Abstract
Exposure to social and environmental stressors may influence behavior as well as autonomic and cardiovascular regulation, potentially leading to depressive disorders and cardiac dysfunction including elevated sympathetic drive, reduced parasympathetic function, and ventricular arrhythmias. The cellular mechanisms that underlie these interactions are not well understood. One mechanism may involve alterations in the expression of Connexin43 (Cx43) and Connexin45 (Cx45), gap junction proteins in the heart that play an important role in ensuring efficient cell-to-cell coupling and the maintenance of cardiac rhythmicity. The present study investigated the hypothesis that long-term social isolation, combined with mild environmental stressors, would produce both depressive behaviors and altered Cx43 and Cx45 expression in the left ventricle of prairie voles – a socially monogamous rodent model. Adult, female prairie voles were exposed to either social isolation (n=22) or control (paired, n=23) conditions (4 weeks), alone or in combination with chronic mild stress (1 week). Social isolation, versus paired control conditions, produced significantly (P < 0.05) increased depressive behaviors in a 5-min forced swim test, and chronic mild stress exacerbated (P < 0.05) these behaviors. Social isolation (alone) reduced (P < 0.05) total Cx43 expression in the left ventricle; whereas chronic mild stress (but not isolation) increased (P < 0.05) total Cx45 expression and reduced (P < 0.05) the Cx43/Cx45 ratio, measured via Western blot analysis. The present findings provide insight into potential cellular mechanisms underlying altered cardiac rhythmicity associated with social and environmental stress in the prairie vole.
Keywords: cardiac arrhythmic susceptibility, chronic mild stress, connexins, depression, microtus, social isolation
Introduction
Increased attention has been devoted to understanding the link between affective states, such as depression, and cardiovascular disease (CVD). These conditions are bidirectionally associated (Glassman 2007; Lichtman et al. 2008). Depression is an important risk factor for CVD in medically healthy individuals and in patients with established cardiac dysfunction, independent of traditional cardiovascular risk factors (Penninx et al. 2001; Carney and Freedland 2003; Frasure-Smith and Lespérance 2003). Conversely, CVD is associated with increased levels of mood disorders (Freedland et al. 2003). Disrupted homeostatic mechanisms may underlie the association between mood and cardiovascular disorders, including, among others, autonomic nervous system dysfunction and cardiac rhythm disturbances (Johnson and Grippo 2006; de Jonge et al. 2010).
Further, social and environmental factors, such as actual and perceived social isolation, influence the relationships among autonomic dysregulation, mood, and CVD (Cacioppo et al. 2002; Kiecolt-Glaser et al., 2010; Grippo 2011; Steptoe et al. 2013). Individuals with smaller social networks or who are less socially integrated display increased depressive symptomatology compared to those who are more socially integrated (Rutledge et al. 2008). Social isolation and fewer social connections also are associated with cardiovascular risk factors, including coronary artery calcification, increased blood glucose levels, hypertension, and diabetes mellitus; and contribute to increased cardiovascular mortality (Kaplan et al. 1988; Eng et al. 2002; Rutledge et al. 2004; Kop et al. 2005; Rutledge et al. 2008; Ramsay et al. 2008).
Studies with animal models have provided valuable insight into the potential interactions among social stressors, mood disorders, and CVD. Of relevance, the socially monogamous prairie vole has been used in this context given its display of behavioral, social, and physiological traits similar to humans. Unlike most other rodent species, the prairie vole is among only 3% of mammalian species that is actively engaged in its social context, exhibiting traits of social monogamy such as forming long-term pair bonds, displaying bi-parental care, and living in extended family groups (Carter and Keverne 2002). This species also exhibits autonomic characteristics that mimic those of humans and larger primates (but are unlike other rodents such as rats and mice), including high parasympathetic and low sympathetic regulation of the heart at rest (Grippo et al. 2007b). Prairie voles are highly sensitive, both behaviorally and physiologically, to disruptions in the social environment. Social isolation and the disruption of established social bonds in this species result in depressive and anxiety-like behaviors, an increased responsiveness to acute stressors, and physiological alterations that mirror those observed in CVD, including increased heart rate, decreased heart rate variability, impaired vascular relaxation, and autonomic imbalance (Grippo et al. 2007c; Peuler et al. 2012; McNeal et al. 2014). These findings provide evidence for the utility of the prairie vole as a model for investigating mechanisms underlying responses to social stressors.
Social stressors also produce ventricular and supraventricular arrhythmias in prairie voles (Grippo et al. 2010; Grippo et al. 2012). Socially isolated prairie voles exposed to an acute swimming stressor exhibit depressive behaviors and a dramatically higher arrhythmic burden (resulting from increased ventricular and supraventricular arrhythmias) relative to socially paired animals (Grippo et al. 2012). Similarly, social isolation (versus social pairing) followed by acute social crowding produces increased arrhythmias and heart rate, and reduced heart rate variability (Grippo et al. 2010). The specific processes, however, that mediate these cardiac arrhythmias as a function of social stressors are not well understood and may be informed by investigations of cellular cardiac mechanisms.
Although there are a number of cellular mechanisms that could be responsible for the increased cardiac arrhythmias resulting from social stressors, one possibility includes an alteration in the expression of the gap junction protein Connexin43 (Cx43). Cx43 is a 43 kDa protein highly expressed within mammalian ventricles at the intercalated discs. It plays a substantial role in ensuring efficient cell-to-cell coupling and maintaining cardiac rhythms (Kanter et al., 1992; Bernstein and Morley 2006; Imanaga 2009). Ventricular arrhythmias are more easily provoked in animals deficient in Cx43 (Lerner et al. 2000), while significant decreases in the expression of Cx43 have been implicated in the pathogenesis of ventricular arrhythmias following infarction (Takamatsu 2008) and heart failure (Ai and Pogwizd 2005). Interestingly, cardiovascular deconditioning produced through hindlimb unloading increases the incidence of ventricular arrhythmias and is associated with increased expression of Cx43 in rats (Moffitt et al. 2013), as does forced restraint (Unuma et al. 2010). In addition, changes in Cx43 expression and phosphorylation vary considerably over the time course of development of heart failure in dogs (Akar et al. 2007). These data taken together indicate that changes in Cx43 expression may follow a complex trajectory in a number of pathological states, and that disruption of Cx43 expression appears to be a critical factor in disrupting cardiac rhythmicity.
Unlike Cx43, another gap junction protein, Cx45, although absolutely required for embryonic development, is expressed in relatively low levels in the working myocardium (Bao et al. 2011). However, studies have shown that Cx45 expression is increased in human heart failure (Yamada et al. 2003) and it increases arrhythmic susceptibility (Betsuyaka et al. 2006). Since Cx45 can form heterotypic and heteromeric channels with Cx43 (Verheule and Kaese 2013), increased expression of Cx45 relative to Cx43 may decrease the size of gap junctions (Grikscheit et al. 2008), and a decreased ratio of Cx43/45 may induce a pro-arrhythmic state under pathological conditions (Yamada et al. 2004).
Given previous findings regarding Cx43 and Cx45 in other species, we examined the hypothesis that a combination of social and environmental stressors would produce disruptions in Cx43 and Cx45 protein expression in the left ventricle of prairie voles. We further hypothesized that social stressors would produce depressive behaviors, confirming social isolation as a useful model for the study of depression (Grippo et al. 2007a; Grippo et al. 2008), and that the novel combination of social and environmental stressors in this model would exacerbate depressive behaviors. To investigate these hypotheses, adult prairie voles were exposed to 4 weeks of social isolation followed by a period of mild environmental stressors for one week, after which a behavioral measure of depression (helplessness) and analysis of Cx43 and Cx45 protein levels and their ratio in the left ventricle were performed in the same animals.
Methods
Animals
Forty-five adult female prairie voles, descendants of a wild stock caught near Champaign, Illinois, were used in the present study. Prairie voles had a mean (± standard error of the mean, SEM) age of 70±4 days and a body weight of 36±1 grams. All prairie voles were maintained on a 14h/10 h light/dark cycle (lights on at 06:00h), with a mean ± SEM ambient temperature of 25±2°C and relative humidity of 40±5%. Prairie voles were allowed food (Purina rabbit chow) and water ad libitum, unless otherwise noted. Offspring were removed from breeding pairs at 21 days of age and housed in same-sex sibling pairs until the commencement of the study. For all procedures described herein, one prairie vole from each sibling pair was studied. All procedures were conducted according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the local University Institutional Animal Care and Use Committees.
Social Isolation
Prairie voles were randomly divided into paired (control; n=23) or socially isolated (n=22) groups. Paired control prairie voles were continually housed with the siblings for 4 weeks, while isolated prairie voles were separated from their respective siblings and housed individually without visual, auditory or olfactory cues. Handling and cage changing were matched between the two groups.
Chronic Mild Stress (CMS)
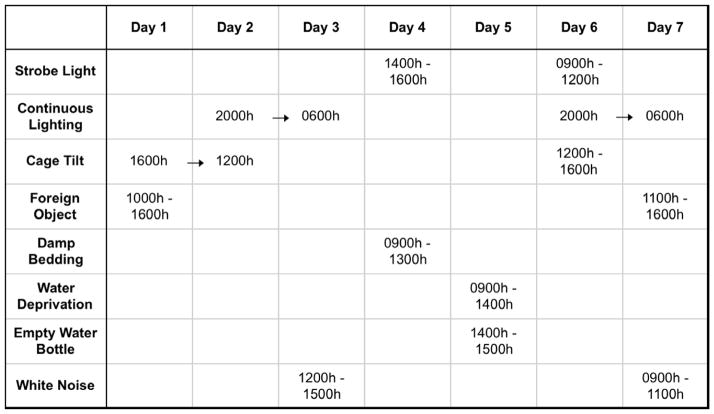
Following the 4-week period of social isolation or pairing, half of the prairie voles in each group were exposed to 7 days of chronic mild stress (CMS; n=14 paired and n=11 isolated), while the other half remained undisturbed (n=9 paired and n=11 isolated). The CMS procedure was a modification of methods which have been used previously to induce depressive behaviors in rodents (Grippo et al. 2005). Briefly, all prairie voles were exposed to a series of mild stressors on an unpredictable schedule for varying durations throughout the light and dark periods (Figure 1 gives details), including: (1) continuous overnight lighting; (2) strobe light (300 flashes/min); (3) white noise (90 dB); (4) exposure to an empty water bottle following a brief period of water deprivation; (5) tilted cage (40º tilt along the vertical axis); (6) damp bedding (100 ml water poured into the bottom of the cage; and (7) a foreign object in the cage a brick, 15 cm × 7.6 cm × 6.3 cm; this stressor was used in place of the paired housing stressor, which has been employed previously in CMS paradigms in other rodents (Grippo et al. 2005) but would have interfered with the social housing manipulation in the present study.
Figure 1.
Details of the chronic mild stress (CMS) procedure used in the present study design.
Forced Swim Test (FST)
Twenty-four hours following the last stressor in the CMS paradigm, investigation of swimming behavior in the forced swim test (FST) was used as an operational index of depressive behaviors. All prairie voles were exposed to a 5-minute FST using procedures described elsewhere (Cryan et al., 2005), during the light period (approximately 3–5 hours after light onset). Briefly, a clear, cylindrical Plexiglas tank (46 cm height; 20 cm diameter) was filled to a depth of 18 cm with tap water (25–26° C). Each prairie vole was placed individually into the tank for 5 minutes. The tank was cleaned thoroughly and filled with clean water prior to testing each prairie vole. Each prairie vole was returned to its home cage (paired or isolated) immediately following the 5-minute swim period, and was allowed access to a heat lamp for 15 minutes.
Behaviors during the FST were recorded using a digital video camera, and scored offline manually by two trained observers who were blind to the experimental conditions. The behaviors were defined as: (1) swimming: directed, coordinated movements of the forelimbs and hindlimbs without breaking the surface of the water; (2) struggling: forelimbs breaking the surface of water; (3) climbing: attempts to climb the walls of the tank; and (4) immobility: no limb or body movements (floating) or using limbs solely to remain afloat without corresponding trunk movements. Struggling, climbing and swimming were summed to provide one index of active coping behaviors, and immobility was used as the operational index of depressive behavior, according to previous tests of validity and reliability (Cryan et al., 2005).
Collection of Cardiac Tissue
Five days following the FST, all paired and isolated animals were killed using an overdose of a 5:1 ratio of ketamine (67 mg/kg, dissolved at 100 mg/ml in distilled water, sc; NLS Animal Health, Owings Mills, MD) and xylazine (13.33 mg/kg, dissolved at 100 mg/ml in distilled water, sc; NLS Animal Health), during the light period (approximately 3–5 hours following light onset). All prairie voles were anesthetized within 1 minute of being removed from the housing room. The heart was immediately removed and sectioned into 4 chambers. Each chamber was immediately weighed, and then frozen using liquid nitrogen. The tissue was stored at −80° C.
Analysis of Connexin43 (Cx43) and Connexin45 (Cx45) Protein Expression
To determine the levels of Cx43 and Cx45 protein expression, Western blot analysis was performed on left ventricle tissue from prairie voles in paired and isolated groups in the presence and absence of CMS. Frozen tissue was homogenized on ice with a tissue grinder in lysis buffer [10mM Tris, 1% (v/V) Triton X-100, 5mM disodium EDTA, 50mM NaCl, 30mM Sodium Pyrophosphate, 50mM NaF, 0.1mM Sodium Orthovanadate, 0.3mM PMSF, and protease inhibitor cocktail (AEBSF 104 mM, Aprotinin 80 μM, Bestatin 4 mM,E-64 1.4 mM, Leupeptin 2 mM, Pepstatin A, 1.)]. Subsequently, the homogenates were pulse sonicated at 5 watts while on ice for 15 minutes. The lysate was cleared by centrifugation at 21000 x g for 15 minutes. The supernatant was collected for further analysis and the pellet discarded. Lysate was resolved on a 10% polyacrylamide gel and transferred to a polyvinylidene fluoride membrane. Membranes were probed for total Cx43 expression using a polyclonal Cx43 antibody (C6219, Sigma Aldrich, St. Louis, MO) or Cx45 antibody (Lecanda et al. 1998). To control for loading, membranes were probed for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) with a polyclonal anti-GAPDH antibody (G9545, Sigma Aldrich). To visualize protein, membranes were incubated with an anti-rabbit-HRP conjugated secondary antibody (W4011, Fisher Scientific, Pittsburgh, PA) and subsequently treated with Super SignalChemiluminescent reagent (Thermo Scientific, Rockford, IL). Membranes were exposed to x-ray film. Protein expression was measured as a function of optical density using Image Lab (BioRad, Contra Costa, CA). Data are expressed as the ratio of Cx43 normalized to GAPDH, Cx45 normalized to GAPDH, and the ratio of the normalized Cx43 to Cx45 values. Each sample in the experimental groups (paired + CMS, isolated, and isolated + CMS) was then normalized to the average intensity of the control group (paired) and expressed as a value relative to the control group.
Data Analyses
Data are presented as means ± SEM for all analyses and figures. A value of P < 0.05 was considered to be statistically significant. Two-factor, independent-groups analyses of variance (ANOVA) were used for all comparisons between housing (paired or isolated) and stress (CMS or no stress). Fisher’s Least Significant Difference post-hoc analyses and Student’s t-tests with a Bonferroni correction were conducted for pairwise comparisons.
Results
Depressive Behaviors
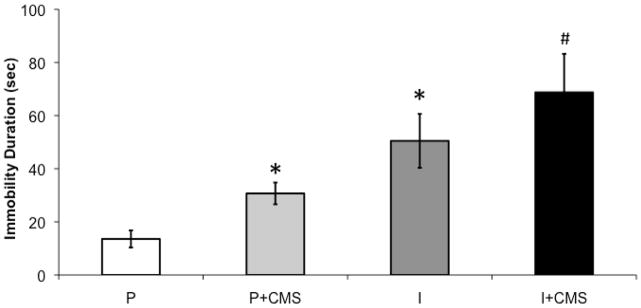
Social isolation was associated with increased depressive behaviors in the FST, relative to social pairing (control conditions); and CMS exacerbated depressive behaviors in both paired and isolated groups (Figure 2). An independent-groups ANOVA yielded main effects of both housing [F(1,41) = 30.37, P < 0.001] and stress [F(1,41) = 5.96, P < 0.02]. Fisher’s post-hoc analyses, with P < 0.05 defined as the critical probability value, indicated a step-wise effect of CMS and social isolation, such that all 3 experimental groups displayed significantly greater levels of immobility relative to the paired (control) group (P < 0.05 for all comparisons); the isolated and isolated + CMS groups displayed significantly greater levels of immobility relative to the paired + CMS group (P < 0.05); and the isolated + CMS group displayed greater levels of immobility relative to the isolated group (P < 0.05). However, the immobility levels of the isolated group did not differ significantly from the paired + CMS group (P > 0.05).
Figure 2.
The impact of social isolation and chronic mild stress (CMS) on depressive behaviors in the 5-min forced swim test (FST). Mean (± standard error of the mean, SEM) duration of immobility in the FST in paired (P, n=9), paired + CMS (P+CMS, n=14), isolated (I, n=11), and isolated + CMS (I+CMS, n=11). Statistical symbols indicate value is significantly different (P < 0.05, using 2-factor, independent groups ANOVA and Fisher’s Least Significant Difference post-hoc analyses) from: * = paired group only; # = all other groups. Note: the remainder of 300 seconds is composed of active coping behaviors.
Connexin43 (Cx43) and Connexin45 (Cx45) Protein Expression
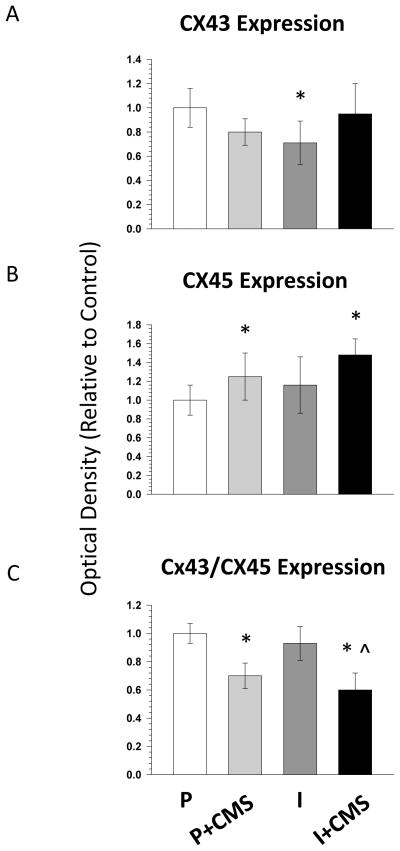
Social isolation (but not CMS) was associated with reduced Cx43 expression; whereas CMS (but not social isolation) was associated with increased Cx45 expression and a reduced Cx43/Cx45 ratio in the left ventricle (Figures 3 and 4). An independent-groups ANOVA for total Cx43 expression yielded a main effect of housing [F(1,31) = 4.60, P < 0.04]. The isolated group displayed significantly lower levels of Cx43 relative to the paired (control) group [t(16] = 2.82, P < 0.01].
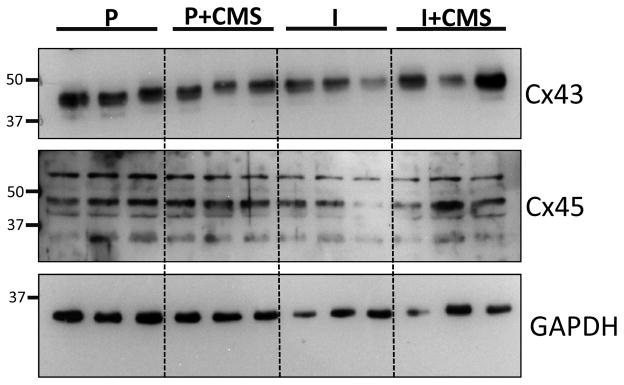
Figure 3.
A representative Western blot of connexin43 (Cx43) and connexin45 (Cx45) expression in the left ventricle, relative to the loading control (glyceraldehyde 3-phosphate dehydrogenase, GAPDH), for a set of paired (P) and isolated (I) prairie voles in the presence and absence of chronic mild stress (CMS).
Figure 4.
The impact of social isolation and chronic mild stress (CMS) on connexin43 (Cx43) and connexin45 (Cx45) expression in the left ventricle. Expression levels of Cx43 (A) and Cx45 (B) were determined by Western blot and normalized to the GAPDH loading control. In addition, the ratio of Cx43/Cx45 (C) was determined after normalization of Cx43 and Cx45 to GAPDH. Data are represented as the mean and standard error of the mean (SEM) for each group relative to control (paired). Quantification (mean ± SEM) of Cx43 and Cx45 expression for paired (P, n=8), paired + CMS (P+CMS, n=9), isolated (I, n=9), and isolated + CMS (I+CMS, n=9). Statistical symbol indicates the value is significantly different (P< 0.05, using 2-factor, independent groups ANOVA and Student’s t-tests with a Bonferroni correction) from: * = paired group; ^ = isolated group.
An independent-groups ANOVA for total Cx45 expression yielded a main effect of stress [F(1,31) = 4.63, P < 0.04]. The paired + CMS group [t(16) = 2.51, P < 0.02] and the isolated + CMS group [t(16) = 3.18, P < 0.006] displayed increased levels of Cx45 relative to the paired (control) group.
An independent-groups ANOVA for the Cx43/Cx45 ratio yielded a main effect of stress [F(1,31) = 8.13, P < 0.008]. The paired + CMS group displayed a significantly reduced Cx43/Cx45 ratio relative to the paired (control) group [t(16) = 2.13, P < 0.05]. The isolated + CMS group displayed a significantly reduced Cx43/Cx45 ratio relative to the isolated group [t(16) = 2.31, P < 0.03], and relative to the paired (control) group [t(16) = 2.07, P < 0.05].
Body Weight and Left Ventricular Weight
Neither social isolation nor CMS affected body weight, left ventricular weight, or left ventricular/body weight ratio. There were no significant differences in any of these variables among the 4 groups (P > 0.05 for all comparisons; data not shown).
Discussion
The current findings provide novel insight into cardiac Cx43 and Cx45 protein expression, and their association with depressive behaviors, in prairie voles exposed to social and environmental stressors. Several social stressors, including long-term social isolation, the short-term disruption of established social bonds, social crowding, and intersexual aggression, have produced behavioral disturbances relevant to mood and cardiovascular disorders in prairie voles (Grippo et al. 2007c; Bosch et al. 2009; Grippo et al. 2010; McNeal et al. 2014). The present study demonstrates that social stress (but not CMS) alters Cx43 expression, whereas CMS (but not social stress) alters Cx45 expression and the Cx43/C45 ratio. These findings can inform our understanding of the influence of stress on behavior and electrical communication in the heart.
Both social isolation and CMS, separately, have been shown to produce various depressive behaviors in rodent models, including anhedonia and helplessness (Grippo et al., 2002; Willner 2005; Grippo et al. 2007c; McNeal et al. 2014). This is the first study to investigate a combination of CMS and social isolation in the prairie vole, and therefore the FST was specifically chosen here as a validated operational measure of depressive behaviors (Cryan et al., 2005). The present study indicates that a combination of social isolation and CMS has an additive effect on depressive behaviors in the FST. Social isolation led to an increase in helpless behavior (immobility) and a reduction in active coping behavior in the FST when compared to social pairing (control), similar to previous studies that have investigated the effects of social isolation on behavior in prairie voles (Grippo et al. 2008; Bosch et al. 2009). However, a combination of social isolation and CMS produced significantly greater levels of immobility than social isolation alone. The current findings provide further evidence for the behavioral consequences of social stressors in rodents, and indicate that the addition of mild environmental stressors is an important consideration in the context of investigating behavioral responses to the social environment.
In contrast to the effects of social isolation and CMS on behavior, the combination of these two manipulations did not have the same additive influence on Cx43 and Cx45 expression in the left ventricle. Although social isolation was associated with a reduction in Cx43 expression compared to social pairing (control), the combination of CMS with social isolation did not further alter the expression of this protein versus social isolation alone. In contrast to Cx43 expression, however, CMS was associated with a significant increase in Cx45 expression, as well as a significant decrease of the ratio of Cx43 to Cx45 in the left ventricle, but social isolation did not further influence Cx45 or the Cx43/Cx45 ratio. This pattern of responses indicates that social isolation and CMS may have differential influences on the expression of Cx43 and Cx45 in the left ventricle of the prairie vole. Further exploration of these potential differential influences is warranted.
The present results may provide insight into a mechanism through which social isolation and CMS have arrhythmogenic effects on the heart, which has been demonstrated in our previous studies using both rats and prairie voles (Grippo et al. 2004; Grippo et al. 2010; Grippo et al. 2012) The present findings may contribute to the understanding of previously demonstrated alterations in electrophysiological functioning following chronic social stress in a rodent model of depression (Carnevali et al. 2013). The precise interactions of social and environmental stress, depressive behaviors, and altered Cx43 and Cx45 expression are not well defined, however previous findings from a number of studies indicate that appropriate Cx43 communication is important for maintaining cardiac rhythmicity (Beardslee et al. 1998; Lerner et al. 2000; Bernstein and Morley 2006; Mayama et al. 2007), and a reduction in the Cx43/Cx45 ratio may induce a pro-arrhythmic state (Yamada et al. 2003; Yamada et al. 2004; Betsuyaka et al. 2006). The present findings also lend further support to previous data indicating that various forms of psychological and physiological stressors, such as hindlimb unloading (Moffitt et al. 2013) and restraint (Unuma et al. 2010), disrupt Cx43 expression in the heart. However, whether stress influences cardiac function directly to alter Cx43 and Cx45 expression, or indirectly via neurocardiac communication, is yet to be determined.
One possible explanation is that exposure to stressors alters cardiac autonomic balance which then elicits disruptions in Cx43 and Cx45 protein expression in the heart. Previous data from rats (Grippo et al. 2002) and prairie voles (Grippo et al. 2007c; McNeal et al. 2014) indicate that social isolation and/or CMS induces cardiac autonomic imbalance such that there is a shift toward sympathetic dominance and away from cardiac parasympathetic (vagal) dominance. This cardiac autonomic imbalance has been previously well-documented to increase predisposition to cardiac arrhythmias (Verrier and Antzelevich 2004; Zipes 2008), and this is corroborated by data indicating increased arrhythmogenesis in animal models of each chronic stress and isolation (Sgoifo et al. 1998; Grippo et al. 2010; Grippo et al. 2012). Previous data suggest that increasing parasympathetic activity through vagus nerve stimulation increases the expression and phosphorylation of Cx43 (Ando et al. 2005; Yue et al. 2006; Bellafiore et al. 2007; Zhao et al. 2010), while direct cardiac sympathetic nerve stimulation decreases the expression of Cx43 in the left ventricle (Jiang et al. 2008). Similarly, exposure of cultured cardiomyocytes to acetylcholine up-regulates the expression of Cx43 (Ando et al. 2005), while exposure to β-adrenoreceptor agonists increases the non-phosphorylated form of Cx43 (Xia et al. 2009), which is considered to provoke cardiac arrhythmias (Takamatsu 2008; Imanaga 2009). In addition, human heart failure, a pathological condition notorious for sympathetic elevation, results in increased expression of Cx45 (Yamada et al. 2003) indicating that this may be part of the pathogenesis of ventricular arrhythmias in heart failure since the overexpression of Cx45 in mice induces gap junction uncoupling and increased predisposition to ventricular tachycardia (Betsuyaka et al. 2006). These data taken together indicate that the stressors associated with isolation and/or CMS result in cardiac autonomic imbalance which may in turn serve to alter the ratio of Cx43 to Cx45, thereby rendering the heart more vulnerable to sustaining arrhythmias.
While the present study focused exclusively on Cx43 and Cx45 expression in the left ventricle, it is possible that social isolation and/or CMS may alter the expression of connexin proteins in the brain, thereby impairing neuronal function and disrupting emotion-related pathways associated with depressive disorders (Sun et al. 2012). For instance, recent studies have demonstrated that chronic unpredictable stress in rats is associated with altered Cx43 expression in the prefrontal cortex (Sun et al. 2012) and hippocampus (Li et al. 2010); and that a Cx43-mimetic peptide infused into the brain, which blocks gap junctions, produces depressive behaviors (Sun et al. 2012).
In summary, the present study has demonstrated that social isolation in prairie voles reduces Cx43 protein expression in the left ventricle, as well as increasing depressive behaviors, which were exacerbated by the addition of CMS. However, CMS increased Cx45 protein expression and reduced the ratio of Cx43 to Cx45 in the left ventricle. A combination of social isolation and CMS may serve to alter autonomic control of the heart, in turn negatively affecting the expression of Cx43 and Cx45, thereby increasing the vulnerability to cardiac arrhythmias. Disruptions of connexin proteins in the left ventricle may thus be one cellular mechanism through which social stressors are arrhythmogenic and produce a cascade of cardiovascular dysfunction. The current study is a first step in the investigation of cellular cardiac consequences of social and environmental stressors in the prairie vole model. Additional studies are necessary to determine the precise pathways through which social and environmental stressors influence connexin protein expression in the heart. For example, further investigation of the interactions of behaviors and connexin protein expression, both in the brain and the heart, can provide insight into signaling mechanisms responsible for Cx43 and Cx45 alterations. Taken together with the present data, these additional studies will inform our understanding of the mechanisms through which stress contributes to behavioral and physiological alterations associated with mood disorders and CVD.
Acknowledgments
This research was supported by National Institutes of Health grants HL112350 (AJG), HL108475 (M-ALS), and MH77581 (AJG); Northern Illinois University (AJG); and the Iowa Osteopathic Educational Research Fund (JAM). The authors would like to thank Michael Koval, PhD, of Emory University, for the generous donation of Connexin45 antibody.
Footnotes
Declaration of Interest
The authors declare no financial or personal relationships with individuals or organizations that would influence the subject matter or materials discussed in this manuscript. The sources of funding for this work include the following: National Institutes of Health grants HL112350 (AJG), HL108475 (M-ALS), and MH77581 (AJG); Northern Illinois University (AJG); and the Iowa Osteopathic Educational Research Fund (JAM).
Reference List
- Ai X, Pogwizd SM. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res. 2005;96:54–63. doi: 10.1161/01.RES.0000152325.07495.5a. [DOI] [PubMed] [Google Scholar]
- Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H1223–H1230. doi: 10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- Ando M, Katare RG, Kakinuma Y, Zhang D, Yamasaki F, Muramoto K, Sato T. Efferent vagal nerve stimulation protects heart against ischemia-induced arrhythmias by preserving connexin43 protein. Circulation. 2005;112:164–170. doi: 10.1161/CIRCULATIONAHA.104.525493. [DOI] [PubMed] [Google Scholar]
- Bao B, Jiang J, Yanase T, Nishi Y, Morgan JR. Connexon-mediated cell adhesion drives microtissue self-assembly. FASEB J. 2011;25:255–264. doi: 10.1096/fj.10-155291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardslee MA, Laing JG, Beyer EC, Saffitz JE. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- Bellafiore M, Sivverini G, Palumbo D, Macaluso F, Bianco A, Palma A, Farina F. Increased cx43 and angiogenesis in exercised mouse hearts. Int J Sports Med. 2007;28:749–755. doi: 10.1055/s-2007-964899. [DOI] [PubMed] [Google Scholar]
- Bernstein SA, Morley GE. Gap junctions and propagation of the cardiac action potential. Adv Cardiol. 2006;42:71–85. doi: 10.1159/000092563. [DOI] [PubMed] [Google Scholar]
- Betsuyaka T, Nnebe NS, Sundset R, Patibandla S, Krueger CM, Yamada KA. Overexpression of cardiac connexin45 increases susceptibility to ventricular tachyarrhythmias in vivo. Am J Physiol Heart Circ Physiol. 2006;290:163–171. doi: 10.1152/ajpheart.01308.2004. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, Van Cauter E, Berntson GG. Loneliness and health: potential mechanisms. Psychosom Med. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Carnevali L, Trombini M, Rossi S, Graiani G, Manghi M, Koolhaas JM, Quaini F, Macchi E, Nalivaiko E, Sgoifo A. Structural and electrical myocardial remodeling in a rodent model of depression. Psychosom Med. 2013;75:42–51. doi: 10.1097/PSY.0b013e318276cb0d. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54:241–247. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Keverne EB. The neurobiology of social affiliation and pair bonding. Horm Brain Behav. 2002;1:299–337. [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- de Jonge P, Rosmalen JGM, Kema IP, Doornbos B, van Melle JP, Pouwer F, Kupper N. Psychophysiological biomarkers explaining the association between depression and prognosis in coronary artery patients: A critical review of the literature. Neurosci Biobehav Rev. 2010;35:84–90. doi: 10.1016/j.neubiorev.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Eng PM, Rimm EB, Fitzmaurice G, Kawachi I. Social ties and change in social ties in relation to subsequent total and cause-specific mortality and coronary heart disease incidence in men. Am J Epidemiol. 2002;155:700–709. doi: 10.1093/aje/155.8.700. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F. Depression and other psychological risks following myocardial infarction. Arch Gen Psychiatry. 2003;60:627–636. doi: 10.1001/archpsyc.60.6.627. [DOI] [PubMed] [Google Scholar]
- Freedland KE, Rich MW, Skala JA, Carney RM, Dávila-Román VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med. 2003;65:119–128. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- Glassman AH. Depression and cardiovascular comorbidity. Dialogues Clin Neurosci. 2007;9:9–17. doi: 10.31887/DCNS.2007.9.1/ahglassman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grikscheit K, Thomas N, Bruce AF, Rothery S, Chan J, Severs NJ, Dupont E. Coexpression of connexin 45 with connexin 43 decreases gap junction size. Cell Commun Adhes. 2008;15:185–193. doi: 10.1080/15419060802013943. [DOI] [PubMed] [Google Scholar]
- Grippo AJ. The utility of animal models in understanding links between psychosocial processes and cardiovascular health. Soc Personal Psychol Compass. 2011;5(4):164–179. doi: 10.1111/j.1751-9004.2011.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007a;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Cardiac regulation in the socially monogamous prairie vole. Physiol Behav. 2007b;90:386–393. doi: 10.1016/j.physbeh.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol Psychiatry. 2007c;62:1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Sgoifo A, Jepson AJ, Bates SL, Chandler DL, McNeal N, Preihs K. The integration of depressive behaviors and cardiac dysfunction during an operational measure of depression: investigating the role of negative social experiences in an animal model. Psychosom Med. 2012;74:612–619. doi: 10.1097/PSY.0b013e31825ca8e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Santos CM, Johnson RF, Beltz TG, Martins JB, Felder RB, Johnson AK. Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. Am J Physiol Heart Circ Physiol. 2004;286:H619–H626. doi: 10.1152/ajpheart.00450.2003. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Sgoifo A, Mastorci F, McNeal N, Trahanas DM. Cardiac dysfunction and hypothalamic activation during a social crowding stressor in prairie voles. Autonom Neurosci Basic Clin. 2010;156:44–50. doi: 10.1016/j.autneu.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 2008;25:E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaga I. Pathological remodeling of cardiac gap junction connexin 43-with special reference to arrhythmogenesis. Pathophysiology. 2009;17:73–81. doi: 10.1016/j.pathophys.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Jiang H, Hu X, Lu Z, Wen H, Zhao D, Tang Q, Yang B. Effects of sympathetic nerve stimulation on ischemia-induced ventricular arrhythmias by modulating connexin43 in rats. Arch Med Res. 2008;39:647–654. doi: 10.1016/j.arcmed.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Grippo AJ. Sadness and broken hearts: neurohumoral mechanisms and co-morbidity of ischemic heart disease and psychological depression. J Physiol Pharmacol. 2006;57(Suppl 11):5–29. [PubMed] [Google Scholar]
- Kanter HL, Saffitz JE, Beyer EC. Cardiac myocytes express multiple gap junction proteins. Circ Res. 1992;70:438–444. doi: 10.1161/01.res.70.2.438. [DOI] [PubMed] [Google Scholar]
- Kaplan GA, Salonen JT, Cohen RD, Brand RJ, Syme SL, Puska P. Social connections and mortality from all causes and from cardiovascular disease: prospective evidence from eastern Finland. Am J Epidemiol. 1988;128:370–380. doi: 10.1093/oxfordjournals.aje.a114977. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin J-P, Hantsoo L. Close relationships, inflammation, and health. Neurosci Biobehav Rev. 2010;35:33–38. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kop WJ, Berman DS, Gransar H, Wong ND, Miranda-Peats R, White MD, Shin M, Bruce M, Krantz DS, Rozanski A. Social network and coronary artery calcification in asymptomatic individuals. Psychosom Med. 2005;37:343–352. doi: 10.1097/01.psy.0000161201.45643.8d. [DOI] [PubMed] [Google Scholar]
- Lecanda F, Towler DA, Ziambaras K, Cheng SL, Koval M, Steinberg TH, Civitelli R. Gap junctional communication modulates gene expression in osteoblastic cells. Mol Biol Cell. 1998;9:2249–2258. doi: 10.1091/mbc.9.8.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner DL, Yamada KA, Schuessler RB, Saffitz JE. Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in Cx43-deficient mice. Circulation. 2000;101:547–552. doi: 10.1161/01.cir.101.5.547. [DOI] [PubMed] [Google Scholar]
- Li DQ, Li XJ, Duan JF, Cai W. Wuling Capsule promotes hippocampal neurogenesis by improving expression of connexin 43 in rats exposed to chronic unpredictable mild stress. J Chinese Integr Med. 2010;8:662–669. doi: 10.3736/jcim20100710. [DOI] [PubMed] [Google Scholar]
- Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lespérance F, Mark DB, Sheps D, Taylor CB, Froelicher ES. Depression and coronary heart disease: recommendations for screening, referral, and treatment. Circulation. 2008;118:1–8. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- Mayama T, Matsumura K, Lin H, Ogawa K, Imanaga I. Remodelling of cardiac gap junction connexin 43 and arrhythmogenesis. Exp Clin Cardiol. 2007;12:67–76. [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Scotti ML, Wardwell J, Chandler DL, Bates SL, LaRocca MA, Trahanas DM, Grippo AJ. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Autonom Neurosci Basic Clin. 2014;180:9–16. doi: 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt JA, Henry MK, Welliver KC, Jepson AJ, Garnett ER. Hindlimb unloading results in increased predisposition to cardiac arrhythmias and alters left ventricular connexin 43 expression. Am J Physiol Regul Integr Comp Physiol. 2013;304:R362–R373. doi: 10.1152/ajpregu.00391.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BWJH, Beekman ATF, Honig A, Deeg DJH, Schoevers RA, van Eijk JTM, van Tilburg W. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- Peuler JD, Scotti ML, Phelps LE, McNeal N, Grippo AJ. Chronic social isolation in the prairie vole induces endothelial dysfunction: implications for depression and cardiovascular disease. Physiol Behav. 2012;106:476–484. doi: 10.1016/j.physbeh.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay S, Ebrahim S, Whincup P, Papacosta O, Morris R, Lennon L, Wannamethee SG. Social engagement and the risk of cardiovascular disease mortality: results of a prospective population-based study of older men. Ann Epidemiol. 2008;18:476–483. doi: 10.1016/j.annepidem.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Linke SE, Olson MB, Francis J, Johnson BD, Bittner V, York K, McClure C, Kelsey SF, Reis SE, Cornell CE, Vaccarino V, Sheps DS, Shaw LJ, Krantz DS, Parashar S, Merz CN. Social networks and incident stroke among women with suspected myocardial ischemia. Psychosom Med. 2008;70:282–287. doi: 10.1097/PSY.0b013e3181656e09. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Reis SE, Olson M, Owens J, Kelsey SF, Pepine CJ, Mankad S, Rogers WJ, Bairey Merz CN, Sopko G, Cornell CE, Sharaf B, Matthews KA. Social networks are associated with lower mortality rates among women with suspected coronary disease: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation study. Psychosom Med. 2004;66:882–888. doi: 10.1097/01.psy.0000145819.94041.52. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, De Boer SF, Buwalda B, Korte-Bouws G, Tuma J, Bohus B, Zaagsma J, Koolhaas JM. Vulnerability to arrhythmias during social stress in rats with different sympathovagal balance. Am J Physiol Heart Circ Physiol. 1998;275:H460–H466. doi: 10.1152/ajpheart.1998.275.2.H460. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci. 2013;110:5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J-D, Liu Y, Yuan Y-H, Li J, Chen N-H. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology. 2012;37:1305–1320. doi: 10.1038/npp.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu T. Arrhythmogenic substrates in myocardial infarct. Pathol Int. 2008;58:533–543. doi: 10.1111/j.1440-1827.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- Unuma K, Shintani-Ishida K, Tsushima K, Shimosawa T, Ueyama T, Kuwahara M, Yoshida K. Connexin-43 redistribution and gap junction activation during forced restraint protects against sudden arrhythmic death in rats. Circ J. 2010;74:1087–1095. doi: 10.1253/circj.cj-09-1019. [DOI] [PubMed] [Google Scholar]
- Verheule S, Kaese S. Connexin diversity in the heart: insights from transgenetic mouse models. Front Pharmacol. 2013;4:81-1–81-14. doi: 10.3389/fphar.2013.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier RL, Antzelevich C. Autonomic aspects of arrhythmogenesis: the enduring and the new. Curr Opin Cardiol. 2004;19:2–11. doi: 10.1097/00001573-200401000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Xia Y, Gong KZ, Xu M, Zhang YY, Guo JH, Song Y, Zhang P. Regulation of gap-junction protein connexin 43 by beta-adrenergic receptor stimulation in rat cardiomyocytes. Acta Pharmacol Sin. 2009;30:928–934. doi: 10.1038/aps.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KA, Kanter EM, Green KG, Saffitz JE. Transmural distribution of connexins in rodent hearts. J Cardiovasc Electrophysiol. 2004;15:710–715. doi: 10.1046/j.1540-8167.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Rogers JG, Sundset R, Steinberg TH, Saffitz J. Up-regulation of connexin45 in heart failure. J Cardiovasc Electrophysiol. 2003;14:1205–1212. doi: 10.1046/j.1540-8167.2003.03276.x. [DOI] [PubMed] [Google Scholar]
- Yue P, Zhang Y, Du Z, Xiao J, Pan Z, Wang N, Yu H, Ma W, Qin H, Wang WH, Lin DH, Yang B. Ischemia impairs the association between connexin 43 and M3 subtype of acetylcholine muscarinic receptor (M3-mAChR) in ventricular myocytes. Cell Physiol Biochem. 2006;17:129–136. doi: 10.1159/000092074. [DOI] [PubMed] [Google Scholar]
- Zhao J, Su Y, Zhang Y, Pan Z, Yang L, Chen X, Liu Y, Lu Y, Du Z, Yang B. Activation of cardiac muscarinic M3 receptors induces delayed cardioprotection by preserving phosphorylated connexin43 and up-regulating cyclooxygenase-2 expression. Br J Pharmacol. 2010;159:1217–1225. doi: 10.1111/j.1476-5381.2009.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipes DP. Heart-brain interactions in cardiac arrhythmias: role of the autonomic nervous system. Cleve Clin J Med. 2008;75(Suppl 2):S94–S96. doi: 10.3949/ccjm.75.suppl_2.s94. [DOI] [PubMed] [Google Scholar]