL-13 in ILC2s via the activation of CRTH2.113 Mast cells are the one of the major sources of PGD2. Xue et al. also looked at the role of mast cell products on the activation of ILC2s and found that supernatants of degranulated mast cells induced ILC2 migration and production of type 2 cytokines in ILC2.113 Mast cells also produce other pro allergic arachidonic acid metabolites called CysLTs including LTC4 upon degranulation.114 Doherty et al. found that CysLTR1 was expressed on ILC2s, and LTC4 and LTD4 strongly induced the production of type 2 cytokines in ILC2s.115 Since mast cells are highly accumulated and many of them are activated and degranulated in eosinophilic NPs,48,97 degranulation of mast cells may further enhance the recruitment of ILC2s and the production of type 2 cytokines in ILC2s via release of PGD2 and LTC4/D4 from mast cells and activation of CRTH2 and CysLTR1 on ILC2s in eosinophilic NPs (Fig. 1).
B-lineage cells
We have described immune cells that produce type 2 cytokines and cells that control type 2 cytokine production in these cells. In the last section, we will focus on the B-lineage cells that can be activated by type 2 cytokines in eosinophilic NPs.
In general, B cell maturation and expansion occur in germinal centers of the lymphoid organs. However, there are compelling reasons to believe that local proliferation and activation of B-lineage cells, B cells, plasmablasts and plasma cells, are of central pathogenic importance in several airway inflammatory diseases.116 The production of IgA and IgE from B-lineage cells is known to be critical to allergic disease via the activation of airway eosinophils and mast cells, respectively, in response to antigen exposure.116 Several groups have shown that extensive class switch recombination and antigen specific IgA and IgE production can occur in the nasal and bronchial mucosa as part of inflammatory diseases such as allergic rhinitis and asthma.117–120 Due to the recognized importance of IgA and IgE in the airway, the local activation of B-lineage cells in the airway has been proposed to be an important event in the pathogenesis of allergic airway diseases.
In the case of CRS, B cell accumulation can be found in the lamina propria of NPs (Fig. 2H). Van Zele et al. found that CD20+ B cells and CD138+ plasma cells are elevated in NPs by immunohistochemistry.6 Several groups also found B cell clusters and B cell follicle-like structures in NPs.121–124 However, unlike the germinal center, the structure of these local follicles was not well organized (Fig. 2I). In addition, we also found B cell clusters and follicles in the nasal mucosa of CRSsNP and control subjects and found that they were not elevated in NPs.125 The role of B cell clusters and follicles in nasal mucosa needs further investigation. Our group also characterized B-lineage cells in CRS by flow cytometry and found that B-lineage cells, B cells, plasmablasts and plasma cells, were all elevated in NPs compared to uncinate process tissues from control subjects and patients with CRSsNP.125
In addition to accumulation of B-lineage cells in NPs, many groups reported the elevation of IgE in NPs. Gevaert P et al. found that total IgE, Staphylococcus aureus enterotoxin-specific IgE and IgE positive cells were elevated in NPs.121,123 Our group also evaluated the immunoglobulin isotypes, IgM, IgG, IgA and IgE in the nasal mucosa of CRS and found that all isotypes (except IgG3) were significantly elevated in NPs.125 In contrast, we could not find the elevation of immunoglobulin isotypes, except IgE, in serum of patients with CRSwNP. In addition, we found that only less than 10% of the antibodies found in NP tissues can be attributed to vascular leak.125 These results indicate that the antibodies found in NP tissues were produced locally. Moreover, several groups found the elevation of ɛ-germline gene transcripts suggesting that IgE class switch recombination can occur in NPs.123 Interestingly, Cao et al. and Baba et al. reported that local IgE production and IgE class switch recombination were found only in eosinophilic NPs but not in non-eosinophilic NPs in China and Japan.97,124
Several factors are involved in B-lineage cell responses.116 As described above, type 2 cytokine IL-13 is known to be elevated in eosinophilic NPs. IL-13 is a critical inducer of IgE class switch recombination and IgE production in B cells.116 We also found that B cell-activation factor of the TNF family (BAFF) was significantly elevated in NP tissues and nasal lavage from patients with CRSwNP.126 Gevaert et al. confirmed elevation of BAFF in NPs in Belgium.123 BAFF is an essential factor for B cell maturation and survival. BAFF also induces T cell-dependent and independent immunoglobulin class switching and production.65,116 Importantly, expression of BAFF positively correlated with B cell marker CD20 and one of the BAFF receptors, TACI, in sinus mucosa suggesting that BAFF may be a key B cell activation factor in NPs.126 Since BAFF cannot induce migration of B-lineage cells, we also looked at chemokines and found elevation of the B cell chemokine CXCL12 (SDF-1) and CXCL13 (BCL) in NPs.122 This data indicates that chemokines CXCL12 and CXCL13 may contribute to the initial recruitment of B-lineage cells, BAFF may be involved in the proliferation and activation of B-lineage cells and IL-13 is a key factor for the local production of IgE in NPs.
IgE induces allergic inflammation by activation of mast cells and basophils and plays an important role in allergic diseases including asthma. Omalizumab, which is a humanized anti-human IgE monoclonal antibody, is approved and known to be effective in the treatment of patients with severe allergic asthma.127 As described above, mast cells and IgE are highly elevated in eosinophilic NPs. We also found that basophils were elevated in NPs in the US.128 Gevaert et al. performed clinical trials of anti-IgE in patients with CRSwNP who were comorbid with asthma. They found that Omalizumab showed significant reduction in NP score and had a beneficial effect on QOL scores.129 This suggests that IgE can be an important therapeutic target for patients with eosinophilic NPs.
As mentioned above, the cytokine BAFF is elevated in NPs. BAFF is now known as a pathogenic factor for autoimmune diseases.130–132 More importantly anti-BAFF (Belimumab) has very recently been approved for the treatment of lupus.133 We therefore looked at the presence of autoantibodies in CRS. We found that anti-dsDNA antibody and anti-BP-180 antibody were significantly elevated in NPs.134,135 Since we could not find elevation of auto-antibodies in the serum of polypoid patients, this should be a local phenomenon. Interestingly we found that anti-dsDNA antibodies were more elevated in NPs who had revision surgery.135 This indicates that autoantibodies may be a pathogenic factor and/or biomarker of severe NPs. Further study will be required to identify the pathogenic role of autoantibodies in NPs.
Conclusions
This review primary focused on immune cells and cytokines that control initiation and amplification of inflammation in CRS. Although CRSsNP is known to be characterized by type 1 inflammation with elevated levels of IFN-γ, CRSsNP is a heterogeneous disease and sub-classification of this disease and further investigation of the inflammatory patterns in each group might be required in the future. Although the majority of CRSwNP cases in western countries show type 2 inflammation and eosinophilia, about 50% of CRSwNP in Asian countries show a non-eosinophilic phenotype. However, the prevalence of eosinophilic NPs has increased in Asia in the past 10–20 years and may continue to increase in the future. Inflammation in eosinophilic CRSwNP is controlled by type 2 cytokines including IL-5 and IL-13 that can be produced from Th2, mast cells and ILC2 in eosinophilic NP. Epithelial cytokines including TSLP might control production of type 2 cytokines in these cell types. Recent clinical trials showed the beneficial effect on eosinophilic NPs and/or asthma by monoclonal antibodies against IL-5, IL-4Rα, IgE and TSLP suggesting that they can be novel therapeutic targets for eosinophilic CRSwNP.
Acknowledgments
This research was supported in part by NIH grants, R01 AI104733, R21 HL113913, U19 AI106683 and R37 HL068546 and by a grant from the Ernest S. Bazley Trust.
The author acknowledges Ms. Julie Poposki for proofreading of this review. The author extends my gratitude to Ms. Jacqueline Schaffer for her input and guidance in preparation of the illustration in this review.
Abbreviations
- BAFF
B cell-activation factor of the TNF family
- CRS
chronic rhinosinusitis
- CRSsNP
CRS without nasal polyps
- CRSwNP
CRS with nasal polyps
- CRTH2
chemoattractant receptor-homologous molecule expressed on Th2 cells
- CysLT
cysteinyl leukotriene
- DCs
dendritic cells
- mDCs
myeloid DCs
- DP2
PGD2 receptor 2
- ILC2s
group 2 innate lymphoid cells
- MC-T
mast cell-tryptase
- MMP
matrix metalloproteinase
- MC-TC
mast cell-tryptase/chymase
- NPs
nasal polyps
- PG
prostaglandin
- t-PA
tissue plasminogen activator
- TSLP
thymic stromal lymphopoietin
Footnotes
Peer review under responsibility of Japanese Society of Allergology.
Conflict of interest
The author has no conflict of interest to declare.
References
- 1.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol. 2011;120:423–7. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22:549–59. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012:1–298. 3 p preceding table of contents. [PubMed] [Google Scholar]
- 5.Bachert C, Van Bruaene N, Toskala E, Zhang N, Olze H, Scadding G, et al. Important research questions in allergy and related diseases: 3-chronic rhinosinusitis and nasal polyposis e a GALEN study. Allergy. 2009;64:520–33. doi: 10.1111/j.1398-9995.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–9. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 7.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132:593–600. e12. doi: 10.1016/j.jaci.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Bruaene N, Derycke L, Perez-Novo CA, Gevaert P, Holtappels G, De Ruyck N, et al. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:253–9. 9.e1–2. doi: 10.1016/j.jaci.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Meng J, Qiao X, Liu Y, Liu F, Zhang N, et al. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125:1061–8. doi: 10.1016/j.jaci.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Shi LL, Xiong P, Zhang L, Cao PP, Liao B, Lu X, et al. Features of airway remodeling in different types of Chinese chronic rhinosinusitis are associated with inflammation patterns. Allergy. 2013;68:101–9. doi: 10.1111/all.12064. [DOI] [PubMed] [Google Scholar]
- 11.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med. 2013;187:49–57. doi: 10.1164/rccm.201207-1292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Bruaene N, Bachert C. Tissue remodeling in chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2011;11:8–11. doi: 10.1097/ACI.0b013e32834233ef. [DOI] [PubMed] [Google Scholar]
- 13.Yang YC, Zhang N, Van Crombruggen K, Hu GH, Hong SL, Bachert C. Transforming growth factor-beta1 in inflammatory airway disease: a key for understanding inflammation and remodeling. Allergy. 2012;67:1193–202. doi: 10.1111/j.1398-9995.2012.02880.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediat Inflamm. 2013;2013:928315. doi: 10.1155/2013/928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watelet JB, Bachert C, Claeys C, Van Cauwenberge P. Matrix metalloproteinases MMP-7, MMP-9 and their tissue inhibitor TIMP-1: expression in chronic sinusitis vs nasal polyposis. Allergy. 2004;59:54–60. doi: 10.1046/j.1398-9995.2003.00364.x. [DOI] [PubMed] [Google Scholar]
- 16.Regateiro FS, Howie D, Cobbold SP, Waldmann H. TGF-beta in transplantation tolerance. Curr Opin Immunol. 2011;23:660–9. doi: 10.1016/j.coi.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Al-Alawi M, Hassan T, Chotirmall SH. Transforming growth factor beta and severe asthma: a perfect storm. Respir Med. 2014;108:1409–23. doi: 10.1016/j.rmed.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Sejima T, Holtappels G, Kikuchi H, Imayoshi S, Ichimura K, Bachert C. Cytokine profiles in Japanese patients with chronic rhinosinusitis. Allergol Int. 2012;61:115–22. doi: 10.2332/allergolint.10-OA-0290. [DOI] [PubMed] [Google Scholar]
- 19.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2013;132:584–92. e4. doi: 10.1016/j.jaci.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Rosso M, Fibbi G, Pucci M, Margheri F, Serrati S. The plasminogen activation system in inflammation. Front Biosci. 2008;13:4667–86. doi: 10.2741/3032. [DOI] [PubMed] [Google Scholar]
- 21.Bagoly Z, Katona E, Muszbek L. Factor XIII and inflammatory cells. Thromb Res. 2012;129(Suppl. 2):S77–81. doi: 10.1016/j.thromres.2012.02.040. [DOI] [PubMed] [Google Scholar]
- 22.Muszbek L, Bereczky Z, Bagoly Z, Komaromi I, Katona E. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011;91:931–72. doi: 10.1152/physrev.00016.2010. [DOI] [PubMed] [Google Scholar]
- 23.Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–41. 41e1–3. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124:478–84. 84e1–2. doi: 10.1016/j.jaci.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Park SJ, Kim TH, Jun YJ, Lee SH, Ryu HY, Jung KJ, et al. Chronic rhinosinusitis with polyps and without polyps is associated with increased expression of suppressors of cytokine signaling 1 and 3. J Allergy Clin Immunol. 2013;131:772–80. doi: 10.1016/j.jaci.2012.12.671. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Wang CS, Han DM, Sy C, Huang Q, Sun Y, et al. Differential expression of Toll-like receptor pathway genes in chronic rhinosinusitis with or without nasal polyps. Acta Otolaryngol. 2013;133:165–73. doi: 10.3109/00016489.2012.717713. [DOI] [PubMed] [Google Scholar]
- 27.Hamilos DL, Leung DY, Wood R, Bean DK, Song YL, Schotman E, et al. Eosinophil infiltration in nonallergic chronic hyperplastic sinusitis with nasal polyposis (CHS/NP) is associated with endothelial VCAM-1 upregulation and expression of TNF-alpha. Am J Respir Cell Mol Biol. 1996;15:443–50. doi: 10.1165/ajrcmb.15.4.8879177. [DOI] [PubMed] [Google Scholar]
- 28.Jankowski R, Bouchoua F, Coffinet L, Vignaud JM. Clinical factors influencing the eosinophil infiltration of nasal polyps. Rhinology. 2002;40:173–8. [PubMed] [Google Scholar]
- 29.Claeys S, Van Hoecke H, Holtappels G, Gevaert P, De Belder T, Verhasselt B, et al. Nasal polyps in patients with and without cystic fibrosis: a differentiation by innate markers and inflammatory mediators. Clin Exp Allergy. 2005;35:467–72. doi: 10.1111/j.1365-2222.2005.02215.x. [DOI] [PubMed] [Google Scholar]
- 30.Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with and without nasal polyps. Allergy. 2006;61:1275–9. doi: 10.1111/j.1398-9995.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–8. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Payne SC, Borish L, Steinke JW. Genetics and phenotyping in chronic sinusitis. J Allergy Clin Immunol. 2011;128:710–20. doi: 10.1016/j.jaci.2011.05.022. quiz 21–2. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda K, Shiozawa A, Ono N, Kusunoki T, Hirotsu M, Homma H, et al. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope. 2013;123:E1–9. doi: 10.1002/lary.24154. [DOI] [PubMed] [Google Scholar]
- 34.Kim JW, Hong SL, Kim YK, Lee CH, Min YG, Rhee CS. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg. 2007;137:925–30. doi: 10.1016/j.otohns.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimura K, Kawata R, Haruna S, Moriyama H, Hirakawa K, Fujieda S, et al. Clinical epidemiological study of 553 patients with chronic rhinosinusitis in Japan. Allergol Int. 2011;60:491–6. doi: 10.2332/allergolint.10-OA-0234. [DOI] [PubMed] [Google Scholar]
- 36.Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia W, et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012;129:1522–8. e5. doi: 10.1016/j.jaci.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 37.Kim SJ, Lee KH, Kim SW, Cho JS, Park YK, Shin SY. Changes in histological features of nasal polyps in a Korean population over a 17-year period. Otolaryngol Head Neck Surg. 2013;149:431–7. doi: 10.1177/0194599813495363. [DOI] [PubMed] [Google Scholar]
- 38.Katotomichelakis M, Tantilipikorn P, Holtappels G, De Ruyck N, Feng L, Van Zele T, et al. Inflammatory patterns in upper airway disease in the same geographical area may change over time. Am J Rhinol Allergy. 2013;27:354–60. doi: 10.2500/ajra.2013.27.3922. [DOI] [PubMed] [Google Scholar]
- 39.Hamilos DL, Leung DY, Wood R, Cunningham L, Bean DK, Yasruel Z, et al. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J Allergy Clin Immunol. 1995;96:537–44. doi: 10.1016/s0091-6749(95)70298-9. [DOI] [PubMed] [Google Scholar]
- 40.Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997;158:3902–8. [PubMed] [Google Scholar]
- 41.Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–41. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 42.Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–95. e1–8. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 43.Beck LA, Stellato C, Beall LD, Schall TJ, Leopold D, Bickel CA, et al. Detection of the chemokine RANTES and endothelial adhesion molecules in nasal polyps. J Allergy Clin Immunol. 1996;98:766–80. doi: 10.1016/s0091-6749(96)70126-4. [DOI] [PubMed] [Google Scholar]
- 44.Uguccioni M, Loetscher P, Forssmann U, Dewald B, Li H, Lima SH, et al. Monocyte chemotactic protein 4 (MCP-4), a novel structural and functional analogue of MCP-3 and eotaxin. J Exp Med. 1996;183:2379–84. doi: 10.1084/jem.183.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jahnsen FL, Haye R, Gran E, Brandtzaeg P, Johansen FE. Glucocorticosteroids inhibit mRNA expression for eotaxin, eotaxin-2, and monocyte-chemotactic protein-4 in human airway inflammation with eosinophilia. J Immunol. 1999;163:1545–51. [PubMed] [Google Scholar]
- 46.Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–12. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olze H, Forster U, Zuberbier T, Morawietz L, Luger EO. Eosinophilic nasal polyps are a rich source of eotaxin, eotaxin-2 and eotaxin-3. Rhinology. 2006;44:145–50. [PubMed] [Google Scholar]
- 48.Takabayashi T, Kato A, Peters AT, Suh LA, Carter R, Norton J, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;130:410–20. e5. doi: 10.1016/j.jaci.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao T, Kojima Y, Koyanagi A, Yokoi H, Saito T, Kawano K, et al. Eotaxin-1, -2, and -3 immunoreactivity and protein concentration in the nasal polyps of eosinophilic chronic rhinosinusitis patients. Laryngoscope. 2009;119:1053–9. doi: 10.1002/lary.20191. [DOI] [PubMed] [Google Scholar]
- 50.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–42. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 51.Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130:845–52. doi: 10.1016/j.jaci.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Comeau MR, Ziegler SF. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3:138–47. doi: 10.1038/mi.2009.134. [DOI] [PubMed] [Google Scholar]
- 53.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31C:31–7. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 55.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–53. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 56.Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010;88:257–68. doi: 10.1038/icb.2009.113. [DOI] [PubMed] [Google Scholar]
- 57.Lam M, Hull L, McLachlan R, Snidvongs K, Chin D, Pratt E, et al. Clinical severity and epithelial endotypes in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013;3:121–8. doi: 10.1002/alr.21082. [DOI] [PubMed] [Google Scholar]
- 58.Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014;69:1154–61. doi: 10.1111/all.12440. [DOI] [PubMed] [Google Scholar]
- 59.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 60.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–87. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–9. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baba S, Kondo K, Kanaya K, Suzukawa K, Ushio M, Urata S, et al. Expression of IL-33 and its receptor ST2 in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2014;124:E115–22. doi: 10.1002/lary.24462. [DOI] [PubMed] [Google Scholar]
- 63.Zhou B, Headley MB, Aye T, Tocker J, Comeau MR, Ziegler SF. Reversal of thymic stromal lymphopoietin-induced airway inflammation through inhibition of Th2 responses. J Immunol. 2008;181:6557–62. doi: 10.4049/jimmunol.181.9.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jessup HK, Brewer AW, Omori M, Rickel EA, Budelsky AL, Yoon BR, et al. In-tradermal administration of thymic stromal lymphopoietin induces a T cell-and eosinophil-dependent systemic Th2 inflammatory response. J Immunol. 2008;181:4311–9. doi: 10.4049/jimmunol.181.6.4311. [DOI] [PubMed] [Google Scholar]
- 65.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–7. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–20. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123:472–8. doi: 10.1016/j.jaci.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 68.Liu T, Li TL, Zhao F, Xie C, Liu AM, Chen X, et al. Role of thymic stromal lymphopoietin in the pathogenesis of nasal polyposis. Am J Med Sci. 2011;341:40–7. doi: 10.1097/MAJ.0b013e3181f20489. [DOI] [PubMed] [Google Scholar]
- 69.Kimura S, Pawankar R, Mori S, Nonaka M, Masuno S, Yagi T, et al. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011;3:186–93. doi: 10.4168/aair.2011.3.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ouyang Y, Fan E, Li Y, Wang X, Zhang L. Clinical characteristics and expression of thymic stromal lymphopoetin in eosinophilic and non-eosinophilic chronic rhinosinusitis. ORL J Otorhinolaryngol Relat Spec. 2013;75:37–45. doi: 10.1159/000346929. [DOI] [PubMed] [Google Scholar]
- 71.Boita M, Garzaro M, Raimondo L, Riva G, Mazibrada J, Pecorari G, et al. Eosinophilic inflammation of chronic rhinosinusitis with nasal polyps is related to OX40 ligand expression. Innate Immun. 2015;21:167–74. doi: 10.1177/1753425914523460. [DOI] [PubMed] [Google Scholar]
- 72.Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–10. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 73.Derycke L, Eyerich S, Van Crombruggen K, Perez-Novo C, Holtappels G, Deruyck N, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One. 2014;9:e97581. doi: 10.1371/journal.pone.0097581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi LL, Song J, Xiong P, Cao PP, Liao B, Ma J, et al. Disease-specific T-helper cell polarizing function of lesional dendritic cells in different types of chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2014;190:628–38. doi: 10.1164/rccm.201402-0234OC. [DOI] [PubMed] [Google Scholar]
- 75.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 76.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–80. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 78.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–4. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 80.Lu N, Wang YH, Arima K, Hanabuchi S, Liu YJ. TSLP and IL-7 use two different mechanisms to regulate human CD4+ T cell homeostasis. J Exp Med. 2009;206:2111–9. doi: 10.1084/jem.20090153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reefer AJ, Hulse KE, Lannigan JA, Solga MD, Wright PW, Kelly LA, et al. Flow cytometry imaging identifies rare T(H)2 cells expressing thymic stromal lymphopoietin receptor in a proallergic milieu. J Allergy Clin Immunol. 2010;126:1049, 58, 58e1–10. doi: 10.1016/j.jaci.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poposki JA, Peterson A, Welch K, Schleimer RP, Hulse KE, Peters AT, et al. Elevated presence of myeloid dendritic cells in nasal polyps of patients with chronic rhinosinusitis. Clin Exp Allergy. 2015;45:384–93. doi: 10.1111/cea.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pezato R, Perez-Novo CA, Holtappels G, De Ruyck N, Van Crombruggen K, De Vos G, et al. The expression of dendritic cell subsets in severe chronic rhinosinusitis with nasal polyps is altered. Immunobiology. 2014;219:729–36. doi: 10.1016/j.imbio.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 85.Yerkovich ST, Roponen M, Smith ME, McKenna K, Bosco A, Subrata LS, et al. Allergen-enhanced thrombomodulin (blood dendritic cell antigen 3, CD141) expression on dendritic cells is associated with a TH2-skewed immune response. J Allergy Clin Immunol. 2009;123:209–16. e4. doi: 10.1016/j.jaci.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 86.Peterson S, Poposki JA, Nagarkar DR, Chustz RT, Peters AT, Suh LA, et al. Increased expression of CC chemokine ligand 18 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;129:119–27. e1–9. doi: 10.1016/j.jaci.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poposki JA, Uzzaman A, Nagarkar DR, Chustz RT, Peters AT, Suh LA, et al. Increased expression of the chemokine CCL23 in eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:73–81. e4. doi: 10.1016/j.jaci.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nardelli B, Tiffany HL, Bong GW, Yourey PA, Morahan DK, Li Y, et al. Characterization of the signal transduction pathway activated in human monocytes and dendritic cells by MPIF-1, a specific ligand for CC chemokine receptor 1. J Immunol. 1999;162:435–44. [PubMed] [Google Scholar]
- 89.Vulcano M, Struyf S, Scapini P, Cassatella M, Bernasconi S, Bonecchi R, et al. Unique regulation of CCL18 production by maturing dendritic cells. J Immunol. 2003;170:3843–9. doi: 10.4049/jimmunol.170.7.3843. [DOI] [PubMed] [Google Scholar]
- 90.Islam SA, Ling MF, Leung J, Shreffler WG, Luster AD. Identification of human CCR8 as a CCL18 receptor. J Exp Med. 2013;210:1889–98. doi: 10.1084/jem.20130240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krysko O, Holtappels G, Zhang N, Kubica M, Deswarte K, Derycke L, et al. Alternatively activated macrophages and impaired phagocytosis of S. aureus in chronic rhinosinusitis. Allergy. 2011;66:396–403. doi: 10.1111/j.1398-9995.2010.02498.x. [DOI] [PubMed] [Google Scholar]
- 92.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 93.Balzar S, Fajt ML, Comhair SA, Erzurum SC, Bleecker E, Busse WW, et al. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am J Respir Crit Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsu Blatman KS, Gonsalves N, Hirano I, Bryce PJ. Expression of mast cell-associated genes is upregulated in adult eosinophilic esophagitis and responds to steroid or dietary therapy. J Allergy Clin Immunol. 2011;127:1307–8. e3. doi: 10.1016/j.jaci.2010.12.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abonia JP, Blanchard C, Butz BB, Rainey HF, Collins MH, Stringer K, et al. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:140–9. doi: 10.1016/j.jaci.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, et al. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. J Allergy Clin Immunol. 2010;125:1046–53. e8. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao PP, Zhang YN, Liao B, Ma J, Wang BF, Wang H, et al. Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not non-eosinophilic, chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2014;44:690–700. doi: 10.1111/cea.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–8. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagarkar DR, Poposki JA, Comeau MR, Biyasheva A, Avila PC, Schleimer RP, et al. Airway epithelial cells activate TH2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin. J Allergy Clin Immunol. 2012;130:225–32. e4. doi: 10.1016/j.jaci.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–8. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 101.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cellsea proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 102.Sonnenberg GF, Mjosberg J, Spits H, Artis D. SnapShot: innate lymphoid cells. Immunity. 2013;39:622, e1. doi: 10.1016/j.immuni.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 103.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 104.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 105.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 108.Walford HH, Lund SJ, Baum RE, White AA, Bergeron CM, Husseman J, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014;155:126–35. doi: 10.1016/j.clim.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–59. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 110.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671–8. e4. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Townley RG, Agrawal S. CRTH2 antagonists in the treatment of allergic responses involving TH2 cells, basophils, and eosinophils. Ann Allergy Asthma Immunol. 2012;109:365–74. doi: 10.1016/j.anai.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 112.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol. 2014;133:899–901. e3. doi: 10.1016/j.jaci.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184–94. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kato A, Chustz RT, Ogasawara T, Kulka M, Saito H, Schleimer RP, et al. Dexamethasone and FK506 inhibit expression of distinct subsets of chemokines in human mast cells. J Immunol. 2009;182:7233–43. doi: 10.4049/jimmunol.0801375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–13. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kato A, Hulse KE, Tan BK, Schleimer RP. B-lymphocyte lineage cells and the respiratory system. J Allergy Clin Immunol. 2013;131:933–57. doi: 10.1016/j.jaci.2013.02.023. quiz 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilonRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99:1492–9. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Takhar P, Smurthwaite L, Coker HA, Fear DJ, Banfield GK, Carr VA, et al. Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis. J Immunol. 2005;174:5024–32. doi: 10.4049/jimmunol.174.8.5024. [DOI] [PubMed] [Google Scholar]
- 119.Fiset PO, Cameron L, Hamid Q. Local isotype switching to IgE in airway mucosa. J Allergy Clin Immunol. 2005;116:233–6. doi: 10.1016/j.jaci.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 120.Takhar P, Corrigan CJ, Smurthwaite L, O’Connor BJ, Durham SR, Lee TH, et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J Allergy Clin Immunol. 2007;119:213–8. doi: 10.1016/j.jaci.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 121.Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–9. doi: 10.1111/j.1398-9995.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 122.Patadia M, Dixon J, Conley D, Chandra R, Peters A, Suh LA, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24:11–6. doi: 10.2500/ajra.2010.24.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, Fear DJ, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68:55–63. doi: 10.1111/all.12054. [DOI] [PubMed] [Google Scholar]
- 124.Baba S, Kondo K, Toma-Hirano M, Kanaya K, Suzukawa K, Ushio M, et al. Local increase in IgE and class switch recombination to IgE in nasal polyps in chronic rhinosinusitis. Clin Exp Allergy. 2014;44:701–12. doi: 10.1111/cea.12287. [DOI] [PubMed] [Google Scholar]
- 125.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131:1075–83. e1–7. doi: 10.1016/j.jaci.2013.01.043. 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–92. e1–2. doi: 10.1016/j.jaci.2008.03.002. 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McKeage K. Omalizumab: a review of its use in patients with severe persistent allergic asthma. Drugs. 2013;73:1197–212. doi: 10.1007/s40265-013-0085-4. [DOI] [PubMed] [Google Scholar]
- 128.Mahdavinia M, Carter RG, Ocampo CJ, Stevens W, Kato A, Tan BK, et al. Basophils are elevated in nasal polyps of patients with chronic rhinosinusitis without aspirin sensitivity. J Allergy Clin Immunol. 2014;133:1759–63. doi: 10.1016/j.jaci.2013.12.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131:110–6. e1. doi: 10.1016/j.jaci.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 130.Zhang J, Roschke V, Baker KP, Wang Z, Alarcon GS, Fessler BJ, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 131.Mariette X, Roux S, Zhang J, Bengoufa D, Lavie F, Zhou T, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren’s syndrome. Ann Rheum Dis. 2003;62:168–71. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–36. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 133.Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–31. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 134.Jeffe JS, Seshadri S, Hamill KJ, Huang JH, Carter R, Suh L, et al. A role for anti-BP180 autoantibodies in chronic rhinosinusitis. Laryngoscope. 2013;123:2104–11. doi: 10.1002/lary.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–206. e1. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


