Abstract
OBJECTIVE
To determine the accuracy of changes in transvaginal sonographic cervical length over time in predicting preterm birth in women with singleton and twin gestations.
DATA SOURCES
PubMed, Embase, Cinahl, Lilacs, and Medion (all from inception to May 31, 2015), bibliographies, Google scholar, and conference proceedings.
STUDY ELIGIBILITY CRITERIA
Cohort or cross-sectional studies reporting on the predictive accuracy for preterm birth of changes in cervical length over time.
STUDY APPRAISAL AND SYNTHESIS METHODS
Two reviewers independently selected studies, assessed risk of bias, and extracted data. Summary receiver operating characteristic curves, pooled sensitivities, specificities and likelihood ratios were generated.
RESULTS
Fourteen studies met the inclusion criteria, of which 7 provided data on singleton gestations (3374 women) and 8 on twin gestations (1024 women). Among women with singleton gestations, the shortening of cervical length over time had a low predictive accuracy for preterm birth at <37 and <35 weeks of gestation with pooled sensitivities, specificities, and positive and negative likelihood ratios ranging from 49–74%, 44–85%, 1.3–4.1, and 0.3–0.7, respectively. In women with twin gestations, the shortening of cervical length over time had a low to moderate predictive accuracy for preterm birth at <34, <32, <30, and <28 weeks of gestation with pooled sensitivities, specificities, and positive and negative likelihood ratios ranging from 47–73%, 84–89%, 3.8–5.3, and 0.3–0.6, respectively. There were no statistically significant differences between the predictive accuracies for preterm birth of cervical length shortening over time and the single initial and/or final cervical length measurement in 8 of 11 studies that provided data for making these comparisons. In the largest and highest quality study, a single measurement of cervical length obtained at 24 or 28 weeks of gestation was significantly more predictive of preterm birth than any decrease in cervical length between these gestational ages.
CONCLUSIONS
Change in transvaginal sonographic cervical length over time is not a clinically useful test to predict preterm birth in women with singleton or twin gestations. A single cervical length measurement obtained between 18–24 weeks of gestation appears to be a better test to predict preterm birth than changes in cervical length over time.
Keywords: shortening in cervical length, singleton gestation, twin gestation, predictive value of test, screening, prematurity, longitudinal studies
INTRODUCTION
Preterm birth is one of the “great obstetrical syndromes” which are characterized by multiple etiologies, a long preclinical stage, frequent fetal involvement, clinical manifestations that are often adaptive in nature, and complex interactions between the fetal and maternal genome and the environment that may predispose to the syndrome.1,2 Transvaginal sonographic measurement of cervical length (CL) provides useful information about one of the mechanisms of disease implicated in the etiology of the preterm parturition syndrome. In 1990, Andersen et al.3 published a seminal study in which a transvaginal sonographic CL below the 50th percentile at 30 weeks of gestation was associated with a 3.7-fold increased risk of preterm birth compared with a CL at or above the 50th percentile. Logistic regression analysis showed a progressive and statistically significant trend toward higher risk of preterm birth with a shorter CL. Moreover, it was reported that a CL <39 mm had a sensitivity of 76% and a specificity of 59% to predict preterm birth at <37 weeks of gestation. Since then, transvaginal sonographic CL has been extensively investigated as a predictor of preterm birth.4–11 Several meta-analyses have now provided compelling evidence that a transvaginal sonographic CL measurement at 18–24 weeks of gestation is one of the strongest and most consistent predictors of preterm birth in asymptomatic women with singleton gestations, regardless of whether they have a history of preterm birth,12–15 and twin gestations.16–18
More recently, analysis of serial measurements of transvaginal sonographic CL has shown that assessment of risk for preterm birth can be further refined. Several studies have reported that shortening of transvaginal sonographic CL over time is associated with an increased risk of preterm birth,9,19–23 whereas other studies have not been able to demonstrate this association.24–26 Recently, there has been a renewed interest in the relationship between CL changes over time and the risk of preterm birth.27 The shortening of transvaginal sonographic CL over time has been proposed as a better predictor of spontaneous preterm birth than a single CL measurement.9 However, to the best of our knowledge, there are no studies that have systematically evaluated the predictive performance of this test.
The primary aim of this study was to determine the accuracy of changes in transvaginal sonographic CL over time to predict preterm birth in women with singleton and twin gestations through the use of formal methods for systematic reviews and meta-analytic techniques.
MATERIALS AND METHODS
This study followed a prospectively prepared protocol and is reported in accordance with recommended methods for systematic reviews of diagnostic test accuracy.28,29 The two authors independently retrieved and reviewed studies for eligibility, assessed their risk of bias, and extracted data. All disagreements encountered in the review process were resolved through consensus.
Data sources and searches
To identify potentially eligible studies, we searched PubMed, Embase, Cinahl, Lilacs, and Medion (all from inception to May 31, 2015) using an existing literature search strategy for systematic reviews of predictive tests for preterm birth.30 Google Scholar, proceedings of congresses on preterm birth, ultrasound in obstetrics and maternal-perinatal medicine, and reference lists of identified studies were also searched. No language restrictions were applied.
Eligibility criteria
The systematic review focused on cohort or cross-sectional studies that reported on the accuracy of changes in transvaginal sonographic CL over time to predict preterm birth in asymptomatic pregnant women with a singleton or twin gestation, and that allowed construction of 2×2 contingency tables. Studies were excluded if they: (1) were case-control studies, because there is consistent evidence that they are associated with higher diagnostic or predictive accuracy compared with cohort studies;31 (2) assessed CL changes over time in women with cervical cerclage or pessary, preterm labor, premature rupture of membranes, or those who were receiving progestogens; (3) were reviews, case series or reports, editorials, or letters without original data; or (4) did not publish accuracy test estimates and sufficient information to calculate them could not be retrieved. For studies that resulted in multiple publications, the data from the one with the largest sample size were used and supplemented if additional information appeared in the others.
Reference standard outcomes
In women with singleton gestations: spontaneous preterm birth at <37 and <35 weeks of gestation; in women with twin gestations: spontaneous preterm birth at <34, <32, <30, and <28 weeks of gestation.
Assessment of risk of bias
Study quality was assessed using a modified version of the QUADAS (Quality Assessment of Diagnostic Accuracy Studies)-2 tool.32 The assessments were judged as “low risk”, “high risk”, or “unclear risk” of bias. The items were evaluated and interpreted were as follows:
Patient selection - “low risk of bias”: women consecutively or randomly selected; “high risk of bias”: convenience sampling (arbitrary recruitment or non-consecutive recruitment).
Description of the test - “low risk of bias”: the study described sufficient details of the technique used for measuring CL such as the plane in which images were obtained, anatomic references for the determination of CL, and number of measurements; “high risk of bias”: if this information was not reported.
Reference standard - “low risk of bias”: spontaneous preterm birth, defined as a preterm delivery after the spontaneous onset of contractions or preterm premature rupture of membranes, regardless of whether the delivery was vaginal, by cesarean section, or, in the case of rupture of membranes, induced; “high risk of bias”: inclusion of both spontaneous and indicated preterm birth in the reference standard.
Blinding - “low risk of bias”: the study clearly stated that clinicians managing the patient did not have knowledge of the CL measurement results; “high risk of bias”: unmasking of clinicians to test results.
Inclusion of women in the analysis - “low risk of bias”: if at least 90% of women recruited into the study were included in the analysis; “high risk of bias”: if less than 90% of women recruited into the study were included in the analysis.
Use of interventions aimed to prevent preterm birth based on the test results -”low risk of bias”: clinicians did not use interventions based on the results of the CL measurements; “high risk of bias”: clinicians used interventions based on the results of the test (e.g. cerclage, pessary, vaginal progesterone).
If there was insufficient information available to make a judgment about these items, then they were scored as “unclear risk of bias”. We did not calculate a summary score estimating the overall quality of each study because of the well-known problems associated with such scores.33
Data extraction
Data were extracted from each article using a specifically designed form for capturing information on study characteristics, patients characteristics (inclusion and exclusion criteria, risk classification for preterm birth, sample size, plurality of pregnancy, and demographics), risk of bias, how the test was carried out (technique used for measuring CL, gestational ages at testing, and cutoff values used for single CL measurements and CL changes over time), and reference standard outcomes. For each study, for all reported cutoff values for single CL measurements and CL changes over time, and for all categories of preterm birth, we then extracted the number of true-positive, false-positive, true-negative, and false-negative test results. When predictive accuracy data were not available, we recalculated them from the reported results including scatter-plot graphs.
Data were extracted separately for singleton (unselected population, and low and high risk for preterm birth) and twin gestations, and for each reference standard outcome assessed. Studies that reported preterm birth at <36 weeks of gestation were grouped with those that reported preterm birth at <37 weeks of gestation, and those reporting preterm birth at <35 weeks of gestation were considered alongside studies reporting preterm birth at <34 weeks of gestation.
Data synthesis
Data from individual studies were synthesized separately for singleton and twin gestations and stratified according to the predefined reference standard outcomes, regardless of the gestational ages at which CL was measured and cutoff values used to define shortening of CL. For singleton gestations, we synthesized data for all women, those at high risk for preterm birth, those at high risk for preterm birth with an initial normal CL, those at low risk for preterm birth, and those from unselected populations.
Data extracted from each study were arranged in 2×2 contingency tables. When any single cell in these tables contained a zero, we added 0.5 to each cell to enable calculation of likelihood ratios (LRs) and confidence intervals (CIs).34 Sensitivity and specificity with 95% CIs were calculated separately for all reported cutoff values and reference standard outcomes reported. We then constructed summary receiver operating characteristic (ROC) curves by means of a bivariate random-effects approach35 and calculated area under the summary ROC curves with their corresponding 95% CIs.36 A two-sided P <.05 was considered to be statistically significant. We used random-effects bivariate regression models to meta-analyze the logit-transformed sensitivity and specificity to obtain pooled estimates and 95% CIs of these variables.35,37 Thereafter, we calculated LRs with 95% CIs from the pooled sensitivities and specificities for each reference standard outcome considered.38 LRs for a positive test result above 10 and LRs for a negative test result below 0.1 are considered to provide strong predictive evidence in most circumstances. Moderate prediction can be achieved with LRs values of 5–10 and 0.1–0.2 whereas those <5 and >0.2 give only minimal prediction.39
We assessed the heterogeneity of the results among studies through visual examination of forest plots of sensitivities and specificities and by means of the quantity I2.40 A substantial level of heterogeneity was defined as an I2 ≥ 50%.50 We explored potential sources of heterogeneity by performing meta-regression analysis of subgroups defined a priori (study′s risk of bias, gestational ages at testing, cutoff values used, sample size, prevalence of the reference standard outcome, and setting).41 We planned to assess publication and location biases,42 but this was not performed because there were <10 studies in each meta-analysis. Finally, we compared the predictive accuracy for spontaneous preterm birth of the initial and/or final CL measurements and the changes in CL over time in individual studies that provided this information. When comparing the performance of two predictive tests, it is more convenient to summarize the predictive accuracy with one single overall measurement. We calculated the Youden index43 for the initial and/or final CL measurements and the shortening of CL over time in each study. This index is formally defined as Sensitivity + Specificity - 1 and its value ranges from 0 for a useless test to 1 for an ideal test. A Z-score test was then used to estimate the statistical significance of the difference between the Youden index of shortening of CL over time and that of the initial or final CL measurement.43 A two-sided P <.05 was considered to be statistically significant.
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC) and Review Manager (RevMan) version 5.3.5 (The Nordic Cochrane Centre, København, Denmark).
RESULTS
Selection, characteristics, and quality of studies
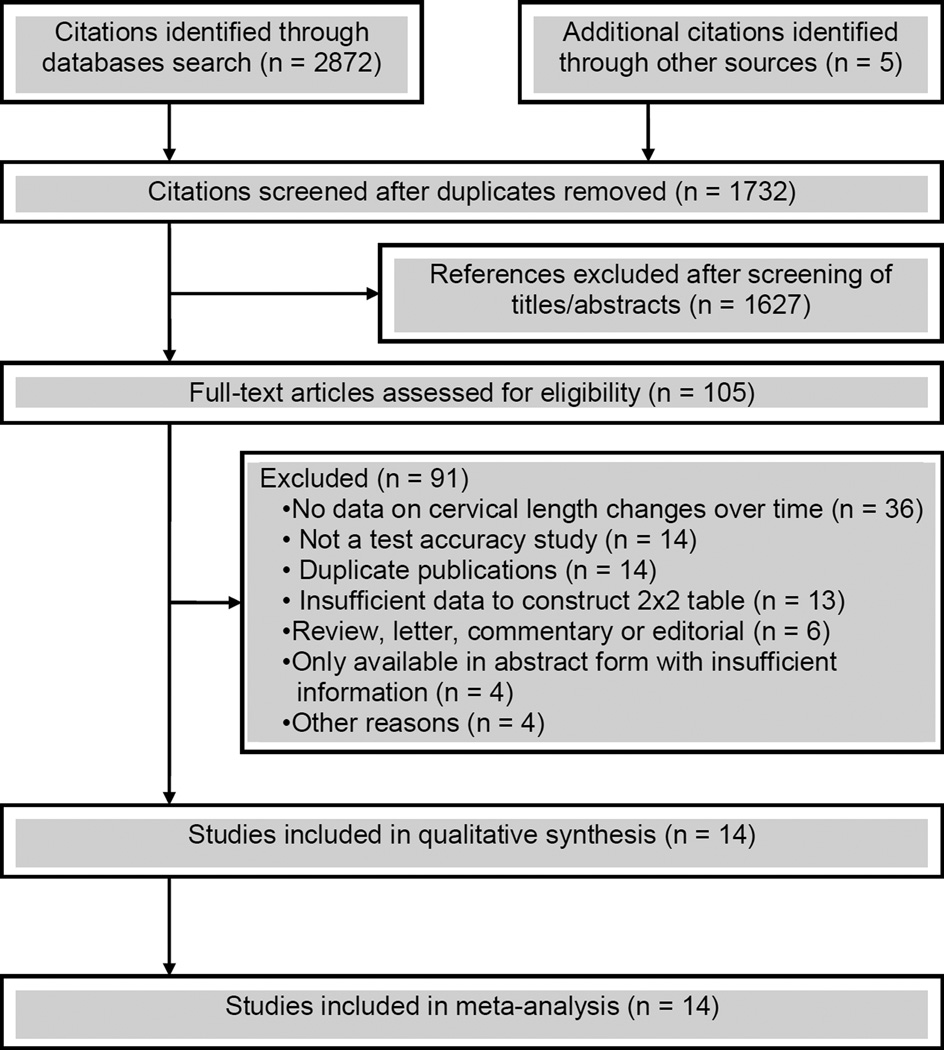
A total of 1732 citations were identified, of which 105 were retrieved for full text review. Of these, 91 were excluded, mainly because they did not provide data on CL changes over time, were not a test accuracy study, were duplicate publications, or provided insufficient data to construct 2×2 tables (Figure 1). Fourteen studies,4,44–56 including a total of 4398 women, met the inclusion criteria of which six provided data on women with singleton gestations (n=3236),4,44,47,49,50,53 seven on women with twin gestations (n=871),45,46,51,52,54–56 and one on women with singleton (n=138) and twin (n=153) gestations.48
Figure 1.
Study selection process
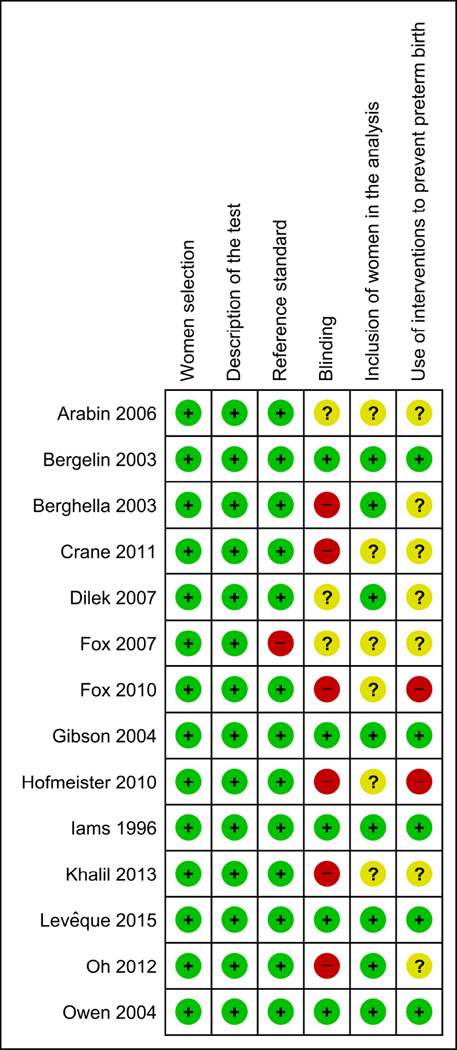
The main characteristics of the included studies are listed in Table 1. Five studies were conducted in the United States, four in European countries, three in Asia, and one each in Canada and Brazil. The sample size ranged from 6849 to 25314 in women with singleton gestations and from 2045 to 20955 in women with twin gestations. Among the seven studies performed in women with singleton gestations, five included exclusively women at high risk for preterm birth, one included only women at low risk, and the remainder included an unselected population. Prophylactic cerclage and major fetal anomalies were reported as exclusion criteria in half of the included studies. The gestational ages at initial and final CL measurement ranged from 10–28 weeks and 20–30 weeks, respectively. The initial CL measurement was carried out at 10–13 weeks in one study, at 14–19 weeks in 7 studies, at 20–24 weeks in 5 studies, and at 25–30 weeks in one study. The final CL measurement was carried out at 20–24 weeks in six studies and at 25–30 weeks in eight studies. The test result was considered abnormal if there was a shortening of CL over time ≥20%,45,51,54,56 or ≥25%,55 or ≥13%,54 or >10%,53 or >2 mm/wk,52 or ≥2.5 mm/wk,46 or ≥6.6 mm,50 or ≥6 mm,4 or >5, >10 and >15 mm.48 Two studies that included women with an initial CL ≥25 mm44 or >30 mm47 used a shortening of CL to <25 mm44 or ≤30 mm47 at follow up transvaginal sonography to indicate an abnormal test result. Any decrease in CL defined abnormality in two studies.4,49 Seven studies used ROC curve analysis to determine the optimal cutoff value for defining an abnormal change in CL over time.46,50–52,54–56 The remaining studies used arbitrary cutoff values to define an abnormal test result. Twelve studies provided data on the predictive accuracy of shortening in CL over time for preterm birth at <34 or <35 weeks of gestation, 4 each on preterm birth at <37 and <32 weeks of gestation, and 3 each on preterm birth at <30 and <28 weeks of gestation. The risk of bias in each included study is shown in Figure 2. Five studies (36%) fulfilled all six criteria. Nine studies (64%) had two or more methodological flaws. The most common deficiencies were related to blinding of clinicians to the test results and the use of interventions aimed to prevent preterm birth based on the test results.
TABLE 1.
Characteristics of studies included in the systematic review
| Study, year (country) |
No. of women |
Inclusion criteria | Exclusion criteria | Gestational ages at testing (wks) |
Abnormal test result |
Reference standard outcome |
|---|---|---|---|---|---|---|
| Iams,4 1996 (United States) |
2531 | Singleton gestation (unselected population) |
Multiple gestation, cerclage, placenta previa, major fetal anomaly |
24 (range, 22–24) and 28 (range, 26–29) |
Any decrease in CL; shortening of CL ≥6 mm |
Spontaneous preterm birth <35 wk |
| Berghella,44 2003 (United States) |
173a | Singleton gestation at high risk (≥1 previous spontaneous preterm birth between 14 and 34 wk or ≥2 dilatation and curettage procedures, Müllerian anomaly, cone biopsy, diethylstilbestrol exposure) with an initial CL ≥25 mm |
Placenta previa, current drug abuse, severe fetal anomalies |
10–13 and then every 2 to 4 wks up to 23 wks 6 days′ gestation |
Shortening of CL to <25 mm at 14- 24 wks |
Spontaneous preterm birth <35 wk |
| Bergelin,45 2003 (Sweden) |
20 | Twin gestation | Pregnancy complications | 24 and 28 | Shortening of CL ≥20% |
Spontaneous preterm birth <34 wk |
| Gibson,46 2004 (United Kingdom) |
91 | Twin gestation | Twin–to-twin transfusion syndrome, fetal anomalies |
18, 24, and 28 | Shortening of CL ≥2.5 mm/wk between 18 and 28 wks’ gestation |
Spontaneous preterm birth <35 wk |
| Owen,47 2004 (United States) |
137b | Singleton gestation at high risk (previous spontaneous preterm birth before 32 wk of gestation) with an initial CL >30 mm |
Chronic medical or obstetric conditions, history of substance abuse, uterine anomalies, history- indicated cerclage |
16–18 and then every two wk up to 23 wk 6 days′ gestation |
Shortening of CL to ≤30 mm at 19- 24 wks |
Spontaneous preterm birth <35 wk |
| Arabin,48 2006 (The Netherlands) |
291 | Singleton gestation at high risk (previous spontaneous preterm birth or uterine anomaly) (N=138) and twin gestation (N=153) |
Unreported | 15–19 and 20–24 | Shortening of CL >5, >10 and >15 mmc |
Spontaneous preterm birth <36 wk |
| Fox,49 2007 (United States) |
68 | Singleton gestation with a CL ≤25 mm at 16–28 wk of gestation and expectant management |
Cerclage | 16–28 (median, 22) and within 3 wk of the initial measurement (median, 23) |
Any decrease in CL |
Preterm birth <34 and <37 wk |
| Dilek,50 2007 (Turkey) |
257 | Singleton gestation (low risk) | History of preterm birth, preterm PROM, cervical incompetence, multiple gestation, previously detected cervical funneling, Müllerian anomalies |
16 and 24 | Shortening of CL ≥6.6 mm |
Spontaneous preterm birth <37 wk |
| Fox,51 2010 (United States) |
121 | Twin gestation | Monoamniotic twins, major fetal anomalies, preterm labor, aneuploidy, twin–to- twin transfusion syndrome |
18–24 and within 2–6 wk of the initial measurement (98% at or before 25 wk) |
Shortening of CL ≥20% |
Spontaneous preterm birth <32, <28, <30, and <34 wk |
| Hofmeister,52 2010 (Brazil) |
124 | Twin gestation | Monoamniotic twins, twin- to-twin transfusion syndrome, polyhydramnios, intrauterine fetal death, fetal malformation, iatrogenic preterm birth Cerclage |
18–21 and 22–25 | Shortening of CL >2 mm/wk |
Spontaneous preterm birth <34, <28, <30, and <32 wk |
| Crane,53 2011 (Canada) |
70 | Singleton gestation at high risk (previous spontaneous preterm birth or excisional cervical procedure, or uterine anomaly) with a CL <30 mm at 20–28 wk of gestation |
Cerclage | 20–28 (mean, ~26) and within 3 wk of the initial measurement (mean, ~28) |
Shortening of CL >10% |
Spontaneous preterm birth <35, <37, <34, and <32 wk |
| Oh,54 2012 (Korea) |
190 | Twin gestation with a CL >25 mm at 20–24 wk of gestation |
Prophylactic cerclage, PROM, preterm labor, major fetal anomalies, twin–to-twin transfusion syndrome, placenta previa, monoamniotic placenta |
20–24 (mean, 21.9) and within 4–5 wk of the initial measurement (mean, 26.0) |
Shortening of CL ≥13% and ≥20% |
Spontaneous preterm birth <32 and <34 wk |
| Khalil,55 2013 (Saudi Arabia) |
209 | Twin gestation with a CL >25 mm at first measurement |
CL ≤25 mm on the first ultrasound, monoamniotic twins, preterm labor with or without PROM, abnormal vaginal discharge, severe twin–to-twin transfusion syndrome, aneuploidy, major fetal anomalies, elective cerclage |
20–23 and within 3–5 wk of the initial measurement |
Shortening of CL ≥25% |
Spontaneous preterm birth <32, <34, <30, and <28 wk |
| Levêque,56 2015 (France) |
116 | Twin gestation | Prophylactic cerclage, placenta previa, major fetal anomalies, twin-to-twin transfusion syndrome, PROM, undetermined gestational age |
22 (range, 21–23) and 27 (range, 26–28) |
Shortening of CL >20% |
Spontaneous preterm birth <34 wk |
CL, cervical length; PROM, premature rupture of the membranes
From the 183 women included in the study, we excluded 10 with an initial CL <25 mm without data on CL changes over time
From the 183 women included in the study, we excluded 46 with an initial CL ≤30 mm without data on CL changes over time
In the meta-analyses performed, we used predictive values for “shortening in CL >10 mm” with women in recumbent position
Figure 2.
Methodological quality of studies included in the systematic review
Predictive accuracy for preterm birth in singleton and twin gestations
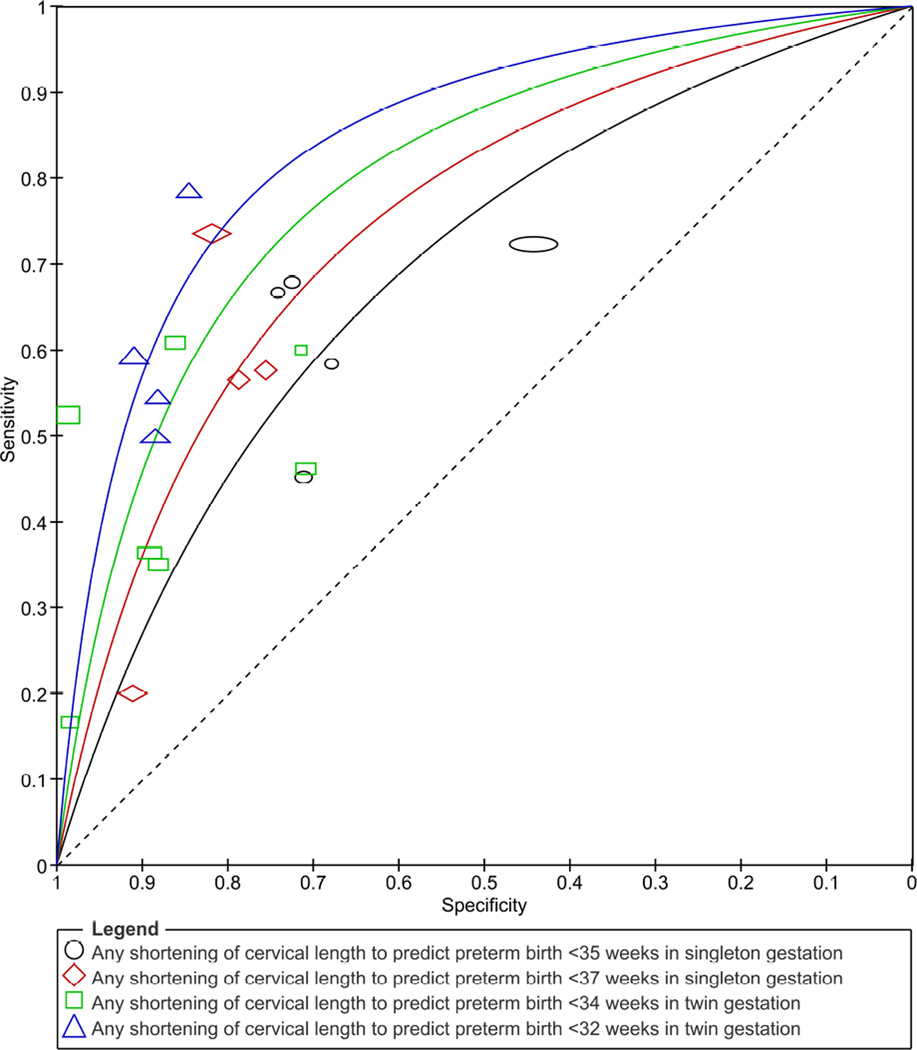
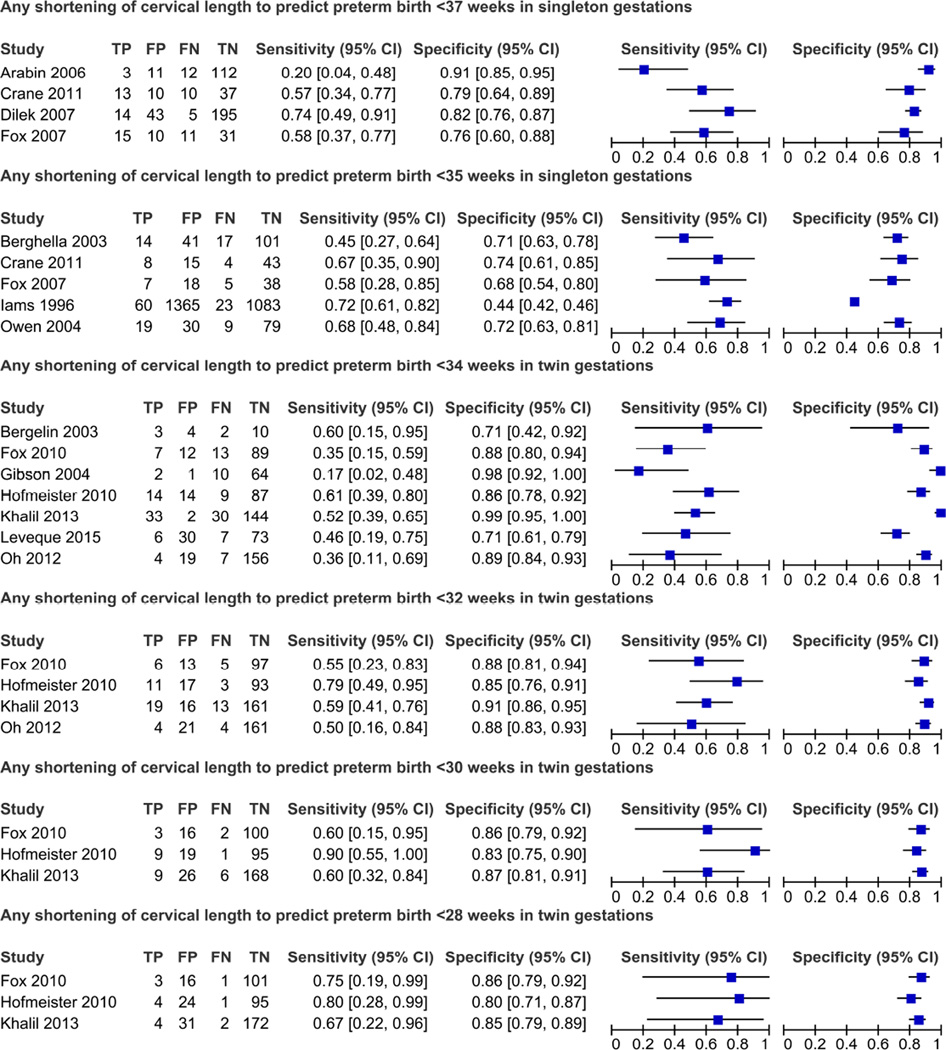
Figure 3 shows the summary ROC curves of changes in CL over time to predict spontaneous preterm birth. The shortening of CL over time had a higher predictive accuracy for preterm birth among women with twin gestations with areas under the summary ROC curves (95% CI) of 0.81(0.74–0.89) for preterm birth at <32 weeks of gestation and 0.76 (0.69–0.83) for preterm birth at <34 weeks of gestation. Among women with singleton gestations, the areas under the summary ROC curves to predict preterm birth at <35 and <37 weeks of gestation were 0.64 (0.56–0.72) and 0.71 (0.62–0.80), respectively. The sensitivity and specificity of any shortening of CL to predict preterm birth in the single studies are shown in Figure 4. Pooled estimates of accuracy of CL changes over time for the prediction of spontaneous preterm birth are presented in Table 2. Overall, regardless of the risk status of women, reference standard outcome assessed and definition of an abnormal test result, the predictive ability of shortening of CL over time for preterm birth was low among women with singleton gestations (pooled sensitivities, specificities, and positive and negative LRs ranging from 49–74%, 44–85%, 1.3–4.1, and 0.3–0.7, respectively) and low to moderate among women with twin gestations (pooled sensitivities, specificities, and positive and negative LRs ranging from 47–73%, 84–89%, 3.8–5.3, and 0.3–0.6, respectively). In women with singleton gestations, the pooled positive and negative LRs of any shortening of CL to predict preterm birth at <37 and <35 weeks of gestation were 3.2 and 0.6, and 1.3 and 0.7, respectively. In women with twin gestations, the pooled positive and negative LRs of any shortening of CL to predict preterm birth at <34 and <32 weeks of gestation were 4.0 and 0.6 and 5.3 and 0.5, respectively. There was a substantial level of heterogeneity among studies in the meta-analyses of preterm birth at <34 weeks of gestation among women with twin gestations, which was explained entirely by the study of Khalil et al.55 In fact, when we removed this study from the meta-analyses, the I2 was 0% and the pooled positive likelihood ratios decreased from 4.0 to 3.0 for any shortening of CL, and from 3.8 to 2.5 for shortening of CL ≥20–25%.
Figure 3.
Summary receiver operating characteristic curves of changes in cervical length over time to predict spontaneous preterm birth
Figure 4.
Forest plots of any shortening of cervical length to predict preterm birth in singleton and twin gestations
TABLE 2.
Pooled estimates for changes in cervical length over time to predict preterm birth
| Population | Outcome | Abnormal test result | No. of studies/ total number of women |
Sensitivity (%) (95% CI) |
Specificity (%) (95% CI) |
Positive likelihood ratio (95% CI) |
Negative likelihood ratio (95% CI) |
I2 (%) |
|---|---|---|---|---|---|---|---|---|
| Singleton gestation (all) |
Preterm birth <37 wk | Any shortening of CL | 448–50,53/532 | 54 (43–65) | 84 (80–87) | 3.2 (2.2–4.4) | 0.6 (0.4–0.9) | 0 |
| Preterm birth <35 wk | Any shortening of CL | 54,44,47,49,53/2979 | 65 (57–72) | 48 (46–50) | 1.3 (1.1–1.4) | 0.7 (0.6–0.9) | 25 | |
| Singleton gestation at high risk |
Preterm birth <37 wk | Any shortening of CL | 348,49,53/275 | 49 (37–61) | 85 (80–90) | 3.3 (2.2–5.0) | 0.6 (0.5–0.8) | 0 |
| Preterm birth <35 wk | Any shortening of CL | 444,47,49,53/448 | 58 (47–68) | 72 (67–76) | 2.0 (1.6–2.6) | 0.6 (0.4–0.8) | 12 | |
| Singleton gestation at high risk with an initial normal CL |
Preterm birth <35 wk | Shortening of CL to <25 or <30 mm at ≤24 wks |
244,47/310 | 56 (43–68) | 72 (66–77) | 2.0 (1.5–2.7) | 0.6 (0.4–0.8) | 63 |
| Singleton gestation (unselected) |
Preterm birth <35 wk | Any decrease in CL | 14/2531 | 72 (62–81) | 44 (42–46) | 1.3 (1.1–1.5) | 0.6 (0.4–0.9) | NA |
| Singleton gestation at low risk |
Preterm birth <37 wk | Shortening of CL ≥6.6 mm |
150/257 | 74 (51–88) | 82 (77–86) | 4.1 (2.8–6.0) | 0.3 (0.1–0.7) | NA |
| Twin gestation | Preterm birth <34 wk | Any shortening of CL | 745,46,51,52,54–56 /852 |
47 (39–55) | 88 (86–91) | 4.0 (2.0–8.3) | 0.6 (0.5–0.8) | 66 |
| Shortening of CL ≥20- 25% |
545,51,54–56 /651 | 47 (38–57) | 87 (84–90) | 3.8 (2.8–5.1) | 0.6 (0.5–0.7) | 76 | ||
| Preterm birth <32 wk | Any shortening of CL | 451,52,54,55 /644 | 61 (49–73) | 88 (85–91) | 5.3 (3.4–7.2) | 0.5 (0.3–0.6) | 0 | |
| Shortening of CL ≥20- 25% |
351,54,55/520 | 56 (42–69) | 89 (86–92) | 5.2 (3.7–7.5) | 0.5 (0.4–0.7) | 0 | ||
| Preterm birth <30 wk | Any shortening of CL | 351,52,55 /454 | 70 (52–83) | 86 (82–89) | 4.8 (3.5–6.8) | 0.4 (0.2–0.6) | 0 | |
| Preterm birth <28 wk | Any shortening of CL | 351,52,55/454 | 73 (48–89) | 84 (80–88) | 4.5 (3.1–6.6) | 0.3 (0.2–0.7) | 0 |
CI, confidence interval; CL, cervical length; NA, not applicable
Eleven studies (three among women with singleton gestations4,48,50 and eight among women with twin gestations45,46,48,51,52,54–56) provided data that allowed us to compare the predictive accuracy for preterm birth of CL shortening over time and the final CL measurement (Table 3). In addition, five of these studies4,46,50,52,56 also allowed comparison of the predictive accuracy for preterm birth of CL shortening over time and the initial CL measurement. In eight studies,45,46,48,50–52,54,56 there were no statistically significant differences between the predictive accuracies of shortening of CL over time and the single initial and/final CL measurement. Two studies46,56 showed no differences in the predictive accuracy of shortening in CL over time and the single initial CL measurement. In one study,55 a shortening of CL ≥25% was significantly more predictive of preterm birth at <34 weeks of gestation than the single final measurement of CL taken at 23–28 weeks (P=0.03). In two studies, the shortening of CL over time was significantly more predictive of preterm birth than the single initial CL measurement taken at 16 weeks (P=.02 for preterm birth at <37 weeks of gestation),50 and at 18–21 weeks (P=.02 for preterm birth at <32 weeks of gestation and P=.04 for preterm birth at <34 weeks of gestation).52 In the study by Iams et al,4 the largest and highest quality study included in the review, a single measurement of CL taken at 24 or 28 weeks was significantly more predictive of spontaneous preterm birth at <35 weeks of gestation than any decrease in CL between these gestational ages (P=.049 at 24 weeks and P=.008 at 28 weeks). In this study,4 there were no significant differences in the accuracy to predict preterm birth at <35 weeks between a single measurement of CL taken at 24 or 28 weeks of gestation and a shortening of CL ≥6 mm. Overall, the predictive performance of shortening in CL over time was not significantly superior to a single measurement (initial or final) of CL taken at 20–24 weeks of gestation in any study.
TABLE 3.
Comparison of the predictive accuracy for preterm birth of cervical length at first and/or last measurement and change in cervical length over time in individual studies using the Youden index
| Study |
Outcome: preterm birth |
Cervical length at initial measurement | Cervical length at final measurement | Change in cervical length over time | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cut-off value, (GA at testing, wks) |
Youden index |
P valuea | Cut-off value, (GA at testing, wks) |
Youden index |
P valuea | Abnormal test result | Youden index |
||
| Singleton gestation | |||||||||
| Iams,4 1996 | <35 wk | 25 mm (24) | 0.295 | 0.049 | 25 mm (28) | 0.362 | 0.008 | Any decrease in CL | 0.165 |
| <35 wk | 25 mm (24) | 0.295 | 0.44 | 25 mm (28) | 0.362 | 0.12 | Shortening of CL ≥6 mm | 0.241 | |
| Arabin,48 2006 | <36 wk | Unreported (15–19) | NC | NA | 25 mm (20–24) | 0.080 | 0.66 | Shortening of CL >10 mm | 0.140 |
| Dilek,50 2007 | <37 wk | 35.3 mm (16) | 0.200 | 0.02 | 34.3 mm (24) | 0.657 | 0.56 | Shortening of CL ≥6.6 mm | 0.556 |
| Twin gestation | |||||||||
| Bergelin,45 2003 | <34 wk | Unreported (24) | NC | NA | 25 mm (28) | 0.129 | 0.56 | Shortening of CL ≥20% | 0.314 |
| Gibson,46 2004 | <35 wk | 25 mm (18) | 0.128 | 0.87 | 25 mm (28) | 0.042 | 0.43 | Shortening of CL ≥2.5 mm/week | 0.151 |
| Arabin,48 2006 | <36 wk | Unreported (15–19) | NC | NA | 20 mm (20–24) | 0.080 | 0.06 | Shortening of CL >10 mm | 0.285 |
| Fox,51 2010 | <32 wk | Unreported (18–24) | NC | NA | 25 mm (20–25) | 0.300 | 0.55 | Shortening of CL ≥20% | 0.427 |
| <34 wk | Unreported (18–24) | NC | NA | 25 mm (20–25) | 0.250 | 0.90 | Shortening of CL ≥20% | 0.231 | |
| Hofmeister,52 2010 |
<32 wk | 5th percentile (18–21) | 0.264 | 0.02 | 5th percentile (22–25) | 0.478 | 0.35 | Shortening of CL >2 mm/week | 0.631 |
| <34 wk | 5th percentile (18–21 ) | 0.206 | 0.04 | 5th percentile (22–25) | 0.344 | 0.36 | Shortening of CL >2 mm/week | 0.470 | |
| Oh,54 2012 | <32 wk | Unreported (20–24) | NC | NA | 30 mm (24–29) | 0.503 | 0.98 | Shortening of CL ≥13% | 0.507 |
| <34 wk | Unreported (20–24) | NC | NA | 30 mm (24–29) | 0.482 | 0.50 | Shortening of CL ≥13% | 0.350 | |
| Khalil,55 2013 | <32 wk | Unreported (20–23) | NC | NA | 25 mm (23–28) | 0.367 | 0.28 | Shortening of CL ≥25% | 0.503 |
| <34 wk | Unreported (20–23) | NC | NA | 25 mm (23–28) | 0.317 | 0.03 | Shortening of CL ≥25% | 0.510 | |
| Levêque,56 2015 | <34 wk | 35 mm (22) | 0.093 | 0.71 | 25 mm (27) | 0.412 | 0.23 | Shortening of CL ≥20% | 0.170 |
CL, cervical length; GA, gestational age; NA, not applicable; NC, not calculable
For the difference with Youden index of change in cervical length over time
COMMENT
Main findings
The results of our systematic review shows that, overall, the change in CL over time has a low predictive accuracy for preterm birth at <35 and <37 weeks of gestation in women with singleton gestations, and a low to moderate predictive accuracy for preterm birth at <34, <32, <30 and <28 weeks of gestation in women with twin gestations. Subgroup analyses according to risk status of women and the cutoff value used to define an abnormal test result did not improve predictive accuracy. In addition, data from individual studies suggest that the predictive ability for preterm birth of CL shortening over time does not differ significantly from that of a single CL measurement obtained at 18–23 or 24–28 weeks of gestation.
Several systematic reviews have assessed the predictive accuracy for spontaneous preterm birth of single CL measurements in asymptomatic women with singleton12–15 and twin gestations.16–18 Overall, among women with singleton gestations, a single CL ≤25 mm had a pooled positive likelihood ratio of 6.3 when performed at <20 weeks of gestation, and 4.4–4.7 when performed at 20–24 weeks of gestation, for predicting spontaneous preterm birth at <34 or <35 weeks of gestation. The corresponding pooled negative likelihood ratios ranged from 0.6–08. Among women with twin gestations, a CL ≤20 mm at 20–24 weeks of gestation had pooled positive and negative likelihood ratios of 10.1 and 0.6, respectively, to predict preterm birth at <32 weeks of gestation, and 9.0 and 0.7, respectively, to predict spontaneous preterm birth at <34 weeks of gestation. In the current study, we found that a shortening in CL over time had pooled positive likelihood ratios of 1.3–3.2 and 4.0–5.3 to predict spontaneous preterm birth in women with singleton and twin gestations, respectively. The corresponding pooled negative likelihood ratios varied between 0.5 and 0.7.The reasons for the relatively poor performance of the change in CL over time to predict spontaneous preterm birth adequately are not clear. Factors that could have affected the predictive accuracy of changes in CL over time for preterm birth include the timing and interval of CL measurements, obstetrical history, concurrent risk factors for preterm birth, the baseline risk of preterm birth in the population studied, the inclusion of women who used interventions aimed to prevent preterm birth, and the cutoff values used to define a positive test result.
Strengths and limitations of the study
The strength of our review lies in the rigorous methodological criteria used for performing a systematic review of predictive test accuracy. These included, among others: (1) the use of a prospective protocol designed to address a highly specific research question; (2) the extensive literature searches without language restrictions; (3) the study quality assessment that was based on strict predetermined criteria; (4) the quantitative synthesis of the evidence; (5) the use of contemporary statistical methods to obtain summary measures of predictive accuracy; (6) the exploration of potential sources of heterogeneity; and (7) the comparison of the predictive ability for preterm birth of the changes in CL over time and the single CL measurements taken at the initial and/or final transvaginal sonographic examination. Our review is subject to some potential limitations. First, only approximately one third of the studies included in the review could be considered at low risk of bias. In more than half of the included studies there was a lack of blinding of test results and omission of information on whether women received any interventions aimed to prevent preterm birth based on test results. This is particularly relevant because the use of preventive therapies could introduce bias in the assessment of the test’s predictive accuracy. Second, there were considerable differences in cutoff values for defining abnormal changes in CL over time among studies, which limited our ability to make comparisons. Third, 13 studies were excluded because they did not report sufficient information to construct a 2×2 table resulting in a potential loss of relevant data. Ten of these studies reported that shortening in CL over time was associated with a significant increased risk of preterm birth or that mean or median rate of CL shortening over time was significantly higher in women who delivered preterm as compared to those who delivered at term. The remaining three studies found no association between CL shortening over time and the risk of preterm birth. Finally, the statistical power of some of our meta-analyses was limited by the small number of studies within each subgroup and the relatively small sample size of some included studies.
To be clinically useful, a predictive test should be associated with an intervention that reduces the risk of preterm birth and perinatal morbidity and mortality. Currently, there is strong evidence that the administration of vaginal progesterone to women with a sonographic CL ≤25 mm in the midtrimester, with or without previous spontaneous preterm birth, and singleton gestation is associated with a significant reduction in the risk of both spontaneous preterm birth and neonatal morbidity and mortality.57 We previously demonstrated that either vaginal progesterone or cerclage are equally efficacious in reducing preterm birth at <32 weeks of gestation and perinatal morbidity and mortality among women with a sonographic CL <25 mm at midtrimester, previous spontaneous preterm birth, and singleton gestation.58 Moreover, there is growing evidence that vaginal progesterone also reduces the risk of perinatal morbidity and mortality in women with a twin gestation and a sonographic CL ≤25 mm in the midtrimester.57,59 In addition, there is some evidence indicating that cervical pessary could be useful in reducing preterm birth among women with a singleton gestation and a CL ≤25 mm at 20–23 weeks of gestation.60 At present, there is no effective intervention to prevent preterm birth in women with a singleton or twin gestation and shortening of CL over time. Vaginal progesterone might be a reasonable therapy for these patients. In fact, a secondary analysis from a large randomized controlled trial in women with a singleton gestation and a prior preterm birth showed that patients treated with vaginal progesterone had significantly less CL shortening over an approximate 8-week interval than those treated with placebo.61
Implications for practice and research
Currently, CL change as a function of time cannot be considered a clinically useful test to predict spontaneous preterm birth in women with singleton or twin gestations. A single CL measurement taken at 18–24 weeks of gestation appears to be a better test to predict preterm birth than changes in CL over time, and seems to be more cost-effective (due to fewer transvaginal sonographic examinations) than serial CL measurements.
Further well-designed prospective studies are required to more rigorously define “abnormal” CL change over time in different populations and to evaluate its predictive performance for preterm birth in women with singleton or twin gestations, mainly in those with a sonographic CL >25 mm at 18–24 weeks of gestation. If such studies demonstrate that a positive test result accurately identifies those who will deliver prematurely, the next step will be to demonstrate that use of changes in CL is associated with reductions in the risk of spontaneous preterm birth by means of randomized controlled trials in which women with an “abnormal” CL change over time are allocated to receive an intervention or placebo/no intervention. Moreover, the identification of distinct patterns of change in CL as a function of gestational age and the assessment of their predictive ability for preterm birth in women with singleton and twin gestations calls for further research.
Acknowledgments
Financial support: This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: the authors report no conflicts of interest.
REFERENCES
- 1.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859–867. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 4.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 5.Berghella V, Tolosa JE, Kuhlman K, Weiner S, Bolognese RJ, Wapner RJ. Cervical ultrasonography compared with manual examination as a predictor of preterm delivery. Am J Obstet Gynecol. 1997;177:723–730. doi: 10.1016/s0002-9378(97)70259-x. [DOI] [PubMed] [Google Scholar]
- 6.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–317. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 7.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks’ gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92:902–907. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 8.Hassan SS, Romero R, Berry SM, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 9.Owen J, Yost N, Berghella V, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340–1348. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 10.de Carvalho MH, Bittar RE, Brizot Mde L, Bicudo C, Zugaib M. Prediction of preterm delivery in the second trimester. Obstet Gynecol. 2005;105:532–536. doi: 10.1097/01.AOG.0000154157.22500.1d. [DOI] [PubMed] [Google Scholar]
- 11.To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362–367. doi: 10.1002/uog.2773. [DOI] [PubMed] [Google Scholar]
- 12.Honest H, Bachmann LM, Coomarasamy A, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervical transvaginal sonography in predicting preterm birth: a systematic review. Ultrasound Obstet Gynecol. 2003;22:305–322. doi: 10.1002/uog.202. [DOI] [PubMed] [Google Scholar]
- 13.Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol. 2008;31:579–587. doi: 10.1002/uog.5323. [DOI] [PubMed] [Google Scholar]
- 14.Honest H, Forbes CA, Durée KH, et al. Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess. 2009;13:1–627. doi: 10.3310/hta13430. [DOI] [PubMed] [Google Scholar]
- 15.Domin CM, Smith EJ, Terplan M. Transvaginal ultrasonographic measurement of cervical length as a predictor of preterm birth: a systematic review with meta-analysis. Ultrasound Q. 2010;26:241–248. doi: 10.1097/RUQ.0b013e3181fe0e05. [DOI] [PubMed] [Google Scholar]
- 16.Conde-Agudelo A, Romero R, Hassan SS, Yeo L. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:128.e1–128.e12. doi: 10.1016/j.ajog.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim AC, Hegeman MA, Huis In ‘T Veld MA, Opmeer BC, Bruinse HW, Mol BW. Cervical length measurement for the prediction of preterm birth in multiple pregnancies: a systematic review and bivariate meta-analysis. Ultrasound Obstet Gynecol. 2011;38:10–17. doi: 10.1002/uog.9013. [DOI] [PubMed] [Google Scholar]
- 18.Kindinger L, Ashrafian H, Poon L, et al. Prediction of preterm delivery with cervical length in twin pregnancy: A meta-analysis and systematic review. Reprod Sci. 2014;21(Suppl 1):256A. [Google Scholar]
- 19.Guzman ER, Mellon C, Vintzileos AM, Ananth CV, Walters C, Gipson K. Longitudinal assessment of endocervical canal length between 15 and 24 weeks’ gestation in women at risk for pregnancy loss or preterm birth. Obstet Gynecol. 1998;92:31–37. doi: 10.1016/s0029-7844(98)00120-3. [DOI] [PubMed] [Google Scholar]
- 20.Naim A, Haberman S, Burgess T, Navizedeh N, Minkoff H. Changes in cervical length and the risk of preterm labor. Am J Obstet Gynecol. 2002;186:887–889. doi: 10.1067/mob.2002.123058. [DOI] [PubMed] [Google Scholar]
- 21.Carvalho MH, Bittar RE, Brizot ML, Maganha PP, Borges da Fonseca ES, Zugaib M. Cervical length at 11–14 weeks’ and 22–24 weeks’ gestation evaluated by transvaginal sonography, and gestational age at delivery. Ultrasound Obstet Gynecol. 2003;21:135–139. doi: 10.1002/uog.32. [DOI] [PubMed] [Google Scholar]
- 22.Ozdemir I, Demirci F, Yucel O, Erkorkmaz U. Ultrasonographic cervical length measurement at 10–14 and 20–24 weeks gestation and the risk of preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2007;130:176–179. doi: 10.1016/j.ejogrb.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Moroz LA, Simhan HN. Rate of sonographic cervical shortening and the risk of spontaneous preterm birth. Am J Obstet Gynecol. 2012;206:234.e1–234.e5. doi: 10.1016/j.ajog.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Ong S, Smith A, Smith N, Campbell D, Wilson A. Cervical length assessment in twin pregnancies using transvaginal ultrasound. Acta Obstet Gynecol Scand. 2000;79:851–853. [PubMed] [Google Scholar]
- 25.Antsaklis A, Daskalakis G, Papantoniou G, Mesogitis N, Antsaklis S. The role of cervical length change from first to second trimester of pregnancy for the prediction of preterm delivery. J Matern Fetal Neonatal Med. 2012;25(Suppl 2):55. [Google Scholar]
- 26.Bastek JA, Hirshberg A, Chandrasekaran S, et al. Biomarkers and cervical length to predict spontaneous preterm birth in asymptomatic high-risk women. Obstet Gynecol. 2013;122:283–289. doi: 10.1097/AOG.0b013e31829ab714. [DOI] [PubMed] [Google Scholar]
- 27.Iams JD. Cervical length--time to report the rate of change? Am J Obstet Gynecol. 2014;211:443. doi: 10.1016/j.ajog.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326:41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Cochrane Diagnostic Test Accuracy Working Group. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149:889–897. doi: 10.7326/0003-4819-149-12-200812160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honest H, Bachmann LM, Khan K. Electronic searching of the literature for systematic reviews of screening and diagnostic tests for preterm birth. Eur J Obstet Gynecol Reprod Biol. 2003;107:19–23. doi: 10.1016/s0301-2115(02)00265-8. [DOI] [PubMed] [Google Scholar]
- 31.Whiting PF, Rutjes AW, Westwood ME, Mallett S. A systematic review classifies sources of bias and variation in diagnostic test accuracy studies. J Clin Epidemiol. 2013;66:1093–1104. doi: 10.1016/j.jclinepi.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 33.Whiting P, Harbord R, Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med Res Methodol. 2005;5:19. doi: 10.1186/1471-2288-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44:763–770. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 35.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. 2002;21:1237–1256. doi: 10.1002/sim.1099. [DOI] [PubMed] [Google Scholar]
- 37.Menke J. Bivariate random-effects meta-analysis of sensitivity and specificity with the Bayesian SAS PROC MCMC: methodology and empirical evaluation in 50 meta-analyses. Med Decis Making. 2013;33:692–701. doi: 10.1177/0272989X13475719. [DOI] [PubMed] [Google Scholar]
- 38.Zwinderman AH, Bossuyt PM. We should not pool diagnostic likelihood ratios in systematic reviews. Stat Med. 2008;27:687–697. doi: 10.1002/sim.2992. [DOI] [PubMed] [Google Scholar]
- 39.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271:703–707. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 40.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med. 2002;21:1525–1537. doi: 10.1002/sim.1185. [DOI] [PubMed] [Google Scholar]
- 42.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 44.Berghella V, Talucci M, Desai A. Does transvaginal sonographic measurement of cervical length before 14 weeks predict preterm delivery in high-risk pregnancies? Ultrasound Obstet Gynecol. 2003;21:140–144. doi: 10.1002/uog.28. [DOI] [PubMed] [Google Scholar]
- 45.Bergelin I, Valentin L. Cervical changes in twin pregnancies observed by transvaginal ultrasound during the latter half of pregnancy: a longitudinal, observational study. Ultrasound Obstet Gynecol. 2003;21:556–563. doi: 10.1002/uog.150. [DOI] [PubMed] [Google Scholar]
- 46.Gibson JL, Macara LM, Owen P, Young D, Macauley J, Mackenzie F. Prediction of preterm delivery in twin pregnancy: a prospective, observational study of cervical length and fetalfibronectin testing. Ultrasound Obstet Gynecol. 2004;23:561–566. doi: 10.1002/uog.1048. [DOI] [PubMed] [Google Scholar]
- 47.Owen J, Yost N, Berghella V, et al. Can shortened midtrimester cervical length predict very early spontaneous preterm birth? Am J Obstet Gynecol. 2004;191:298–303. doi: 10.1016/j.ajog.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 48.Arabin B, Roos C, Kollen B, van Eyck J. Comparison of transvaginal sonography in recumbent and standing maternal positions to predict spontaneous preterm birth in singleton and twin pregnancies. Ultrasound Obstet Gynecol. 2006;27:377–386. doi: 10.1002/uog.2694. [DOI] [PubMed] [Google Scholar]
- 49.Fox NS, Jean-Pierre C, Predanic M, Chasen ST. Short cervix: is a follow-up measurement useful? Ultrasound Obstet Gynecol. 2007;29:44–46. doi: 10.1002/uog.3902. [DOI] [PubMed] [Google Scholar]
- 50.Dilek TU, Yazici G, Gurbuz A, et al. Progressive cervical length changes versus single cervical length measurement by transvaginal ultrasound for prediction of preterm delivery. Gynecol Obstet Invest. 2007;64:175–179. doi: 10.1159/000106486. [DOI] [PubMed] [Google Scholar]
- 51.Fox NS, Rebarber A, Klauser CK, Peress D, Gutierrez CV, Saltzman DH. Prediction of spontaneous preterm birth in asymptomatic twin pregnancies using the change incervical length over time. Am J Obstet Gynecol. 2010;202:155.e1–155.e4. doi: 10.1016/j.ajog.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Hofmeister C, Brizot ML, Liao A, Francisco RP, Zugaib M. Two-stage transvaginal cervical length screening for preterm birth in twin pregnancies. J Perinat Med. 2010;38:479–484. doi: 10.1515/jpm.2010.088. [DOI] [PubMed] [Google Scholar]
- 53.Crane JM, Hutchens D. Follow-up cervical length in asymptomatic high-risk women and the risk of spontaneous preterm birth. J Perinatol. 2011;31:318–323. doi: 10.1038/jp.2010.149. [DOI] [PubMed] [Google Scholar]
- 54.Oh KJ, Park KH, Jeong EH, Lee SY, Ryu A, Kim SN. The change in cervical length over time as a predictor of preterm delivery in asymptomatic women with twin pregnancies who have a normal mid-trimester cervical length. Twin Res Hum Genet. 2012;15:516–521. doi: 10.1017/thg.2012.27. [DOI] [PubMed] [Google Scholar]
- 55.Khalil MI, Alzahrani MH, Ullah A. The use of cervical length and change in cervical length for prediction of spontaneous pretermbirth in asymptomatic twin pregnancies. Eur J Obstet Gynecol Reprod Biol. 2013;169:193–196. doi: 10.1016/j.ejogrb.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 56.Levêque C, Vayssière C, Favre R, et al. Cervical length in asymptomatic twin pregnancies: prospective multicenter comparison of predictive indicators. J Matern Fetal Neonatal Med. 2015;28:37–40. doi: 10.3109/14767058.2014.900038. [DOI] [PubMed] [Google Scholar]
- 57.Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124.e1–124.e19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conde-Agudelo A, Romero R, Nicolaides K, Vaginal progesterone vs, et al. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol. 2013;208:42.e1–42.e18. doi: 10.1016/j.ajog.2012.10.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schuit E, Stock S, Rode L, et al. Effectiveness of progestogens to improve perinatal outcome in twin pregnancies: an individual participant data meta-analysis. BJOG. 2015;122:27–37. doi: 10.1111/1471-0528.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goya M, Pratcorona L, Merced C, et al. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomised controlled trial. Lancet. 2012;379:1800–1806. doi: 10.1016/S0140-6736(12)60030-0. [DOI] [PubMed] [Google Scholar]
- 61.O’Brien JM, Defranco EA, Adair CD, et al. Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2009;34:653–659. doi: 10.1002/uog.7338. [DOI] [PubMed] [Google Scholar]