Abstract
Recent studies show the importance of mitochondrial dysfunction in the initiation and progression of acute kidney injury (AKI). However, no biomarkers exist linking renal injury to mitochondrial function and integrity. To this end, we evaluated urinary mitochondrial DNA (UmtDNA) as a biomarker of renal injury and function in humans with AKI following cardiac surgery. mtDNA was isolated from the urine of patients following cardiac surgery and quantified by qPCR. Patients were stratified into no AKI, stable AKI and progressive AKI groups based on Acute Kidney Injury Network (AKIN) staging. UmtDNA was elevated in progressive AKI patients, and was associated with progression of patients with AKI at collection to higher AKIN stages. To evaluate the relationship of UmtDNA to measures of renal mitochondrial integrity in AKI, mice were subjected to sham surgery or varying degrees of ischemia followed by 24 hours of reperfusion. UmtDNA increased in mice after 10-15 minutes of ischemia and positively correlated with ischemia time. Furthermore, UmtDNA was predictive of AKI in the mouse model. Finally, UmtDNA levels were negatively correlated with renal cortical mtDNA and mitochondrial gene expression. These translational studies demonstrate that UmtDNA is associated with recovery from AKI following cardiac surgery by serving as an indicator of mitochondrial integrity. Thus, UmtDNA may serve as valuable biomarker for the development of mitochondrial targeted therapies in AKI.
Keywords: acute kidney injury, ischemia reperfusion, mitochondria
INTRODUCTION
Acute kidney injury (AKI) is a serious clinical problem, particularly among surgical and critically ill patients1, 2. Despite advances in diagnosis and patient management, incidence of AKI continues to increase and outcomes continue to be poor. An expanding array of risk factors for AKI and expanded use of nephrotoxic drugs have contributed to its increased incidence and prevalence. AKI is associated with high levels of short and long term mortality, and increases patient risk of progression to chronic kidney disease (CKD) and end stage renal disease (ESRD)3-5. Survivors of AKI, particularly those with ESRD requiring dialysis, are plagued by a variety of chronic sequelae contributing to decreased quality of life and increased utilization of healthcare resources.
Cardiac surgery requiring cardiopulmonary bypass (CPB) is a common cause of AKI in humans. Estimates of incidence of AKI in patients following cardiac surgery are as high as 30%6, 7. Furthermore, AKI is one of the most significant negative predictors of patient outcome in this population8, 9. Despite the high incidence and severity of CPB-induced AKI, methods for early detection following surgery remain poor. Additionally, there are no effective therapeutic strategies to prevent or promote recovery from AKI in these patients10-12. These facts highlight the need to better understand mechanisms of CPB-induced AKI and to develop novel diagnostic tools and therapies.
Renal injury following CPB is multifactorial and poorly understood; however, ischemia-reperfusion (I/R) injury is believed to play a significant role13, 14. Poor renal perfusion due to reduced cardiac output, increased systemic inflammation, hemodynamic alterations, volume depletion and perioperative administration of nephrotoxic drugs, contribute to I/R-induced renal injury following cardiac surgery15, 16. Mitochondrial damage and dysfunction is a key pathophysiological component of renal tubular injury during both the initiation and recovery phases of ischemic AKI17, 18. Organ reperfusion following ischemia leads to rapid opening of the mitochondrial permeability transition pore (MPTP) causing mitochondrial membrane depolarization, increased production of reactive oxygen and nitrogen species, and release of apoptotic proteins. Oxidative damage of mitochondrial respiratory complexes and subsequent reduction in mitochondrial function contributes to tissue ATP depletion inhibiting energy-dependent cellular repair mechanisms19. Additionally, generation of new, functional mitochondria through the process of mitochondrial biogenesis (MB) is persistently suppressed following renal injury in mice20, 21.
These reports highlight mitochondria as an intriguing diagnostic and therapeutic target for AKI in animals; however, correlative data in humans is limited due to limited availability of renal tissue for analysis. Current assays for mitochondrial function including tissue ATP and oxygen consumption measurements require invasive tissue biopsies22. Due to the profound role of mitochondria in tissue repair, biomarkers of mitochondrial dysfunction following CPB may serve as better prognostic measures of AKI progression and recovery compared to existing biomarkers, enabling modification of patient management and development of new mitochondrial-targeted therapies for AKI. To this end, we conducted proof of concept experiments that examine the efficacy of urinary mitochondrial DNA (UmtDNA) as a predictive biomarker of AKI development and progression in humans with CPB-induced AKI. Furthermore, we validated UmtDNA as a biomarker of renal mitochondrial integrity in a mouse model of renal I/R injury. Fragments of mtDNA, referred to as mtDNA damage associated molecular patterns (DAMPs), are released into circulation following injury23, 24. Released mtDNA can modulate oxidative and inflammatory injury at distant sites through activation of Toll-like receptor signaling25. While numerous studies have evaluated serum and plasma levels of mtDNA as predictors of injury and disease progression, no reports exist of the evaluation of UmtDNA as a specific marker of renal damage/dysfunction.
RESULTS
UmtDNA is associated with AKI progression following cardiac surgery
To assess UmtDNA as a biomarker of renal dysfunction in humans, UmtDNA levels were measured by qPCR in urine collected as a component of an NIDDK-funded multicenter trial (DK080234) to determine prognostic biomarkers of AKI following cardiac surgery26. Samples were collected through the Southeastern Acute Kidney Injury Network (SAKInet) consortium. The patient cohort included patients who developed AKI and those who did not develop AKI following surgery. Patients were enrolled before surgery for baseline measurements and urine was collected approximately 1 d following surgery (mean collection time = 1.26 d, median collection time = 1 d). Table 1 contains the demographic and clinical characteristics of the subjects. Renal function was evaluated at collection and during follow-up by serum creatinine, and patients were staged using the Acute Kidney Injury Network (AKIN) criteria.
Table 1.
Patient demographics and clinical characteristics of cardiac surgery patients.
| AKI Status |
||||
|---|---|---|---|---|
| Patient demographics | No AKI | Stable AKI | Progressive AKI | P-value |
| Total patients | 17 | 63 | 31 | |
| Female | 3 (18 %) | 13 (22 %) | 15 (48 %) | 0.02 |
| Black | 3 (18 %) | 16 (27 %) | 6 (19 %) | 0.59 |
| Age (years) | 65 ± 16 | 66 ± 11 | 68 ± 12 | 0.66 |
| Weight (kg) | 81 ± 28 | 93 ± 20 | 85 ± 28 | 0.11 |
| History | ||||
| CHF | 6 (36 %) | 14 (24 %) | 11 (35 %) | 0.42 |
| Prev. cardiac surgery | 2 (12 %) | 9 (15 %) | 10 (32 %) | 0.10 |
| Diabetes mellitus | 8 (47 %) | 20 (34 %) | 14 (45 %) | 0.45 |
| COPD | 2 (12 %) | 3 (5 %) | 4 (13 %) | 0.39 |
| Peripheral vascular disease | 0 (0 %) | 6 (10 %) | 1 (3 %) | 0.22 |
| Stroke | 0 (0 %) | 4 (7 %) | 2 (6 %) | 0.55 |
| Surgical parameters | ||||
| CABG | 16 (94 %) | 42 (71 %) | 23 (74 %) | 0.15 |
| Valve replacement | 4 (24 %) | 22 (37 %) | 15 (48 %) | 0.23 |
| Bypass | 14 (82 %) | 46 (78 %) | 27 (87 %) | 0.57 |
| Bypass Time | 117 ± 49 | 139 ± 65 | 150 ± 90 | 0.31 |
| Outcomes | ||||
| Baseline creatinine | 1.3 ± 0.4 | 1.2 ± 0.3 | 1.3 ± 0.5 | 0.41 |
| Collection creatinine | 1.4 ± 0.4 | 2.0 ± 0.7 | 1.8 ± 0.8 | 0.0083 |
| Max creatinine | 1.4 ± 0.4 | 2.1 ± 0.8 | 2.9 ± 1.5 | <0.0001 |
| RRT | 1 (6 %) | 1 (2 %) | 5 (16 %) | 0.031 |
| Mortality | 1 (6 %) | 1 (2 %) | 7 (23 %) | 0.0029 |
| Days to discharge | 6.8 ± 2.6 | 9.3 ± 8.6 | 15.1 ± 11.4 | 0.0031 |
| Days to max creatinine | 5.6 ± 4.5 | 2.3 ± 1.7 | 4.1 ± 2.6 | <0.0001 |
No AKI (AKIN 0 throughout follow-up), Stable AKI (AKIN 1+ at collection, collection AKIN = maximum AKIN), Progressive AKI (AKIN 1+ at collection, maximum AKIN > collection AKIN). CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CABG, coronary artery bypass graft; RRT, renal replacement therapy. P-values were determined using the Chi-Square test or ANOVA, as appropriate.
The urine mtDNA/nDNA ratio was not elevated in patients with AKI (AKIN1+time of collection) (median=147, IQR 102-610) vs. no AKI (AKIN 0) (median=202, IQR 59-604), and no differences were observed in UmtDNA levels between healthy or any AKIN stage patients based on either AKIN at collection or maximum AKIN stage achieved (Suppl. 1).
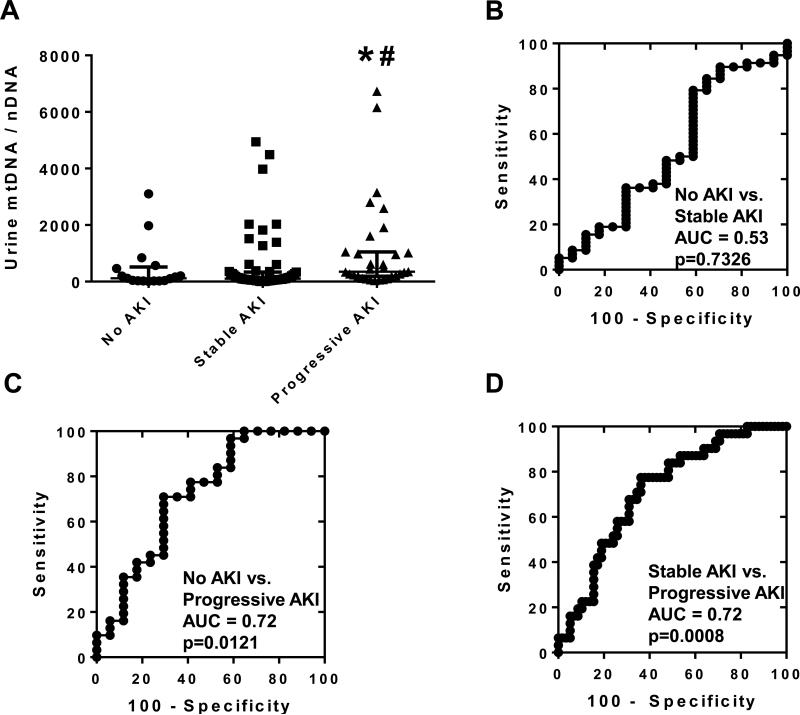
To assess the efficacy of UmtDNA in predicting worsening of AKI, patients were classified into 3 groups based on disease progression: no AKI (AKIN 0 throughout follow-up), stable AKI (AKIN 1+ at collection, maximum AKIN = collection AKIN), or progressive AKI (AKIN 1+ at collection, maximum AKIN > collection AKIN). No patients with AKIN 0 at collection that subsequently developed AKI were observed in the cohort. UmtDNA was significantly elevated in patients with progressive AKI (median = 353, IQR 187 - 1053) vs. no AKI (median = 118, IQR 26 – 517), or stable AKI (median = 104, IQR 50 – 340) (Fig. 1A).
Figure 1. Urinary mtDNA copy number is associated with progression of renal dysfunction from collection in patients with cardiopulmonary bypass (CBP)-induced-AKI.
Urine was collected from patients following CBP. Urinary mtDNA levels were measured via real time qPCR for ND1, and corrected to the nuclear control gene β-actin. Patients were stratified into 3 groups based on collection and maximum AKIN staging: no AKI (n=17), stable AKI (n=63) and progressive AKI (n=31) (A). Data are expressed as median +/− IQR. Multiple comparisons were performed using a Kruskal-Wallis test followed by Dunn's test. *p<0.05 vs. No AKI, #p<0.05 vs. stable AKI. AUROC analysis was performed comparing the 3 groups of patients (B-D).
ROC curve analysis was performed to assess UmtDNA as a predictive test for AKI progression. There was no difference between no AKI and stable AKI patients (AUC=0.53, p=0.73) (Fig. 1B). Elevated UmtDNA was a significant predictor of progressive AKI compared to no AKI (AUC=0.72, p=0.012) (Fig. 1C) and stable AKI patients (AUC=0.72, p=0.0008) (Fig. 1D). Evaluation of the primary endpoint of AKI progression in patients with established AKI at collection (stable vs. progressive AKI), by minimizing the distance to the point for sensitivity and specificity of 100%, revealed an optimal cutoff at 185 at which the test had a sensitivity and specificity of 77% and 64%, respectively. A cutoff of 2310 yielded the highest positive likelihood ratio (LR+ = 3.1). Seven of ninety-four AKI patients had urinary mtDNA/nDNA ratios greater than 2310, of which four met the outcome of progressive AKI. The sensitivity and specificity of the test using this cutoff was 16% and 95%, respectively. The lowest negative likelihood ratio was achieved at a cutoff of 58 (LR− = 0.11). Nineteen patients had urinary mtDNA/nDNA ratios less than 58, of which eighteen did not meet the outcome of progressive AKI. Using this cutoff, the test had a sensitivity and specificity of 97% and 30%, respectively.
UmtDNA does not correlate with existing markers of renal damage and dysfunction following cardiac surgery
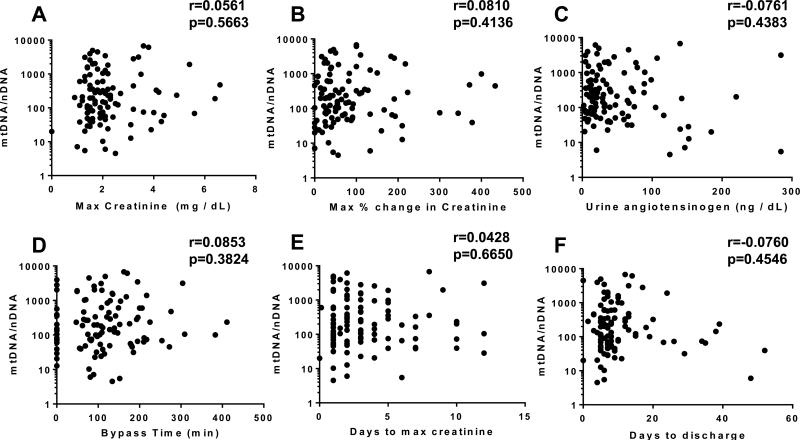
To evaluate the performance of UmtDNA as an indicator of renal function, we compared UmtDNA with serum creatinine in the cohort. Correlation analysis of UmtDNA revealed no correlation with maximum serum creatinine (Fig. 2A) (r=0.0733, p=0.4533), or maximum % change in serum creatinine from baseline (Fig. 2B) (r=−0.0013, r=0.9894). Due to the limited sensitivity of serum creatinine as a marker of renal injury, we also compared UmtDNA levels to levels of urinary angiotensinogen, a marker demonstrated to perform as well as urinary KIM-1 and NGAL as an indicator of renal damage and progression 26, 27. UmtDNA was not correlated with urinary angiotensinogen levels following CPB (Fig. 2C) (r=0.0681, p=0.4877). Furthermore, UmtDNA did not correlate with bypass time (Fig. 2D) (r=0.0370, p=0.7053), days to maximum creatinine (Fig. 2E) (r=0.0190, p=0.8467), or days to discharge (Fig. 2F) (r=0.0760, p=0.4546).
Figure 2. Urinary mtDNA copy number is not correlated with degree of CBP-induced AKI in humans.
Urinary mtDNA/nDNA was not correlated with maximum serum creatinine (A), maximum % change in serum creatinine from baseline (B), urinary angiotensinogen levels (C) and cardiopulmonary bypass time (D) (n=111 for all analyses). Linear regression was performed and Spearman's Rank order coefficients were calculated.
UmtDNA is elevated in mice after I/R-induced AKI and correlates with ischemia time
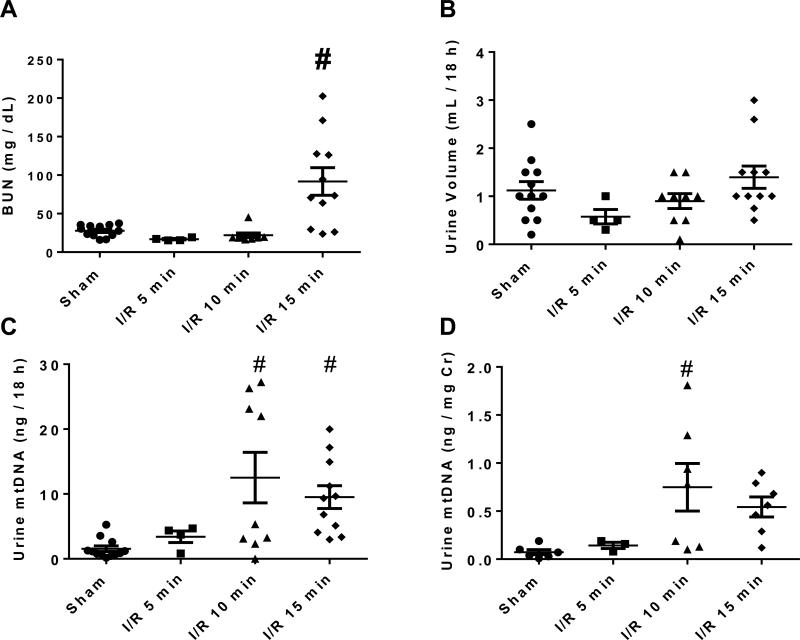
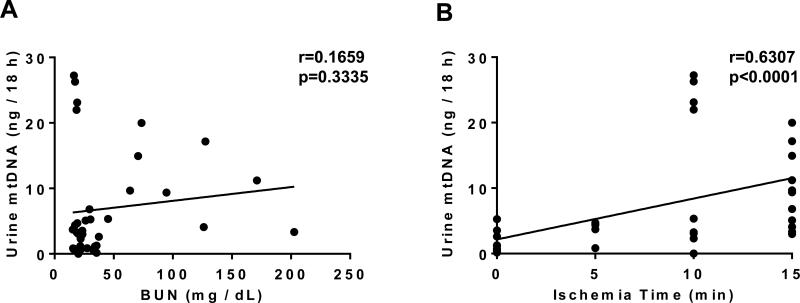
To explore the biological correlates of UmtDNA in the kidney, we used a mouse model of renal I/R injury. Mice were subjected to sham surgery (n=12) or bilateral renal pedicle ligation for 5 (n=4), 10 (n=9) or 15 min (n=11) corresponding to mild, moderate and severe renal histological damage28. Mice that produced no or insufficient urine for detection of UmtDNA (n=6) were excluded from further analyses. BUN was significantly elevated at 24 h only in the 15 min I/R group, and no difference was observed in urine output between all groups (Fig. 3A, B). Total output of UmtDNA over the course of an 18 h collection period increased significantly in the 10 or 15 min I/R groups (Fig. 3C). UmtDNA was not normalized to nDNA due to undetectable levels of urinary β-actin in mice. Normalization of UmtDNA to urine creatinine demonstrated an increase vs. sham only in the 10 min I/R group (Fig. 3D). UmtDNA correlated with time of ischemia (r=0.63, p<0.0001); however it did not correlate with renal function assessed by BUN (Fig. 4A, B) (r=0.17, p=0.33). Correction of UmtDNA levels for urine creatinine did not alter/improve these correlations (Suppl. 2).
Figure 3. Urinary mtDNA levels are increased in mice following renal I/R.
Male C57BL/6 mice underwent sham surgery or ischemia (5, 10 or 15 min) followed by reperfusion. Mice were placed in metabolic cages from 6-24 h after reperfusion for urine collection. Blood was collected and mice were euthanized 24 h after reperfusion. Renal function was evaluated by BUN (A) and 18 h urine output (B). Urinary mtDNA levels were measured by real time PCR for ND1 and are expressed as total urinary output in 18 h (C) and urinary output corrected for urinary creatinine levels (D). Data are expressed are mean +/− SEM. # indicates statistical significance at p<0.05 vs. sham controls; sham (n=12), 5 min I/R (n=4), 10 min I/R (n=9), 15 min I/R (n=11).
Figure 4. Total urinary output of mtDNA is correlated with degree of ischemia, but not BUN.
Urinary mtDNA levels expressed as total urinary output per 18 h were correlated with BUN levels (A) or ischemia time (B) (n=36). Linear regression was performed and Spearman's Rank order coefficients were calculated.
UmtDNA is a predictor of AKI development in mice after I/R
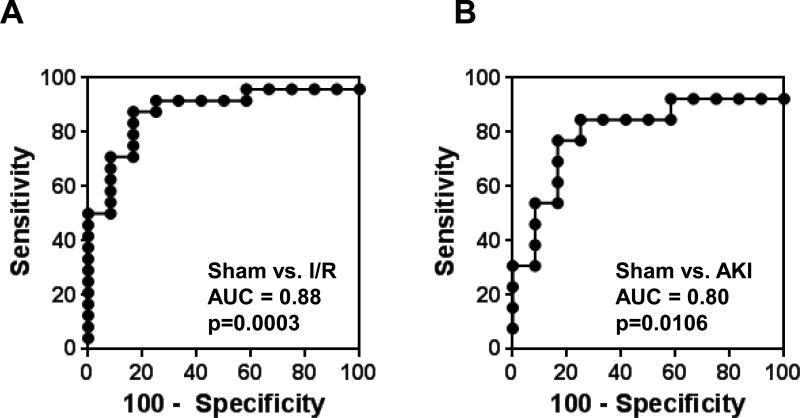
ROC curve analysis was performed to assess the ability of UmtDNA to predict AKI development in mice following renal I/R. UmtDNA was significantly predictive of AKI development in mice undergoing any degree of ischemia vs. sham control mice (AUC=0.88, p=0.0003) (Fig. 5A). An optimal cutoff of 2.8 ng mtDNA/18 h yielded a sensitivity and specificity of 88% and 83%, respectively.
Figure 5. Total 18 h urinary output of mtDNA is a biomarker of renal injury following I/R in mice.
ROC curves were constructed for total 18 h urinary output of mtDNA comparing sham surgical mice (n=12) to all mice that underwent I/R (n=24) (A) or only mice with BUN >2 SD above the historical sham average BUN (n=13) (B).
Several mice with no increase in BUN were observed in the renal I/R group; therefore, mice were reclassified into no AKI and AKI groups based on an increase of >2 SD above our historical sham BUN level (BUN cutoff ≥ 43.4 mg/dL). Mice with BUN levels below this cutoff were excluded. ROC curve analysis demonstrated predictive power for AKI vs. no AKI using UmtDNA in mice after renal I/R (AUC=0.80, p=0.011) (Fig. 5B). The optimal cutoff for this analysis was determined to be 2.8 ng/18 h with a sensitivity and specificity of 77% and 83%, respectively. No differences were observed when analyses were performed corrected for urine creatinine (Suppl. 2).
Renal mitochondrial gene expression and mtDNA copy number are negatively correlated with UmtDNA levels
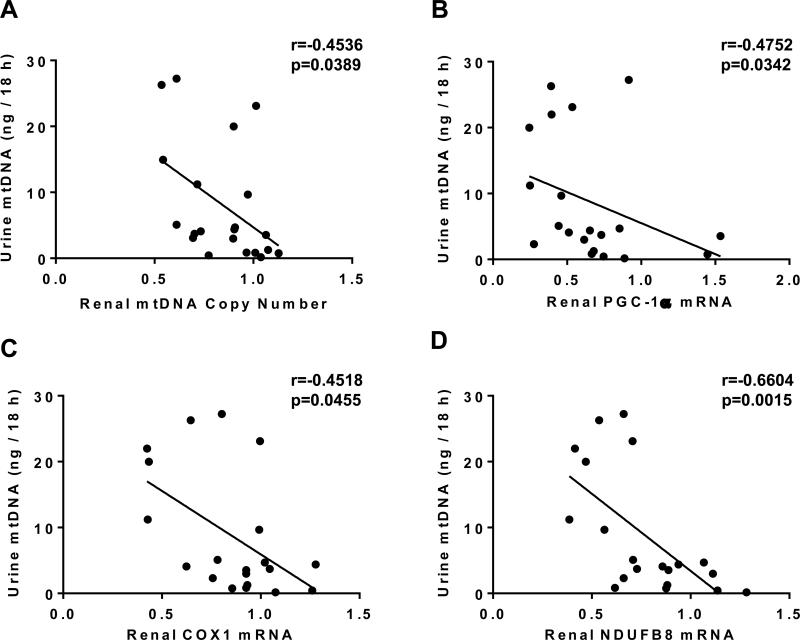
Renal cortical mtDNA copy number and mRNA expression of PGC-1α, COX1 and NDUFB8 were assessed via qPCR and correlated to 18 h urinary output of mtDNA. Renal cortical mtDNA copy number decreased following I/R, and was negatively correlated with UmtDNA output (r=−0.45, p=0.039) (Fig. 6A). Additionally, mRNA expression of PGC-1α, COX1 and NDUFB8 decreased after I/R and their expression was also negatively correlated with UmtDNA output (PGC-1α: r=−0.48, p=0.034; COX1: r=−0.45, p=0.046; NDUFB8: r=−0.66, p=0.0015) (Fig. 6B-D). Analysis performed using UmtDNA corrected for urine creatinine produced modest improvements in these correlations (Suppl. 3).
Figure 6. Total 18 h urinary output of mtDNA correlates with reductions in renal mtDNA copy number and mitochondrial gene expression.
Urinary mtDNA levels expressed as total urinary output per 18 h were correlated with relative renal mtDNA copy number (A), PGC1-α (B), COX1 (C) and NDUFB8 (n=36). Renal mtDNA and mRNA expression were corrected to the nuclear gene β-actin. Linear regression was performed and Pearson correlation coefficients were calculated.
DISCUSSION
Complete recovery from renal injury is dependent on rapid and sustained activation of tissue repair processes, particularly of the renal proximal tubule19, 29, 30. Following injury, coordinated tissue repair responses are activated promoting recovery of sub-lethally injured cells, clearance of necrotic cells and debris, and re-establishment of an intact, polarized renal epithelium. Repair of the renal tubular epithelium is highly energy dependent, thus mitochondrial function is crucial to the structural and functional recovery of the kidney. Due to the significant role of mitochondria in renal repair and recovery, biomarkers of renal mitochondrial function and integrity may be valuable markers of AKI progression; however, practical limitations of existing assays of mitochondrial function have limited our ability to study the link between mitochondrial dysfunction and renal injury in humans17, 19-21, 31-33.
We examined UmtDNA copy number as marker of renal injury in a cohort of patients who developed AKI following cardiac surgery. We found that UmtDNA was associated with increased risk of worsening AKI following initial sample collection, and that UmtDNA predicts AKI progression by ROC analysis (Fig. 1). AKI progression was based on an increase in AKIN stage as determined by serum creatinine from collection baseline. As serum creatinine is a poor early indicator of renal damage/dysfunction, it is possible that some patients classified as AKIN 0-1 at collection had more severe renal damage, potentially affecting the predictive value of UmtDNA for AKI progression in this cohort. However, stratification of patients by AKIN stage at collection or maximum AKIN stage revealed no differences in UmtDNA between any groups suggesting that UmtDNA is not associated with degree of renal damage. These data provide evidence of differences in recovery between these groups rather than initial injury; however, practical limitations of serum creatinine and small sample size temper these conclusions. Larger studies are needed in patients with AKI of various degrees and multiple etiologies in order to validate the predictive power of UmtDNA. In this study we determined LR+ and LR− values as maximum and minimum cutoff values to stratify patients into low and high risk groups. Analysis of our data set using the cutoffs demonstrated that risk of AKI progression could be evaluated with reasonable confidence. These cutoffs value could be used for patient screening for enrollment into future larger scale studies with AKI progression as an endpoint.
Traditional markers of renal dysfunction have limited to no ability to predict progression of disease following CBP-induced AKI34. These limitations have led to the development of new renal injury markers with enhanced predictive power35, 36. However, the prognostic value of newer markers, including KIM-1 and NGAL, is still relatively weak37, 38. A recent study by Arthur et al. examined the ability of a panel of renal biomarkers including KIM-1, NGAL, L-FABP and IL-18 to predict the progression of AKI following cardiac surgery using a different subset of SAKInet patients than used in our study39. IL-18 was determined to be the best predictor of AKI progression (AKIN 1 to AKIN 2/3 or death) with an AUC of 0.74. While our definition of AKI progression differed slightly from that of this study, the predictive performance of UmtDNA compared favorably (AUC = 0.72, stable vs. progressive AKI).
Interestingly, in contrast to many of the markers assessed in the study by Arthur et al., UmtDNA failed to correlate with traditional markers of renal damage and dysfunction (Fig. 2) even acutely. We compared UmtDNA levels to another candidate biomarker of AKI initiation and progression, urinary angiotensinogen. Urinary angiotensinogen has been shown to be a strong predictor of AKI progression in ICU patients26. Furthermore, urinary angiotensinogen measured on day 1 of hospitalization predicted development of AKI in patients with decompensated CHF with high accuracy, and was superior to the performance of urinary NGAL27. Interestingly, UmtDNA was not correlated with urinary angiotensinogen in our cohort. We suggest that UmtDNA is a surrogate marker of renal mitochondrial integrity, representing distinct cellular processes from urinary angiotensinogen and other markers. Due to the role of mitochondrial function and tissue bioenergetics in tissue repair processes, UmtDNA levels may serve as a more direct indicator of the capacity of the kidney for repair and a prognostic indicator of renal functional recovery. The mechanistic relevance of UmtDNA levels in the status of renal mitochondria and renal repair processes warrants further investigation. Furthermore, failure of UmtDNA to correlate with urinary angiotensinogen could be useful in the development of a prognostic biomarker panel, as non-correlated biomarkers are superior for use in combinatorial predictive models39.
As human renal cortical samples from AKI patients are not readily available we chose to explore the physiological link between UmtDNA and renal mitochondrial integrity in a mouse model of renal I/R. Our lab has demonstrated that mitochondrial homeostasis is persistently disrupted following I/R-induced AKI in rodents, and this persistent mitochondrial disruption is associated with an incomplete renal repair process21, 32. UmtDNA levels increased in mice with moderate (10 min ischemia) or severe (15 min ischemia) renal injury (Fig. 3), and correlated with degree of ischemia, but not renal function as assessed by BUN (Fig. 4). These data differ from results from human cardiac surgery patients as no correlation was observed with bypass time. While bypass time is a reasonable indicator of surgical complexity, it is likely a poor surrogate marker of degree of ischemia or renal injury in humans due to heterogeneous patient characteristics and surgical parameters. Of note, when mice without detectable renal injury by BUN were excluded, the predictive power of UmtDNA decreased in our studies. This is likely due to poor performance of BUN as a marker of renal injury resulting in the exclusion of mice with renal injury. UmtDNA levels failed to correlate with BUN indicating that UmtDNA is not an indicator of renal function, but more likely a better indicator of degree of renal damage or progression. Overall, these data demonstrate that mouse I/R provides a reasonable model of the UmtDNA response observed in human patients following CBP, and a platform for examination of renal mitochondrial parameters.
We evaluated mouse renal cortical mtDNA copy number and mRNA expression of PGC-1α, COX1 and NDUFB8 as markers of mitochondrial integrity and biogenesis following renal I/R. Correlation of renal mtDNA copy number and mitochondrial genes with total UmtDNA output demonstrated negative correlations indicating that UmtDNA is reflective of renal mitochondrial disruption following I/R (Fig. 6). Normalization to urine creatinine has been used inconsistently in biomarker evaluations. Correction of biomarkers to urine creatinine concentration has previously demonstrated to improve predictive power40; however, we observed no substantial improvement in predictive power of UmtDNA when corrected for urine creatinine in our studies.
Correlations of UmtDNA with mitochondrial gene expression suggest that the predictive power of UmtDNA for AKI progression may arise from its ability to predict the degree of renal mitochondrial damage and disruption, which could inhibit renal repair processes. However, correlation of suppression of mitochondrial gene expression to elevations of UmtDNA does not causally link mitochondrial integrity to recovery from AKI, and changes in these indicators may not be truly reflective of mitochondrial damage or disruption. Future mechanistic studies are needed to examine how modulation of UmtDNA levels correlates with renal repair and functional recovery after AKI. Nevertheless, many previous animal studies have clearly demonstrated mitochondrial damage and dysfunction as key pathophysiological components of the initiation and recovery from I/R-induced AKI. 28, 41, 42.
Past reports of urinary markers of renal mitochondrial dysfunction are limited to urinary cytochrome c which is elevated following drug-induced AKI43. Cytochrome c is a poor renal biomarker as it is only transiently elevated following injury and thus likely serves as a better predictor of degree of renal damage rather than recovery and disease progression. Our group recently reported that urinary levels of the ATP synthase subunit β serve as a biomarker of CPB-induced AKI and I/R-induced AKI in mice; however, the predictive value in this study was not assessed28.
Our studies provide preliminary evidence of UmtDNA as a novel biomarker reflective of AKI progression linked to renal mitochondrial integrity. The mitochondrial and energy dependence of renal repair processes and renal recovery make UmtDNA a promising tool for exploration of the role of mitochondrial damage and dysfunction in human renal disease and the development of new mitochondrial-targeted therapies.
METHODS
Mouse I/R model
Eight-week-old male C57BL/6 mice (20-25 g) were subjected to sham or I/R surgery by bilateral renal pedicle clamping for 5, 10 or 15 min. as described previously20, 32. Urine was collected for a period of 18 h from 6-24 h post-surgery. Mice were euthanized at 24 h after surgery, and blood and kidneys were collected for analysis. Renal function was evaluated by measurement of urine volume and BUN (Bioassay Systems, Hayward, CA).
Patients and urine samples
Human urine samples were obtained from 111 randomly selected patients who had cardiac surgery at one of the SAKInet institutions, including the Medical University of South Carolina, Duke University and George Washington University, as part of a NIDDK-funded multicenter trial (#DK080234) with the goal of identification of prognostic urine biomarkers of cardiac surgery-induced AKI. Informed consent was obtained in accordance with the institutional review board-approved protocol at each institution. Patients were enrolled prior to undergoing cardiac surgery to determine baseline renal function. Urine was collected approximately 1 d following cardiac surgery (mean = 1.26, median = 1) from both no AKI and AKI patients. Renal function was assessed at urine collection and throughout follow-up by serum creatinine and patients were staged using the Acute Kidney Injury Network (AKIN) staging criteria. Patients were stratified into 3 groups based on changes in AKIN stage to evaluate the prognostic potential of UmtDNA, including No AKI (AKIN 0 throughout follow-up), stable AKI (AKIN 1+ at collection, maximum AKIN = collection AKIN), and progressive AKI (AKIN 1+ at collection, maximum AKIN > collection AKIN). Following collection, protease inhibitors were added to urine samples. Urine was centrifuged at 1,000 × g to remove intact cells and cellular debris, and supernatants were collected and kept frozen at −80°C until analysis. Urine supernatant was used rather than total urine as inclusion of the pellet would lead to the assessment of both soluble mtDNA and mtDNA contained in cells (including lymphocytes and damaged proximal tubular epithelial cells) that have been released into the urine.
Isolation and quantification of urinary mtDNA
Human and mouse urine supernatants were concentrated using Amicon Ultracel-30k centrifugal filters (EMD Millipore, San Diego, CA). DNA was isolated and purified from the concentrate using a Viral RNA Mini Kit (Qiagen, Valencia, CA). Total DNA concentration was determined using the Quant-iT PicoGreen dsDNA reagent (Life Technologies, Carlsbad, CA). qPCR was used for the mitochondrial gene ND1 and the nuclear gene β-actin to determine UmtDNA content with a template input of 0.3-5 ng of total DNA. For human urine, absolute values of mtDNA and nDNA were determined by calculation from a standard curve of total DNA isolated from HK2 cells. For mouse urine, absolute ND1 levels were determined by calculation from a standard curve of total DNA isolated from mouse kidney ranging from 0.1-10 ng. Mouse UmtDNA levels were corrected for urine creatinine (Bioassay Systems, Hayward, CA).
Data and statistical analysis
Data are expressed as means ± SEM for continuous data, or median ± IQR for categorical data. Normality was assessed using the D'Agostino and Pearson omnibus normality test. Multiple comparisons of normally distributed data were analyzed by one-way ANOVA and group means were compared using a Tukey's post-hoc test. Non-parametric analyses were performed using the Kruskal-Wallis test followed by a Dunn's test for multiple comparisons. Single comparisons were analyzed via a Mann-Whitney U-test where appropriate. Receiver operator characteristic (ROC) curve analysis was used to test the predictive ability of UmtDNA for AKI development and progression in mice and humans. Area under the curve (AUC) were computed, and hypothesis tested was performed using a z-test to compare to a baseline AUC of 0.5, indicating no predictive power. Optimal cutoffs were determined by minimizing the distance from the point representing 100% sensitivity and specificity. Maximum positive likelihood ratios and minimum negative likelihood ratios were also determined. Correlation or linear regression analyses were performed on multiple variables and the degree of correlation was determined by calculation of Spearman Rank order coefficients or Pearson correlation coefficients. Data was log transformed before graphing where appropriate. The criterion for statistical significance was p < 0.05 for all comparisons.
Supplementary Material
REFERENCES
- 1.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nature reviews Nephrology. 2014;10:193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 2.Case J, Khan S, Khalid R, et al. Epidemiology of acute kidney injury in the intensive care unit. Critical care research and practice. 2013;2013:479730. doi: 10.1155/2013/479730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney international. 2011;79:1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiffl H. Severity of post-cardiac surgery acute kidney injury and long-term mortality: is chronic kidney disease the missing link? Critical care. 2014;18:424. doi: 10.1186/cc13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu VC, Shiao CC, Chang CH, et al. Long-term outcomes after dialysis-requiring acute kidney injury. BioMed research international. 2014;2014:365186. doi: 10.1155/2014/365186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clinical journal of the American Society of Nephrology : CJASN. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 7.Ryden L, Sartipy U, Evans M, et al. Acute Kidney Injury After Coronary Artery Bypass Grafting and Long-Term Risk of End-Stage Renal Disease. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.114.010622. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Delgado JC, Esteve F, Torrado H, et al. Influence of acute kidney injury on short- and long-term outcomes in patients undergoing cardiac surgery: risk factors and prognostic value of a modified RIFLE classification. Critical care. 2013;17:R293. doi: 10.1186/cc13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machado MN, Nakazone MA, Maia LN. Prognostic value of acute kidney injury after cardiac surgery according to kidney disease: improving global outcomes definition and staging (KDIGO) criteria. PloS one. 2014;9:e98028. doi: 10.1371/journal.pone.0098028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvert S, Shaw A. Perioperative acute kidney injury. Perioperative medicine. 2012;1:6. doi: 10.1186/2047-0525-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellum JA, Lameire N, Group KAGW Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Critical care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akcay A, Turkmen K, Lee D, et al. Update on the diagnosis and management of acute kidney injury. International journal of nephrology and renovascular disease. 2010;3:129–140. doi: 10.2147/IJNRD.S8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munshi R, Hsu C, Himmelfarb J. Advances in understanding ischemic acute kidney injury. BMC medicine. 2011;9:11. doi: 10.1186/1741-7015-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massoudy P, Wagner S, Thielmann M, et al. Coronary artery bypass surgery and acute kidney injury--impact of the off-pump technique. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23:2853–2860. doi: 10.1093/ndt/gfn153. [DOI] [PubMed] [Google Scholar]
- 15.Gude D, Jha R. Acute kidney injury following cardiac surgery. Annals of cardiac anaesthesia. 2012;15:279–286. doi: 10.4103/0971-9784.101874. [DOI] [PubMed] [Google Scholar]
- 16.Mariscalco G, Lorusso R, Dominici C, et al. Acute kidney injury: a relevant complication after cardiac surgery. The Annals of thoracic surgery. 2011;92:1539–1547. doi: 10.1016/j.athoracsur.2011.04.123. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg JM, Venkatachalam MA, Roeser NF, et al. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jassem W, Fuggle SV, Rela M, et al. The role of mitochondria in ischemia/reperfusion injury. Transplantation. 2002;73:493–499. doi: 10.1097/00007890-200202270-00001. [DOI] [PubMed] [Google Scholar]
- 19.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. The Journal of clinical investigation. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jesinkey SR, Funk JA, Stallons LJ, et al. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. Journal of the American Society of Nephrology : JASN. 2014;25:1157–1162. doi: 10.1681/ASN.2013090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stallons LJ, Funk JA, Schnellmann RG. Mitochondrial Homeostasis in Acute Organ Failure. Current pathobiology reports. 2013;1 doi: 10.1007/s40139-013-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfeffer G, Horvath R, Klopstock T, et al. New treatments for mitochondrial disease-no time to drop our standards. Nature reviews Neurology. 2013;9:474–481. doi: 10.1038/nrneurol.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu X, Yao Y, Wu G, et al. The plasma mitochondrial DNA is an independent predictor for post-traumatic systemic inflammatory response syndrome. PloS one. 2013;8:e72834. doi: 10.1371/journal.pone.0072834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinti M, Mussini C, Cossarizza A. Mitochondrial DNA: a proinflammatory 'enemy from within’ during HIV infection? Cell death & disease. 2012;3:307. doi: 10.1038/cddis.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alge JL, Karakala N, Neely BA, et al. Urinary angiotensinogen and risk of severe AKI. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:184–193. doi: 10.2215/CJN.06280612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Chen C, Tian J, et al. Urinary Angiotensinogen Level Predicts AKI in Acute Decompensated Heart Failure: A Prospective, Two-Stage Study. Journal of the American Society of Nephrology : JASN. 2015 doi: 10.1681/ASN.2014040408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitaker RM, Korrapati MC, Stallons LJ, et al. Urinary ATP synthase subunit beta is a novel biomarker of renal mitochondrial dysfunction in acute kidney injury. Toxicological sciences : an official journal of the Society of Toxicology. 2015 doi: 10.1093/toxsci/kfv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu KD, Brakeman PR. Renal repair and recovery. Critical care medicine. 2008;36:S187–192. doi: 10.1097/CCM.0b013e318168ca4a. [DOI] [PubMed] [Google Scholar]
- 30.Guo JK, Cantley LG. Cellular maintenance and repair of the kidney. Annual review of physiology. 2010;72:357–376. doi: 10.1146/annurev.physiol.010908.163245. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg JM, Venkatachalam MA, Roeser NF, et al. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. American journal of physiology Renal physiology. 2000;279:F927–943. doi: 10.1152/ajprenal.2000.279.5.F927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. American journal of physiology Renal physiology. 2012;302:F853–864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall AM, Unwin RJ. The not so ‘mighty chondrion’: emergence of renal diseases due to mitochondrial dysfunction. Nephron Physiology. 2007;105:p1–10. doi: 10.1159/000096860. [DOI] [PubMed] [Google Scholar]
- 34.de Geus HR, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clinical kidney journal. 2012;5:102–108. doi: 10.1093/ckj/sfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devarajan P. Biomarkers for the early detection of acute kidney injury. Current opinion in pediatrics. 2011;23:194–200. doi: 10.1097/MOP.0b013e328343f4dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honore PM, Jacobs R, Joannes-Boyau O, et al. Biomarkers for early diagnosis of AKI in the ICU: ready for prime time use at the bedside? Annals of intensive care. 2012;2:24. doi: 10.1186/2110-5820-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall IE, Coca SG, Perazella MA, et al. Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis. Clinical journal of the American Society of Nephrology : CJASN. 2011;6:2740–2749. doi: 10.2215/CJN.04960511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. Journal of the American Society of Nephrology : JASN. 2012;23:905–914. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arthur JM, Hill EG, Alge JL, et al. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney international. 2014;85:431–438. doi: 10.1038/ki.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ralib AM, Pickering JW, Shaw GM, et al. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. Journal of the American Society of Nephrology : JASN. 2012;23:322–333. doi: 10.1681/ASN.2011040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zsengeller ZK, Ellezian L, Brown D, et al. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2012;60:521–529. doi: 10.1369/0022155412446227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall AM. Maintaining mitochondrial morphology in AKI: looks matter. Journal of the American Society of Nephrology : JASN. 2013;24:1185–1187. doi: 10.1681/ASN.2013050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Small DM, Gobe GC. Cytochrome c: potential as a noninvasive biomarker of drug-induced acute kidney injury. Expert opinion on drug metabolism & toxicology. 2012;8:655–664. doi: 10.1517/17425255.2012.679657. [DOI] [PubMed] [Google Scholar]
- 44.Garrett SM, Whitaker RM, Beeson CC, et al. Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. The Journal of pharmacology and experimental therapeutics. 2014;350:257–264. doi: 10.1124/jpet.114.214700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitaker RM, Wills LP, Stallons LJ, et al. cGMP-selective phosphodiesterase inhibitors stimulate mitochondrial biogenesis and promote recovery from acute kidney injury. The Journal of pharmacology and experimental therapeutics. 2013;347:626–634. doi: 10.1124/jpet.113.208017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jesinkey SR, Korrapati M, Rasbach KA, et al. Atomoxetine prevents dexamethasone-induced skeletal muscle atrophy in mice. The Journal of pharmacology and experimental therapeutics. 2014 doi: 10.1124/jpet.114.217380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.